Stress-related disorders show a very robust sex bias, and thus provide a model for elucidating how sex differences at a molecular level translate to sex differences in expression and prevalence of a disease, said Rita Valentino. For example, major depressive disorder (MDD), anxiety, posttraumatic stress disorder (PTSD), and chronic pain disorders are nearly twice as prevalent in females as in males. Even substance use disorders, which are more prevalent in males but have shown increasing prevalence in females, appear to be linked to stress in females, said Valentino. She added that females with substance use disorders demonstrate a higher propensity for stress-induced relapse and a higher incidence of comorbid stress-related psychiatric disorders.
Valentino added that cognitive and affective features associated with depression, anxiety, PTSD, and pain disorders—negative affect, increased arousal, and reward deficit—are also linked to stress in that they have a common circuitry that interacts with stress circuitry. Comparing sex differences in transcriptional profiles across these disorders may provide clues about what governs the differential expression of symptoms, she said.
DEPRESSION
MDD affects approximately 19 million Americans each year (Kessler et al., 2003). The paucity of effective pharmacological treatments for depression may result from the heterogeneity of the disease, including sex differences, said Marianne Seney, assistant professor in the translational neuroscience program in the Department of Psychiatry at the University of Pittsburgh. She noted that compared with men, women are approximately twice as likely to have a single episode of depression and four times as likely to have recurrent depression. Women also have more symptoms, more severe symptoms, and higher subjective distress. In terms of comorbidities, depressed women have a higher incidence of comorbid anxiety disorders, while depressed men have a higher incidence of substance use disorders, said Seney.
The questions she and other scientists have set out to answer is whether these sex differences reflect the involvement of different biological pathways and/or sex-specific brain pathology, and whether a better understanding of
these pathways and pathology may inform the future development of sex-specific treatments.
Seney suggested two different hypotheses regarding sex differences in the molecular pathology of depression. Men and women could have similar pathological changes moderated by sex-related factors such as gonadal hormones, or different pathologies altogether, she said. To explore these hypotheses, her lab and others have investigated gene-expression changes in brain circuits involved in mood regulation. They have used large-scale transcriptomic studies conducted using unbiased approaches such as micro-array or RNA sequencing technologies.
For example, in what Seney called a tour-de-force study, Eric Nestler’s lab used transcriptomic approaches to analyze a large cohort of well-characterized post-mortem brains across six different brain regions involved in mood regulation (Labonté et al., 2017). The results, she said, were striking: Across the cortical and subcortical brain regions they examined, they found that men and women with depression had distinct transcriptional profiles with very little overlap.
Next, they wanted to examine upstream drivers of these sex-specific differences. For this, they used an innovative technique called weighted gene co-expression network analysis (WGCNA) to identify gene modules (sets of genes whose expression is highly correlated across subjects). WGCNA enables investigators to identify gene networks with common functions and examine the commonalities and differences in expression modules between different populations (e.g., male versus female). This technique enabled Nestler and colleagues to identify sex-specific transcriptional networks in MDD. Within one female-specific module, they identified a candidate “hub” gene called DUSP6, which is highly connected to other genes in the network and differentially expressed in depressed women across all brain regions examined, said Seney. They went on to show in mouse models that reducing expression of DUSP6 in the mouse prefrontal cortex drives stress susceptibility in female, but not male, mice.
Seney’s lab replicated Nestler’s work, demonstrating the same molecular changes using a different set of post-mortem brains. She said this confirmed that the sex-specific transcriptional alterations in MDD is a “real” phenomenon. Interestingly, of the small number of genes that were differentially expressed in both sexes, about 75 percent were changed in opposite directions in depressed men and women (Seney et al., 2018) (see Figure 2-1).
In total, Seney’s lab showed that in more than 1,000 genes, there was a significant interaction of sex and disease, meaning that these 1,000 genes were altered differently in depressed men and women. She noted that only about 1 percent of those genes were differentially expressed in non-psychiatric control subjects. Moreover, mouse studies have suggested that
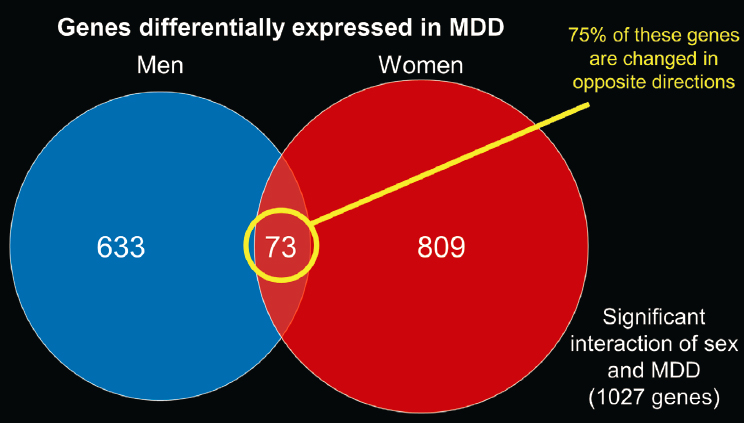
NOTE: MDD = major depressive disorder.
SOURCES: Presented by Marianne Seney, September 23, 2020; adapted from Seney et al., 2018.
some of these sex differences are independent of hormone levels, she said. Her lab went on to show that differentially expressed genes were enriched for synapse structure, function, and organization (decreased expression in men versus increased expression in women) and immune function (increased expression or no change in men versus decreased expression in women). They also showed that depressed men have increased expression of microglia-specific genes (microglia being the resident immune cells of the brain) while depressed women have decreased expression.
Seney suggested that these studies have uncovered potential novel targets for the development of sex-specific depression treatments. They further suggest the possibility that treatments effective in men may be ineffective or even deleterious in women, and vice versa, she said.
POSTTRAUMATIC STRESS DISORDER
As with depression, sex-specific genomic and transcriptomic differences are seen in PTSD, said Matthew Girgenti, research scientist in psychiatry at the Yale School of Medicine. PTSD also has a high degree of comorbidity with depression, he said. Characterized by an uncontrollable and persistent state of fear triggered by a traumatic memory or event, PTSD is particularly prevalent among soldiers, with as many as 30 percent of returning
soldiers found to have PTSD within 1 year of return from deployment, said Girgenti. The prevalence is much lower yet still substantial—about 8 percent—in the general population, according to the National Center for PTSD.1 Given that PTSD is triggered by an outside environmental effect, it is a disorder for which epigenetics likely plays a key role, yet there are also heritable genetic components (Blacker et al., 2019). Moreover, genome-wide association studies (GWASs) with large diverse cohorts of PTSD cases and controls indicate that the heritability of PTSD is much higher in females than in males and nearly as high as schizophrenia in terms of heritability (Nievergelt et al., 2019). According to Girgenti, this means that about 10 percent of women will develop PTSD during their lifetime and are nearly twice as likely as men to develop PTSD after a traumatic event.
The neurobiology of PTSD has focused on regions of the brain that have been implicated in animal studies of fear—the prefrontal cortex, amygdala, and hippocampus (Nees et al., 2018). Girgenti’s lab has conducted a large multi-omics project in post-mortem brain tissue, looking at four areas of the prefrontal cortex—the orbital frontal cortex, anterior cingulate, subgenual, and dorsolateral prefrontal cortex. These studies have shown that gene expression changes overwhelmingly occur in the orbital frontal cortex and dorsolateral prefrontal cortex, with genes involved in synaptic organization tending to be up-regulated, and genes related to glia and gliogenesis tending to be down-regulated, Girgenti said (Girgenti et al., 2021). He noted that this same phenomenon is seen in other neuropsychiatric disorders, including MDD.
Girgenti and colleagues examined the impact of multiple covariates on differential gene expression and found, to their surprise, that the overwhelming amount of differential variance was caused by sex. In females, substantial gene expression changes were seen in three brain regions, while in males, differential gene expression occurred only in one region, and there was very little overlap between male and female brains in terms of their gene expression profiles.
Using WGCNA, they determined that a gene module associated with female PTSD is enriched for markers of endothelial cells, while gene modules associated with male PTSD are enriched for markers of microglia and endothelial cells. Girgenti concluded that there is an overwhelming immune response resulting from the top sex-specific genes in both males and females, but that the genes and pathways differ between the sexes. Digging deeper, they conducted transcriptome-wide association studies (TWASs), which, in combination with GWAS, enabled the identification of one of the most highly significant female-specific, PTSD-associated modules (coral2) and the key driver of this module—a gene involved in interneuron synapse forma-
___________________
1 To learn more, see https://www.ptsd.va.gov (accessed November 27, 2020).
tion called ELFN1. Girgenti suggested that ELFN1 may confer significant genetic liability for PTSD, specifically in females.
The large number of gene expression changes suggested that there would likely be changes in cell-type proportions, said Girgenti. Using bulk sequencing RNA data combined with single-cell data, they demonstrated a significant increase in excitatory neurons and a decrease in astrocytes in females, and an increase in oligodendrocyte precursor cells and endothelial cells in males.
Girgenti and colleagues have also used their transcriptomic data to conduct drug repositioning analyses, a technique that predicts which drugs on the market might target the genes identified as being involved in a particular disorder. This analysis suggested that another gene they linked to PTSD—PLEKHM1—has a shared biological effect with several classes of drugs and thus might be a druggable target.
ADDICTION
Addiction manifests differently in men and women and provides opportunities to better understand basic mechanisms of disrupted sex-specific behavior, said Deena Walker, assistant professor of behavioral neuroscience at Oregon Health & Science University. Men tend to use drugs at a greater rate than women, but women progress to dependence more quickly, report greater craving during withdrawal, are more likely to relapse, and show greater consumption during relapse, putting them at greater risk for overdose, she said (Bobzean et al., 2014). Robust sex differences are also seen in risk of alcohol use disorder, although it is unclear which genetic variants underlie the qualitative difference in risk seen in twin studies or differ in their quantitative effects, said Rohan Palmer, a behavioral geneticist and assistant professor of psychology at Emory University. There is still room for improvement in this area, he said, in part because females account for only about 10 percent of postmortem human brain samples from individuals with overdose-related deaths.
Multiple factors contribute to these sex differences, said Walker. For example, she said neuropharmacological studies indicate that amphetamines induce less release of striatal dopamine in women and that women report less euphoric effects of amphetamines. Adolescent experience and adolescent stress also contribute to sex differences in motivation and reward that influence the risk of addiction, said Walker. Her research as a postdoctoral fellow in Nestler’s lab using an adolescent stress paradigm in mice demonstrated that social isolation drives a big sex difference in behavior: In comparison to group-housed animals where males and females showed an equal preference for cocaine, socially isolated males showed a greater
preference for cocaine while socially isolated females showed a decreased preference (Walker et al., 2020).2
Walker and colleagues examined transcriptional differences in animals displaying sex-specific behavioral responses to cocaine using the social isolation stress model. As was shown earlier in depression and PTSD, in group-housed animals there was little overlap between males and females in terms of the transcriptional response to cocaine. However, there was a gain in the transcriptional response among the socially isolated male animals but not in females, again with little overlap between males and females.
Sexually dimorphic baseline behaviors such as marble burying—males bury more marbles than females—were lost under the stress of social isolation, and these behaviors are also reflected in the transcriptome, said Walker. This observation suggested that the transcriptome might provide information about how behaviors are programmed. To assess changes in global transcriptional structure and potentially identify targets that regulate sex differences in reward, Walker and colleagues used an updated co-expression analytical technique called multiscale embedded gene co-expression network analysis (MEGENA). This technique showed that over the entire transcriptome, socially isolated males gained structure, whereas there was a complete disruption of the structure of the transcriptome in socially isolated females. They also identified one key driver that was conserved across all four groups of mice—Crym, the gene for a thyroid hormone–binding protein. They went on to show that by overexpressing Crym in the medial amygdala, they could induce a large increase in male-specific cocaine conditioned place preference (CPP), which provides an indirect measure of drug reward, and a decrease in female-specific CPP, thus demonstrating that the medial amygdala is crucial for regulating the sex-specific response to cocaine at both the circuit and the molecular level.
PAIN
Chronic pain is one of the most common comorbidities associated with psychiatric disease, said Theodore (Ted) Price, Eugene McDermott Professor and chair of the Department of Neuroscience in the School of Behavioral and Brain Sciences at The University of Texas at Dallas. Yet, while women and men perceive pain similarly, the underlying mechanisms that cause a transition to chronic pain appear to be quite different, he said. He noted that as with other preclinical research areas, there has been a
___________________
2 Made available in preprint format. To learn more, see https://www.biorxiv.org/content/10.1101/2020.02.18.955187v1.full (accessed January 6, 2021).
lag in understanding mechanisms in females because of the historical bias resulting from using only male rodents in studies.
One of the female-specific pain mechanisms being explored in Price’s lab involves the calcitonin gene-related peptide (CGRP), which is involved in the pathophysiology of migraine. In addition, new migraine treatments target CGRP or its receptor. Using an inflammatory pain model, Price and colleagues have shown in female mice that a CGRP-sequestering antibody prevented both the development of mechanical hypersensitivity and the transition to a chronic pain state called hyperalgesic priming. In males, the antibody had no such effect, he said. Several anti-CGRP monoclonal antibodies have recently been approved for the treatment of migraine, said Price, although this and other research suggests that for preventing chronic pain, these drugs may be less effective in men than in women (Moehring and Sadler, 2019).
Transcriptomic studies by Price and others have identified many differences in the peripheral immune system that appear to contribute to the transition from acute to chronic pain, known as pain chronification. However, few transcriptomic differences have been seen in nociceptors—the sensory neurons responsible for initiating the pain pathway in the dorsal root ganglion (DRG). Diana Tavares-Ferreira, a postdoctoral fellow in Price’s lab, tried an alternative approach to identifying sex differences by assessing the “translatome” of the mouse DRG—the mRNA fragments that are translated into peptides. Using a technique called translating ribosome affinity purification (TRAP), she demonstrated striking sex differences, said Price. Among these is an enzyme called prostaglandin-H2 D-isomerase (PTDGS), which synthesizes the pain mediator prostaglandin D2. Price and colleagues showed that PTDGS and prostaglandin D2 levels are much higher in female mice. Moreover, in this study they showed that male mice injected with PTDGS inhibitors exhibited a robust, dose-dependent pain response, while female mice showed no significant effect, suggesting that baseline prostaglandin D2 levels are protective against pain (Tavares-Ferreira et al., 2020). Price commented that although prostaglandins have long been known to play an important role in pain, the sex difference became apparent only by studying both sexes in preclinical studies.
The Price lab has also been studying pain mechanisms in clinical samples in collaboration with colleagues at the MD Anderson Cancer Center. In well-phenotyped patients with cancer that has infiltrated their vertebrae, doctors at MD Anderson surgically remove DRGs. Price and colleagues then conduct histochemical, electrophysiological, and RNA sequencing studies on DRG neurons from pain and non-pain dermatomes (areas of skin supplied by specific nerves from the DRG). After showing that only the neurons from the pain dermatomes showed spontaneous activity in the nociceptors that drive neuropathic pain, they went on to show striking transcriptomic
sex differences, suggesting that different underlying mechanisms may be driving neuropathic pain in males versus females (North et al., 2019). Price suggested that these transcriptomic differences may translate into different therapeutic targets for males and females with neuropathic pain.
Neuropathic pain in males and females also differs at a cellular level, said Price. In males, macrophages are the primary cell type that infiltrates and proliferates in the DRG, whereas in females there is a mix of phenotypically different macrophages, B cells, and possibly T cells, he said. While these different immune cells release different cytokines, the pathways in males and females seem to converge by making nociceptors both spontaneously active and hyperexcitable. This convergence results from the fact that nociceptors express a “dizzying array of receptors,” he said. This could explain why antagonists against specific receptors may have limited effectiveness in different population groups, suggested Price.
ADDITIONAL FACTORS CONTRIBUTING TO SEX DIFFERENCES IN STRESS- AND REWARD-RELATED DISORDERS
While the workshop focused on transcriptomic evidence, genetic regulatory networks are also affected by epigenetic mechanisms triggered by environmental influences, including stress, according to Farah Lubin, associate professor of neurobiology at The University of Alabama at Birmingham. Epigenetics, she said, is the study of both heritable and nonheritable regulation of gene expression that occurs without any alteration in the DNA sequence. Neurons appear to have hijacked epigenetic processes such as DNA methylation, posttranslational histone modification, and non-coding RNAs in order to coordinate transcriptional programming across several brain regions, she said. Long non-coding RNAs (lncRNAs) are of particular importance in brain disorders because they appear to play an important role in regulating higher brain functions, said Orna Issler, an instructor at Icahn School of Medicine at Mount Sinai. In depression, at least one-third of the genes that are differentially expressed are lncRNAs, with very little overlap between lncRNAs regulated in males and females, she said.
Exposure to gonadal hormones, may also drive sex differences, said Marianne Seney. Lubin noted that hormone levels change over the life span and may also help to explain the impact of age on disorders.
To conclude the discussion, Eric Nestler mentioned that there are also societal and other non-biological drivers of sex differences. For example, Issler noted that psychosocial factors lead to differences between how boys and girls deal with stress. Nestler added that the stress on parents coping with child care issues during the COVID-19 pandemic also falls disproportionately on women.
This page intentionally left blank.










