Nuclear energies are so enormous because breaking nuclear bonds for heavy elements (such as uranium  ) and creating them for light elements (such as hydrogen ) and creating them for light elements (such as hydrogen  ) actually reduces the total mass of all of the nuclei involved. The lost mass converts to energy via Einstein's famed equation, E = mc2. This simple string of characters embodies nature's most mysterious manifestation of energy. It declares that energy is matter, multiplied by a truly gigantic number--the speed of light ) actually reduces the total mass of all of the nuclei involved. The lost mass converts to energy via Einstein's famed equation, E = mc2. This simple string of characters embodies nature's most mysterious manifestation of energy. It declares that energy is matter, multiplied by a truly gigantic number--the speed of light  squared. In other words, a minuscule amount of matter packs an astonishing punch if you convert it fully to energy. For example, Hiroshima was eradicated by an atomic bomb that drew its energy from a quantity of matter weighing less than a penny. A cup of water completely converted to energy would provide enough electricity to power a million homes for a year. These are impressive figures. As usual, however, heavenly bodies put our Earthly comparisons to shame. Our Sun is a run-of-the-mill astronomical object as far as energy goes. Even so, every second it converts a mass of hydrogen squared. In other words, a minuscule amount of matter packs an astonishing punch if you convert it fully to energy. For example, Hiroshima was eradicated by an atomic bomb that drew its energy from a quantity of matter weighing less than a penny. A cup of water completely converted to energy would provide enough electricity to power a million homes for a year. These are impressive figures. As usual, however, heavenly bodies put our Earthly comparisons to shame. Our Sun is a run-of-the-mill astronomical object as far as energy goes. Even so, every second it converts a mass of hydrogen  equal to 15 billion cups of water into energy. That 1-second output represents more energy than humans have used in the history of civilization. equal to 15 billion cups of water into energy. That 1-second output represents more energy than humans have used in the history of civilization.
By the LIGHT of a Star
Each type of energy teaches us something different about the universe. However, our best tool for probing the cosmos is electromagnetic energy  . Particles of electromagnetic energy . Particles of electromagnetic energy  called photons called photons   transmit light across the universe. From those signals, faint as they may be, we extract information about the motions of objects, how far away they are, their compositions and ages, and more. We owe our understanding of the universe to photons transmit light across the universe. From those signals, faint as they may be, we extract information about the motions of objects, how far away they are, their compositions and ages, and more. We owe our understanding of the universe to photons   that have journeyed for billions of years across space. We snare these tireless travelers with giant telescopes or other surrogate eyes that we use to stare at the heavens. that have journeyed for billions of years across space. We snare these tireless travelers with giant telescopes or other surrogate eyes that we use to stare at the heavens.
There's much more to light than meets the eye. electromagnetic energy  spans a broad spectrum from high-energy gamma rays spans a broad spectrum from high-energy gamma rays  at one end to low-energy radio waves at one end to low-energy radio waves  at the other. The visible light that humans use to perceive the world falls somewhere in the middle. We have a natural bias toward visible light and the colors of the rainbow that compose it. But visible light opens such a narrow window on our universe that we are practically blind to its wonders. We might as well try to drive on the highway with a windshield painted black except for a half-inch slit in the middle. Fortunately, (continued) at the other. The visible light that humans use to perceive the world falls somewhere in the middle. We have a natural bias toward visible light and the colors of the rainbow that compose it. But visible light opens such a narrow window on our universe that we are practically blind to its wonders. We might as well try to drive on the highway with a windshield painted black except for a half-inch slit in the middle. Fortunately, (continued)
|
The Electromagnetic
Spectrum
On either side of the familiar (but tiny) visible rainbow of red, orange, yellow, green, blue, indigo, and violet lie vast bands of nonvisible radiation. The higher the energy of the radiation, the shorter its wavelength. The spectrum divisions between the different forms of radiation overlap because the names derive in part from how the radiation is generated and the technology used to detect it.
|
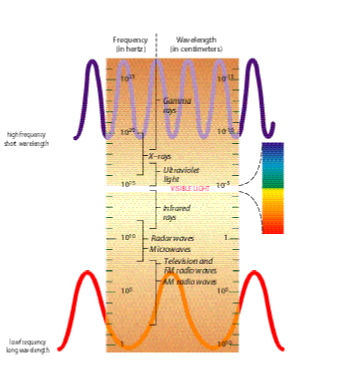 |
|
astronomers have devised ways to expand this limited vision. Through each new window in the electromagnetic spectrum  , the universe displays a different face. , the universe displays a different face.
Gamma rays carry the most energy of any photons   . We rarely see gamma rays arise naturally on Earth. Certain types of radioactive decay produce them, and some energetic thunderstorms spit out gamma rays with their lightning bolts. Otherwise, they come from places in space where matter undergoes sudden and violent changes in form. . We rarely see gamma rays arise naturally on Earth. Certain types of radioactive decay produce them, and some energetic thunderstorms spit out gamma rays with their lightning bolts. Otherwise, they come from places in space where matter undergoes sudden and violent changes in form.
One such setting is the core of our Sun. The thermonuclear reactions that power stars make more gamma rays than all other kinds of energy. However, those photons   must travel for tens of thousands of years to escape the Sun's interior. Along the way, they ricochet off countless atoms must travel for tens of thousands of years to escape the Sun's interior. Along the way, they ricochet off countless atoms  reeling like drunks down a crowded street. The collisions sap energy from the gamma rays and convert each one into thousands of lower-energy photons reeling like drunks down a crowded street. The collisions sap energy from the gamma rays and convert each one into thousands of lower-energy photons   : x-rays, ultraviolet light, and visible light. By the time the photons : x-rays, ultraviolet light, and visible light. By the time the photons   emerge from the Sun, few gamma rays remain. Gamma rays also pour forth in unimaginably energetic bursts emerge from the Sun, few gamma rays remain. Gamma rays also pour forth in unimaginably energetic bursts  from mysterious objects near the fringes of the universe. Discovering how that happens remains an exciting challenge in astronomy. from mysterious objects near the fringes of the universe. Discovering how that happens remains an exciting challenge in astronomy.
X-rays are familiar to anyone who has broken a bone or gone to a dentist. They are less energetic than gamma rays but are strong enough to penetrate our flesh  and most nonmetallic substances. In space, x-rays stream from the sites of stellar storms (continued) and most nonmetallic substances. In space, x-rays stream from the sites of stellar storms (continued) |

