7
Applying Evidence for Patient-Centered Care: Standards and Expectations
INTRODUCTION
Communicating evidence to patients is a critical challenge in transitioning to a health system centered on patients’ individual values and preferences. The public is bombarded with inconsistent health messages and has little background or training in how to evaluate the information presented in television newscasts, daily papers, and on the Internet. Moreover, many messaging campaigns may contribute to this confusion because of oversimplification, emotional appeals, and conflicting advice.
Value and science-based care are concepts that will require rethinking how information is shared with patients. Committing to communicating evidence in all its complexity while ensuring it is understandable and pertinent to individual patient circumstances will be a challenging task. Communication strategies need to be evaluated so that shortfalls can be made clear, and effort should be given to developing new approaches to teach patients about evidence-based medicine.
The papers in this chapter address how to apply standards for evidence in the context of individual patient-centered care. They point to the importance of generating evidence applicable to individual patient outcomes, preferences, and values. This type of evidence is necessary to provide care that is more effective for the individual patient and more efficiently delivered. Additionally, the papers take up the nature of difficulties in communicating this evidence to patients and examine strategies that have proven valuable in informed decision making.
In the first paper, Dale Collins Vidal of the Dartmouth Institute for Health Policy and Clinical Practice points out that the current informed consent process fails to help patients understand the trade-offs—or specific risks and benefits—in comparing treatment options. When competing treatment options are “preference sensitive,” decision making about treatment should incorporate a patient’s values and preferences. To ensure that patients have the tools they need to make an informed choice, providers must adequately communicate the risks, benefits, alternatives, experience, and cost.
In the second paper, Clifford Goodman of The Lewin Group addresses the limitations of evidence hierarchies that have been used for decades. He highlights the limitations of what until now has been considered “best evidence,” for example, meta-analyses and randomized controlled trials (RCTs). He suggests moving away from RCTs as best evidence for a number of reasons, including their focus on population-based care, the time lag in obtaining scientific results, high costs, and the lack of applicability to individual patients. He points to several methods that can better capture evidence applicable to personalized medicine, which is becoming increasingly important with advances in genomic data.
Fran M. Visco of the National Breast Cancer Coalition addresses translating and communicating evidence when the recommendations for care are uncertain. She reviews the barriers to understanding science-driven care, including the adoption of practices that have become standard even though evidence to back them is limited; the oversimplification of messaging that misleads the public; and the promulgation of guidelines that are self-serving. She highlights the pressing need to evaluate how to deliver complex messages about interventions so they can be made pertinent to individual patients.
THE ROLE OF EVIDENCE IN PATIENT-CENTERED CARE
Dale Collins Vidal, M.D., M.S.1
Dartmouth Institute for Health Policy and Clinical Practice
In Crossing the Quality Chasm, the Institute of Medicine (IOM) defines patient-centeredness as “providing care that is respectful of and responsive to individual patient preferences, needs, and values, ensuring that patient values guide all clinical decisions” (IOM, 2001). However, studies reveal that the current informed consent process falls far short of this goal and frequently fails to help patients understand the specific risks and benefits of
____________
1 The author would like to acknowledge Allison J. Hawke, Sue Burg, and Sherry Thornburg for their contributions in preparing this manuscript.
treatment options (Holmboe et al., 2000). This is true even when the decision involves “effective care”—care that is supported by strong evidence and usually depends less on an individual’s personal values and preferences (O’Connor et al., 2007). When treatments are not supported by adequate evidence or when they involve trade-offs that could impact a patient’s quality of life, the traditional informed consent process is inadequate for helping patients make informed treatment decisions. In these situations, the process should more appropriately be framed as informed choice, incorporating a discussion of treatment alternatives, the evidence associated with them, and the patient’s personal values.
To achieve the IOM’s goals around patient-centered decision making, a framework is needed that will allow patients, families, and providers to engage in successful, mutual healthcare interactions, allowing patient and family values to guide all healthcare decisions. This paper presents elements of such a framework and describes tools used by the Dartmouth Hitchcock Medical Center to support it.
Categories of Care
Effective Care
John Wennberg is credited with defining three categories of care: effective, preference-sensitive, and supply-sensitive (Table 7-1). Effective care is that which is supported by high-quality evidence demonstrating that the benefits of a proposed treatment or intervention are large compared with the potential harms. Clinicians, and most patients, agree on the appropriate course of action for effective care. When care is deemed effective, the clinician typically makes a recommendation, and the goal is patient compliance or increased uptake by a population (Wennberg, 2002). Examples include the use of antibiotics for treatment of bacterial pneumonia, screening with pap smears, and preventive flu vaccinations for healthcare workers.
Preference-Sensitive Care
Because of conflicting, uncertain, or insufficient information as well as differing personal preferences, the quality of evidence often does not allow for a clear “right” choice. A quality decision for these “preference-sensitive” medical decisions requires that the patient be knowledgeable about the options and that his or her personal values inform the choice (Sepucha et al., 2004). Examples include elective surgeries such as LASIK eye surgery; use of screening tests, such as prostate-specific antigen for prostate cancer; and prevention of cervical cancer with human papillomavirus vaccinations. An effective care recommendation may become a preference-sensitive deci-
TABLE 7-1 Summary and Description of Categories of Care
| Category of Care | Typically Characterized by: | Goal | Examples | |
| Effective |
• Proven clinical data on treatment effectiveness • Benefits large compared with harms |
• Increase uptake |
• Treatment−antibiotics for community-acquired pneumonia • Screening−pap smear • Prevention−flu shot |
|
| Preference-Sensitive |
• Multiple treatment options • Lack of evidence-based treatment options • Benefits and risks are uncertain • Significant trade-offs for the patient |
• Patient participation • High decision quality • Prevent overuse of options patients do not value |
• Treatment−LASIK surgery • Screening−prostate-specific antigen (PSA) • Prevention−human papillomavirus (HPV) vaccination |
|
| Supply-Sensitive |
• Lack of evidence on comparative effectiveness of treatments • Few guidelines regarding delivery of care • Assumption that more care is better |
• Varies across the country based primarily on capacity • Amplified by fee-for-service model |
• Care for patients with progressive chronic illness (e.g., lung disease, cancer, diabetes, heart failure) |
|
SOURCE: Wennberg et al., 2002.
sion if a patient does not readily accept the clinician’s recommendation. Preference-sensitive care avoids the overuse of options patients do not value. The essential feature common to all preference-sensitive conditions is that choice of treatment is up to the patient (Wennberg, 2002).
Supply-Sensitive Care
Wennberg and colleagues have demonstrated that capacity dictates how healthcare resources are used (Wennberg et al., 2002). Supply-sensitive care exists when the number of available hospital beds drives how often patients are hospitalized rather than cared for in the outpatient setting, or the frequency of referrals to specialists. The Dartmouth Atlas Project2 has
____________
2 See http://www.dartmouthatlas.org/downloads/reports/supply_sensitive.pdf (accessed October 14, 2010).
demonstrated that supply-sensitive care varies widely across the United States. This variation may result in underuse of effective care and improper use of preference-sensitive care. The latter may occur when patients are not provided accurate information about their options and are not encouraged to incorporate their own values and preferences into care decisions.
Healthcare Variation
The Dartmouth Atlas has used Medicare data to map geographic variations in care for more than 20 years. The project reports on resource and medical care use among beneficiaries living in 3,436 hospital service areas—aggregated into 306 hospital referral regions—examining unwarranted variations in the above three categories of clinical care. It has revealed the presence of significant geographic variations in healthcare prices, practices, and providers; patient characteristics; and patient preferences. The project also has found that regions that spend more on health care and have higher rates of utilization do not experience better health outcomes compared with regions spending less on health and using healthcare services less frequently (Fisher et al., 2003).
Although higher rates of effective treatments are preferred, it is difficult to define the “right rate” for preference-sensitive treatments. The question raised by Wennberg and colleagues is, given the variation in the rates of procedures across the United States, which rate is right (Weinstein et al., 1998)? To illustrate the issue, Figure 7-1 shows the variations in spinal surgery across hospital referral regions in the United States. The number of spinal surgeries ranges from fewer than 2 to almost 11 per 1,000 Medicare enrollees. Even among high-performing academic medical centers such as Intermountain, Geisinger, Mayo Clinic, and Dartmouth-Hitchcock Medical Center, there is more than a twofold variation in the rates of spinal surgery. Why is this the case? In this area of preference-sensitive care, the indications for surgery are not universally agreed upon, and the evidence to support the decision-making process is imperfect for both the clinician and the patient. In these cases, it is impossible to know which rate is right, and ideally, well-informed patients should decide whether the intervention is right for them. Thus it is incumbent on the healthcare system to provide patients with accurate, balanced information and encourage them to participate in the decision.
Helping Patients Make Treatment Decisions
Decision Quality
When there is no clear answer as to which treatment is best, the patient should decide. High-quality decisions depend on adequate knowledge,
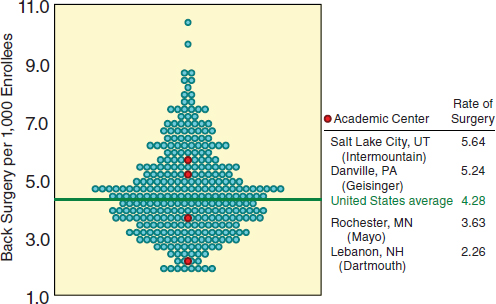
FIGURE 7-1 Turnip plot showing variation in rates of spinal surgeries.
SOURCE: The Dartmouth Atlas of Health Care. Copyright Trustees of Dartmouth College.
clarification of values, and resulting treatment choices that are consistent with the patient’s values (Sepucha et al., 2004). There are, however, real challenges to providing patients with optimal support.
Creating systems and processes to inform patients about the benefits and risks of treatment alternatives, then assessing whether they have the information they need to make high-quality decisions, is an important requirement of today’s healthcare system. This requirement, however, may appear to be in direct conflict with the demand for increased clinical efficiency and documentation that has resulted in ever shorter interactions with patients.
Risks of the Typical Informed Consent Process
In cases in which effective care is indicated, a recommendation from the physician may be appropriate, along with a discussion of the potential benefits and harms with the decision maker. This approach is in keeping with the traditional model of informed consent. Yet studies reveal that the typical informed consent process usually does not help patients understand the specific risks and benefits of treatment options (Holmboe et al., 2000).
Benefits of Shared Decision Making and Patient-Centered Care
Often the choice between competing treatment options is not so clear and requires preference-sensitive decisions. In these situations, the treatment choice should take into account an individual’s values and preferences regarding the potential outcomes. Shared decision making is a process that incorporates evidence-based medicine and requires both patients and physicians to contribute information and participate. Providing decision support and participating in patient-centered care requires skills and core competencies. Several evidence-based practices foster communication and shared decision making at the decision point, including involvement of both the patient and the physician, information sharing by both parties, and reaching an agreement about the treatment options (Charles et al., 1997).
Recent trials of decision-support systems designed to help patients understand their treatment options reveal that informed patient choice results in different patterns of practice from those found with patients who experience usual care. However, medical opinion rather than patient preference tends to dominate the treatment choice (Wennberg, 2002).
Replacing standard informed consent practices with a patient-centered model of informed choice involves incorporating standardized communication of evidence, including risks, benefits, alternatives, experience, and cost. These changes may lead to differences in healthcare utilization and can empower patients to make informed decisions about their care (Krumholz, 2010). Providing patient-centered care depends on the comprehensive training of healthcare providers, increased consumer health literacy, and the successful identification of implementation models (Edwards and Elwyn, 2009).
Use of Information Technology to Feed Forward the Right Patient Information at the Right Time
Dartmouth-Hitchcock Medical Center uses health information technology resources, such as video decision aids and electronic surveys, to collect information from patients about their health history, behaviors, and health preferences in order to provide them with feedback and decision support and facilitate clear communication at the point of care. For example, all patients with breast cancer are electronically screened for psychosocial, financial, and emotional problems (such as anxiety and depression). Using validated surveys such as the Patient Health Questionnaire 9, the computer system can immediately score the survey and identify patients who reach preset clinical thresholds. When a patient reaches that threshold, the computer automatically e-mails Dartmouth-Hitchcock’s social workers to intervene with patients identified as having a need.
In addition, our computer software systems allow us to collect information about patients’ treatment preferences to share with their physician. When patients come in for their appointments, their clinicians are armed with information focused on what is meaningful to the patients. This facilitates discussion between clinician and patient and allows for the creation of a personalized and tailored treatment plan.
Impact of Decision Aids on Patients’ Choices
A number of RCTs have shown the benefits of decision aids in supporting specific decisions. However, few trials have shown the impact on treatment choice. At Dartmouth-Hitchcock, the use of decision aids has resulted in approximately 30 percent of patients changing their initial treatment preference. In an RCT, Deyo and colleagues studied the impact of a video decision aid designed to inform patients about treatment options for back pain. The overall rate of surgery was 22 percent lower in the group viewing the video than in controls (26 percent vs. 33 percent). The researchers concluded that the video decision aid appeared to facilitate decision making and could help ensure informed consent (Deyo et al., 2000).
Summary
Patients and providers should each have the benefit of making decisions with the best available evidence. When treatments are not supported by adequate evidence or when they involve trade-offs that can variably impact a patient’s quality of life, the decision-making process should be structured in a way that supports informed patient choice, by incorporating a discussion of treatment alternatives, the best evidence available, and the patient’s personal values.
At Dartmouth-Hitchcock Medical Center, a patient’s self-reported health information is collected by computer systems. This information is synthesized, and reports are created instantaneously to feed information back to the patient about his/her health behaviors and conditions. This same software can support the provision of sophisticated decision aids when patients are facing preference-sensitive treatment choices. Additionally, information on patients’ treatment preferences—including their understanding of the key facts about the treatment and how they would value the different possible outcomes of care—can be collected and fed forward to their treating clinician at the point of care. This integration of technology, patient information, and evidence provides a framework for patient-centered care and informed choice.
EVIDENCE STANDARDS AND APPLICATION:
RIGHT CARE, RIGHT PATIENT, RIGHT TIME
Clifford Goodman, Ph.D.
The Lewin Group
Standards of evidence for what works in health care are generally appreciated as instrumental to the widely shared goal of getting the right care to the right patient at the right time. Such standards are used to support clinical practice guidelines and other best practices, formulary decisions, coverage policies, and more. These evidence standards are oriented largely toward the tenets of experimental methods and often presented in frameworks or hierarchies of study design. These hierarchies have not been static. They have evolved over the past few decades to better address the particular circumstances of healthcare decisions. However, persistent shortcomings in the practical utility of current approaches for appraising evidence, as well as certain mismatches between evidence hierarchies and the questions they are intended to help answer, suggest that these approaches and hierarchies need to be revisited and reconsidered in a broader context ranging from identification of research priorities bringing research to practice.
Evidence Hierarchies and Their Application
Evidence standards can inform and improve healthcare decisions and policies when they are used to appraise the quality of available evidence on the impacts of healthcare interventions. Although evidence hierarchies have evolved over the last two decades, they are oriented largely toward the relative strength of evidence regarding the causal effect of an intervention on particular health outcomes or other endpoints. In these hierarchies, internal validity is associated with study design, and the resulting evidence hierarchy of primary studies—i.e., those that collect original data—typically places RCTs at the top as the “gold standard” for primary evidence. RCTs are followed, typically, by nonrandomized controlled trials, various prospective and retrospective observational (nonexperimental) studies with concurrent or historical control groups, case series, single cases, and finally (though not a form of evidence per se) expert opinion. In some hierarchies, systematic reviews and meta-analyses of RCTs, where available, are placed above RCTs (Sackett, 1989; USPSTF, 2008).
Notable variations or adaptations of this basic framework recognize that implementation of a study design, not just the nature of the study design itself, affects the quality of the evidence yielded. Accordingly, these variations account for whether a study was “well designed” by employing an acceptable means of randomization, by eliminating or minimizing other
potential biases or threats to internal validity, and by employing adequate statistical power to detect potential treatment effects; what kind of comparison is involved (e.g., “head-to-head” trials of an intervention vs. a standard of care rather than indirect comparison of the two or comparison with placebo); whether the emphasis is on clinically relevant, rather than surrogate, outcomes; and other attributes (Atkins et al., 2005; Harbour and Miller, 2001; McAlister et al., 1999; USPSTF, 2008).
Limitations of Evidence Hierarchies
Despite improvements over the years, characteristics inherent in evidence hierarchies can limit the development and appraisal of evidence necessary for informing decisions about the right care for the right patient at the right time. That there are more than 60 published evidence hierarchies signals a lack of satisfaction or consensus in the field. Apart from inconsistencies across hierarchies in the definitions and categorization of study designs (e.g., multiple and overlapping definitions of “cohort” and “quasi-experimental” studies) and in rankings of study designs (e.g., whether RCTs or systematic reviews reside at the top), most existing hierarchies are limited by the mismatch between their original use and current application and the associated overreliance on the RCT.
Evidence hierarchies are based largely on methodological principles for assessing pharmacological models of therapy, including emphasizing experimental control; placebo control groups where possible; randomized assignment; narrowly defined patient groups; blinding of patients, clinicians, and investigators; and other attributes intended to enhance internal validity. However, this approach can jeopardize external validity—that is, the generalizability of findings to patients, settings, and other circumstances of real-world care. For example, RCTs may provide clear results when assessing the effect of a drug compared with a placebo in a narrowly defined patient population with a specific health problem. However, such RCTs may not provide information relevant for those making choices among alternative therapies used in practice or for those with multiple comorbidities along with the specific health problem. Further, while RCTs and other experimental study designs are intended to address the internal validity of the causal effect of an intervention on outcomes, they have been misapplied to other types of research questions pertaining to healthcare technologies, such as the accuracy of a screening or diagnostic test, the prognostic accuracy of a test, or rare or delayed adverse effects of a therapy.
As is recognized by some evidence hierarchies, relying on study type alone for assessing the quality of evidence can be misleading. For example, poor design or implementation of a high-ranking study type may yield findings that are less valid than those from study types lower on the hier-
archy that are well designed and rigorously implemented. Further, evidence hierarchies often cannot adequately accommodate or combine results from multiple study design types, even when no single study design can answer some evidence questions.
Limitations of Randomized Controlled Trials
RCTs are usually important, and sometimes essential, components of a rigorous evidence base for demonstrating the causal effects of healthcare interventions on outcomes. However, RCTs have important limitations, including some circumstances in which underlying assumptions about them are not valid (Rawlins, 2008; Walach et al., 2006). For example, while undertaking to randomize patients to one intervention or another assumes equipoise between the two, patients and clinicians involved in RCTs often bring preferences for one or the other. Whereas RCTs assume a lack of knowledge about the merits of the interventions in questions, there may indeed be relevant evidence from other sources (e.g., phase II drug trials) that undermines the utility of the null hypothesis in experimentation. RCTs also have a preference for specificity—i.e., that only the specific effects attributable to an intervention are therapeutically valid. Another assumption underlying RCTs that often is not valid relates to the context independence of the effect—i.e., that there is some “true” magnitude of efficacy or a stable effect size independent of the context in which an intervention is used.
Another major weakness concerns incorrectly assuming that the findings about a therapeutic effect from an RCT are externally valid—that is, readily transferable into clinical practice—if the exclusion and inclusion criteria of the trial match the characteristics of a given patient. Although this shortcoming of RCTs is generally well recognized, its extent and significance for patient care are not well understood.
Other methodological problems with RCTs can include relying on intermediate endpoints rather than health outcomes; having inadequate statistical power or duration for assessing benefits and harms, especially those that are rare or delayed; and being unsuccessfully blinded to patients, clinicians, or other investigators. Probability theories may pose problems (especially with frequentist approaches) in the form of arbitrary selection of p-values and the difficulty of applying them to everyday patient care; the multiplicity of endpoints compared, stopping rules, and analysis of subgroups; and resistance to Bayesian approaches.
Furthermore, there are various circumstances in which RCTs can be inappropriate. For example, they may raise bioethical and legal concerns; they may be difficult or impossible to conduct for rare diseases; and they may be unnecessary for very large treatment effects or to establish causa-
tion (e.g., Heimlich maneuver, cardiac defibrillation, laser removal of port wine stains). Additionally, the substantial resources needed to conduct RCTs—large, multicenter, longitudinal trials, sometimes costing hundreds of millions of dollars—limit their broad application to the myriad clinical questions important to providing patient-centered care.
Finally, the RCT’s status as the “gold standard” for establishing the causal effect of an intervention on a health outcome can be extended inappropriately to other purposes. Indeed, other study designs are more suitable for answering certain evidence questions that inform many other aspects of clinical care, as summarized in Box 7-1.
Improving Evidence Hierarchies
Approaches to appraising the quality of evidence must extend beyond single hierarchies of study designs. They must consider the strengths and weaknesses of different study designs for answering specific questions, including the use of certain traditionally lower-ranked observational methods such as examination of large claims databases, patient registries, and electronic health records to supply evidence augmenting that derived from other designs.
BOX 7-1
RCTs Are Not Always the Best Method for Answering Clinical Evidence Questions
For the following situation, other experimental designs may include:
- Prognosis: patient cohort studies with follow-up at uniform points in the clinical course of a disease/disorder
- Identification of risk factors for diseases, disorders, adverse events: case control studies
- Accuracy of a diagnostic test: cohort (or cross-sectional) study of index test vs. gold standard in patients at risk of having a disease/disorder
- Effectiveness of interventions for otherwise fatal conditions: nonrandomized trials, case series
- Rates of recall or procedures precipitated by false-positive screening results: cohort studies
- Complication rates from surgery or other procedures: case series
- Incidence of rare, serious adverse events potentially due to an intervention: surveillance, registries
- Safety, effectiveness of incrementally modified technologies posing no known additional risk: registries
Different Research Questions Call for Different Study Designs
As described above, evidence requirements should address the type of intervention, application, and other attributes of evidence questions. Beyond evidence hierarchies devoted to treatments, hierarchies have been developed for assessing the quality of evidence pertaining to technologies used for screening, diagnosis, and other purposes. For example, the Strength of Recommendation Taxonomy (SORT) (Table 7-2) distinguishes different levels of evidence quality for technologies used in diagnosis, treatment/prevention/screening, and prognosis based on study design and other methodological aspects. In particular, SORT considers the availability of evidence on
TABLE 7-2 Strength of Recommendation Taxonomy (SORT), an Evidence Hierarchy That Includes Explicit Consideration of Intervention Type and Quality of Patient-Oriented Outcomes Assessed
| Study Quality | Diagnosis | Treatment/ | ||
| Level 1− good-quality patient-oriented evidence |
Validated clinical decision rule SR/meta-analysis of high-quality studies High-quality diagnostic cohort study |
SR/meta-analysis of RCTs with consistent findings High-quality individual RCT All-or-none study |
SR/meta-analysis of good-quality cohort studies Prospective cohort study with good follow-up |
|
| Level 2− limited-quality patient-oriented evidence |
Unvalidated clinical decision rule SR/meta-analysis of lower-quality studies or studies with inconsistent findings Lower-quality diagnostic cohort study or diagnostic case-control study |
SR/meta-analysis of lower-quality clinical trials or of studies with inconsistent findings Lower-quality clinical trial Cohort study Case-control study |
SR/meta-analysis of lower-quality cohort studies or with inconsistent results Retrospective cohort study or prospective cohort study with poor follow-up Case-control study Case series |
|
| Level 3− other evidence | Consensus guidelines, extrapolations from bench research, usual practice, opinion, disease-oriented evidence (intermediate or physiologic outcomes only), or case series for studies of diagnosis, treatment, prevention, or screening | |||
SOURCE: Reprinted with permission from “Strength of Recommendation Taxonomy (SORT) Patient-Centered Approach to Grading Evidence in the Medical Literature,” February 1, 2004, American Family Physician. Copyright © 2004 American Academy of Family Physicians. All Rights Reserved.
patient-oriented outcomes (morbidity, mortality, symptom improvement, cost reduction, and patient quality of life) rather than disease-oriented evidence (biomarkers and other intermediate endpoints) (Ebell et al., 2004).
Another evidence-rating approach has been developed by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative to support a coordinated, systematic process for evaluating genomic tests. In this instance, EGAPP developed hierarchies suitable for appraising evidence for each of three main attributes of testing: analytic validity, clinical validity, and clinical utility (Table 7-3). For analytic validity—the ability to identify correctly a gene of interest—Level 1 evidence would consist of a collaborative study using a large panel of well-characterized test samples, or summary data from well-designed external proficiency testing schemes of interlaboratory comparison programs. For appraising evidence for clinical validity—whether the gene is associated with a given disease or other phenotype of interest—Level 1 evidence would consist of well-designed longitudinal cohort studies or a validated clinical decision rule. For clinical utility—which addresses whether a test result affects clinical decision making or patient outcomes—the evidence hierarchy resembles the more traditional ones, in which Level 1 evidence would be a meta-analysis of RCTs that followed patients from testing through clinical decisions to outcomes. Level 2 evidence would be a single RCT, and so on (Teutsch et al., 2009).
Indeed, mapping evidence requirements to clinical analytical frameworks, as is done by EGAPP, the U.S. Preventive Services Task Force (USPSTF), and the Agency for Healthcare Research and Quality’s Evidence-based Practice Centers, illustrates how evidentiary needs can differ based on the decision flow. For example, Figure 7-2 illustrates the evidence framework for determining whether testing for CYP450 polymorphisms in adults entering therapy with selective serotonin reuptake inhibitors is useful in treatment decisions or leads to improved outcomes. Proponents of this test, which uses microarray technology to determine the genotype of a patient’s cytochrome P450 enzymes, recommend its use for guiding the selection of effective medicines. The test’s first uses have been in psychiatry (Teutsch et al., 2009). In Figure 7-2, the numbers correspond to analytical validity (2), clinical validity (3a-c), and clinical utility (4a-c), each of which could be determined using the types of evidence listed in the EGAPP hierarchy shown in Table 7-3.
The Best Scientific Evidence May Derive from a Complementary Set of Methods
The methods in the available toolkit for assessing clinical effectiveness have their respective strengths and weaknesses. Using multiple and complementary methods (Figure 7-3) can offset vulnerabilities and triangulate
TABLE 7-3 Hierarchies of Data Sources and Study Designs for the Components of Evaluation
| Level | Analytic validity | Clinical validity | Clinical utility | |
| 1 |
Collaborative study using a large panel of well-characterized samples Summary data from well-designed external proficiency testing schemes or interlaboratory comparison programs |
Well-designed longitudinal cohort studies Validated clinical decision rule |
Meta-analysis of RCTs |
|
| 2 |
Other data from proficiency testing schemes Well-designed peer-reviewed studies (e.g., method comparisons, validation studies) Expert panel-reviewed FDA summaries |
Well-designed case-control studies |
A single RCT |
|
| 3 |
Less well designed peer-reviewed studies |
Lower quality case-control and cross-sectional studies Unvalidated clinical decision rule |
Controlled trial without randomization Cohort or case-control study |
|
| 4 |
Unpublished and/or non-peer reviewed research, clinical laboratory, or manufacturer data Studies on performance of the same basic methodology, but used to test for a different target |
Case series Unpublished and/or non-peer reviewed research, clinical laboratory or manufacturer data Consensus guidelines Expert opinion |
Case series Unpublished and/or non-peer reviewed studies Clinical laboratory or manufacturer data Consensus guidelines Expert opinion |
|
SOURCE: Reprinted with permission from Teutsch et al., 2009.
findings—starting with results achieved with one method and replicating or augmenting them with other methods. This may constitute a powerful and comprehensive approach for developing a broader body of evidence that is helpful to guide care (Rawlins, 2008; Walach et al., 2006). For example, results of RCTs used to obtain Food and Drug Administration approval could be combined with analyses of patient registry data and longer-term follow-up of outcomes and adverse events through a cohort study to help
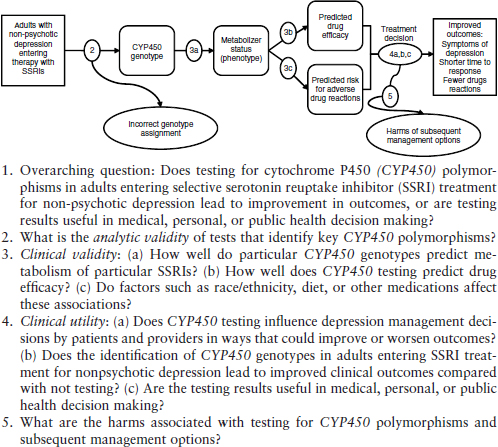
FIGURE 7-2 Analytic framework and key questions for evaluating one application of a genetic test in a specific clinical scenario: Testing for Cytochrome P450 Polymorphisms in Adults With Non-Psychotic Depression Treated With Selective Serotonin Reuptake Inhibitors (SSRIs).
SOURCE: Reprinted with permission from Teutsch et al., 2009.
fill important gaps between what works under ideal conditions and what is needed to support real-world clinical practice.
Such efforts must not simply use multiple study designs, but must also ensure that the methods are complementary. In this respect, it is helpful to consider three types of evidence: direct, mechanistic, and parallel. Direct evidence is derived from experimentation or other studies (randomized or nonrandomized) that reveal a probabilistic association between some intervention and an outcome or result that is causal and not spurious. For a therapy, the size of the effect is not attributable to plausible confounding and exhibits appropriate temporal and/or spatial proximity, as well as dose-responsiveness and reversibility. Mechanistic evidence, playing a subsidiary
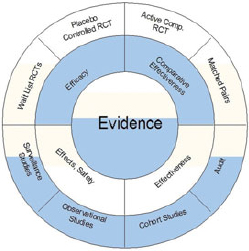
FIGURE 7-3 Circle of complementary methods and data sources. Experimental methods that test specifically for efficacy (upper half of the circle) must be complemented by observational, nonexperimental methods (lower half of the circle) that are more descriptive in nature and describe real-life effects and applicability.
SOURCE: Walach et al., 2006.
or supporting role to direct evidence, advances understanding of the likely causal process—biological, chemical, mechanical, or other—that connects the intervention to the outcomes. Parallel evidence from other studies with similar results that support the causal relationship, such as studies of similar populations and interventions in various healthcare settings over different durations, provides coherence and replicability (Howick et al., 2009).
Another framework for thinking about complementary evidence identifies experimentation, observation, and mathematics (e.g., biostatistics or modeling of therapeutic processes) as playing crucial roles in the development of the evidence base for modern therapeutics (Rawlins, 2008). Discussing this approach, Rawlins (2008) notes that “arguments about the relative importance of each are an unnecessary distraction. Hierarchies of evidence should be replaced by accepting—indeed embracing—a diversity of approaches.” Indeed, strictly hierarchical thinking is increasingly giving way to such approaches as the proposed “circle of methods” illustrated in Figure 7-3 for guiding the use of complementary data and methods (Walach et al., 2006).
Promising Directions
There are many encouraging signs for the ability to ensure that evidence standards and their application are aligned with getting the right care to the right patient at the right time. Clinical decision support systems are being designed to make relevant evidence (and evidence-based decision
aids) readily accessible at the place and time of clinician–patient decisions (Pearson et al., 2009).
The field of comparative effectiveness research (CER) emphasizes the need to rely on multiple evolving methods, including more advanced data infrastructure and linkages among claims data, electronic health records, registries, and other sources. Further, consistent with the intent of Congress and national priority-setting reports for CER, the field should focus on subgroup analyses and priority populations, which will expand the base of well-founded evidence for patient decisions (FCC, 2009; IOM, 2009). Indeed, this focus on CER will help ensure that it not only generates population-based evidence but also supports progress toward personalized medicine—including patients’ genomes; their health states; and the behavioral, environmental, socioeconomic, cultural, and other personal determinants of their response to healthcare interventions (Goodman, 2009).
Several other trends reflect this sharper focus on meeting the needs of decision makers. Patient input has been advanced as central to discussions of CER priority setting, study design, and identification of key outcomes, with citizen councils and forums in Europe, Canada, and the United States offering various models for engaging the public. As noted above with respect to pharmacogenomic testing, evidence appraisals are increasingly attuned to intervention types, applications, and settings. Regulators and payers are more explicit about evidence expectations, including study designs and designation of health outcomes and other study endpoints. Bayesian and adaptive clinical trial designs, the focus of increased interest, offer flexible variations on primary data collection that optimize the use of accumulated findings to derive evidence more efficiently for responsive and nonresponsive patient groups (Orloff et al., 2009).
Finally, much greater and earlier interaction among innovators, regulators, payers, and health technology assessment functions is occurring on evidence expectations or requirements well in advance of regulatory decisions or coverage decisions by payers. Of importance, these discussions are focused on anticipating evidence needs throughout the life cycle of a technology and often include clinician and patient input at the outset. Rather than retrospective reaction to fill evidence gaps or make determinations based on the available evidence, this approach allows for coordination of evidence development that takes advantage of the right methods for the right questions and builds toward a totality of evidence on what works best for individual patients.
TRANSLATION AND COMMUNICATION NEEDS FOR CARE IN THE FACE OF UNCERTAIN EVIDENCE
Fran M. Visco, J.D.
National Breast Cancer Coalition
Approaches to translation and communication needs for health care must take into account the broader context within which the public receives health information. The public receives healthcare information from a variety of sources including healthcare providers, traditional media, the Internet, family and friends, and patient and medical advocacy organizations. For many reasons, the temptation is to provide messages that are short and simple. But when evidence is uncertain, healthcare messages are not simple, and there needs to be a system that is honest about this uncertainty. Health messages must not be presented as absolute or simple when evidence is not. We need to educate the media, the public, and the medical community about evidence, and we need to institute oversight mechanisms to ensure that health communications are based on the best available evidence.
The public must trust that the healthcare system works for their benefit. The public’s expectation is that care is communicated and delivered in a system in which
- patient needs are paramount,
- care is based on evidence,
- risks and benefits will be explained and understood, and
- interventions will change with new knowledge.
The Context in Which the Public Receives Information About Health Care
The public uses various sources for health information. Survey data indicate that these sources vary depending on age and education. For example, a 2009 survey conducted by the Pew Internet and American Life Project found that when asked, “Now thinking about all the sources you turn to when you need information or assistance in dealing with health or medical issues, please tell me if you use any of the following sources”:
- 86 percent of all adults ask a health professional, such as a physician;
- 68 percent ask a friend or family member;
- 57 percent use the Internet;
- 54 percent use books or other printed reference material;
- 33 percent contact their insurance provider; and
- 5 percent use another source not mentioned above.
Although the Pew report found that Internet use did not replace other, traditional sources of health information, significant differences in health information sources did emerge among age groups. For example, adults aged 18–29 are significantly less likely than older adults to consult a health professional (79 percent, compared with 88 percent of those aged 30–49, 89 percent of those aged 50–64, and 89 percent of those aged 65+). Younger adults are more likely than older adults to consult a friend or family member. Seventy-eight percent of adults aged 18–29 and 72 percent of those aged 30–49 consult a friend or family member, compared with 58 percent of those aged 50–64 and 59 percent of those aged 65+ (Fox and Jones, 2009).
The Pew survey also found significant differences in health information sources by education level, with 94 percent of college graduates consulting a health professional, compared with only 83 percent of high school graduates. These findings are similar to those reported in a 2008 Center for Studying Health System Change report. According to that report, health information–seeking behavior differed by education level, with 72 percent of people with a graduate degree seeking information from any source, compared with 42 percent of those without a high school diploma (Tu and Cohen, 2008).
In a survey commissioned by the National Breast Cancer Coalition (NBCC) in 2009, a slightly different pattern of health information sources was found among women seeking information on breast cancer. Among women actively looking for information about breast cancer or its treatments, the four most common sources were the Internet, talking with a friend or relative, talking with a doctor or other medical professional, and magazine articles. Overall, the Internet was by far the most common source, consulted by 71 percent of women as compared with 53 percent who spoke with friends and relatives, 45 percent who spoke with a doctor, and 43 percent who found information in magazines. This pattern was seen in all age groups. In addition, all age groups were more likely to consult a friend or relative than a physician for breast cancer information. Women aged 25–34 were more likely to seek breast cancer information in a magazine (48 percent) than to speak with a physician (40 percent), while women aged 50+ were slightly more likely to speak with a friend or relative (49 percent) or a breast cancer survivor (47 percent) than with a physician (43 percent).
When NBCC survey respondents were asked what they had heard or read about breast cancer and where, television programs were the most likely response (57 percent). The next most common responses were the Internet and advertisements, both of which had increased in usage over the prior two years (from 29 percent to 38 percent, and 15 percent to 34 percent, respectively).
Given these findings on sources of information, no communication strategy can focus simply on physicians and patients; it is also essential to
take into account messages received through print, television, and online media. Also, historical communications about an intervention must be considered in crafting any new messages.
Several recent examples illustrate confusion in healthcare communities and among the public about recommendations for health interventions when
- the intervention became standard with limited or no evidence (e.g., breast self-examination, hormone replacement therapy, use of erythropoiesis-stimulating agents as supportive therapy, management of ductal carcinoma in situ);
- uncertainty exists among healthcare professionals; and
- self-interests of healthcare professions, advocacy groups, and the media appear to trump the evidence.
A Case History: The U.S. Preventive Services Task Force
The recent uproar over the USPSTF recommendations for breast cancer screening captures many of the difficulties of communicating health information today. In 2007, the USPSTF outlined refined methods for developing recommendations that included an outline of communication and dissemination strategies (Guirguis-Blake et al., 2007). Unfortunately, these strategies focused on dissemination to professionals via medical journals and to federal agencies, professional societies, and quality improvement organizations via public meetings. As the recent breast cancer screening example illustrates, these are not the only important audiences. Strategies for disseminating information to the media, policy makers, and the public are also crucial components of any communication plan.
On November 16, 2009, the USPSTF revised its recommendations on screening for breast cancer in the general population (USPSTF, 2009). In summary:
- The USPSTF does not recommend that women automatically begin mammography screening at the age of 40. Instead, it recommends that the decision to start regular, biennial mammography screening before age 50 should be an individual one and take into account patient context, including the patient’s values regarding specific benefits and harms.
- The USPSTF recommends mammography screening every other year for women aged 50–74.
- The USPSTF concludes that evidence is insufficient to determine the harms and benefits of mammography screening in women over 74.
- The USPSTF recommends against healthcare providers teaching breast self-examination.
- Evidence was insufficient for the USPSTF to make a recommendation on clinical breast examination, digital mammography, or magnetic resonance imaging.
These recommendations were not significantly different from those issued in 2002.
The release of these updated recommendations was communicated in an article in the Annals of Internal Medicine, a biweekly medical journal. This typical communication strategy for releasing USPSTF recommendations had not led to mass confusion and hysteria in the previous year when the prostate cancer screening recommendations were updated. But the reaction to the revised breast cancer screening recommendations from the public, policy makers, the media, and the healthcare community was far from typical (Goldberg, 2009).
The timing of the release of the new recommendations was unfortunate, coinciding with a congressional vote on a healthcare reform bill after months of a particularly contentious debate. As a result, the revised screening recommendations were cast as one more example of “big government rationing health care,” which added to the hysteria around the recommendations’ release. Moreover, there have for years been simplistic messages about breast cancer screening—for example, that “early detection saves lives”—from the American Cancer Society, the National Cancer Institute, patient groups, trade associations, and the media. However, the evidence behind these statements and campaigns was not part of any discussion with the public, and the public health experts and primary care physicians with expertise in the area were on the periphery of these messaging campaigns. To further complicate the situation, Congress weighed in and demanded that the National Cancer Institute provide a clear message on screening, despite the lack of strong evidence of overall benefit. Indeed, in 1997 the Senate held hearings and passed a nonbinding resolution in support of mammograms for women under age 50 by a vote of 98-0 (Kassirer, 1997).
Years of communications about screening resulted in an ad campaign’s being converted into absolute truth. In fact, any evidence of the limitations of mammography screening and questioning of the evidence on which these simplistic messages were based was ignored or vilified. For example, a research article reported data indicating that “the natural course of some screen-detected invasive breast cancers is to spontaneously regress” (Zahl et al., 2008). Rather than taking the opportunity to explain the evidence to the public, the American Cancer Society’s director of cancer screening was quoted in USA TODAY as saying, “It’s important that people not wonder if women lost their breasts for no reason. That’s reprehensible conjecture” (Szabo, 2008).
Against this background, when the USPSTF issued its revised recommendations in the Annals of Internal Medicine, many in the media, policy makers, medical trade associations, and healthcare providers attacked the Task Force and the revised guidelines. What did the public hear?
- The American College of Radiology, a medical trade association representing radiologists and the field of medical imaging, stated: “Countless American women will die needlessly from breast cancer each year” (American College of Radiology, 2009).
- The American Cancer Society responded: “With its new recommendations, the USPSTF is essentially telling women that mammography at age 40 to 49 saves lives; just not enough of them” (American Cancer Society, 2009).
- ABC News misrepresented the guidelines with the headline “Stop Annual Mammograms, Govt. Panel Tells Women Under 50” and implied that cost savings, not evidence, motivated the change. “Anecdotally, most people in the United States can think of a woman they know who caught breast cancer through a routine mammogram long before she turned 50. Many patient advocates wonder if money fueled the decision” (Cox, 2009).
- A Fox News Sunday interview included Bernadine Healy, M.D., the former director of the National Institutes of Health, strongly urging women to ignore the USPSTF screening recommendations because they will result in more women dying of breast cancer (Fox News, 2009).
- Secretary of Health and Human Services Sebelius undermined the credibility of the Task Force and its guidelines by stating: “[The U.S. Preventive Services Task Force] has presented some new evidence for consideration, but our policies remain unchanged. My message to women is simple. Mammograms have always been an important life-saving tool in the fight against breast cancer, and they still are today. Keep doing what you have been doing for years—talk to your doctor about your individual history, ask questions, and make the decision that is right for you” (Goldberg, 2009).
- Daniel Kopans, M.D., a radiologist and director of breast imaging at Massachusetts General Hospital, is often quoted in the media along the lines of his letter to the editors of the Annals of Internal Medicine, which stated: “Suggesting that these guidelines are based on clear evidence is not supported by the facts…. I believe that some of the advisors to the USPSTF have major, undisclosed, career interests in the guidelines. They have received funding for what I believe are nihilistic approaches, constituting more insidious con-
flicts of interest than the obvious conflicts of radiologists, such as myself…. Task Force members had no expertise in mammography screening or even breast cancer care…. In sum, the new USPSTF guidelines are unscientific, endanger women through false analyses, and should be withdrawn” (Kopans, 2010).
Unfortunately, these attacks dominated the media and the public perception of the updated guidelines, despite the fact that public health and primary care experts supported the recommendations:
- The American College of Physicians, a professional organization for physicians specializing in internal medicine, had issued clinical practice guidelines in 2007 for mammography screening among women aged 40–49 that encouraged physicians to carefully assess individual women’s risks for breast cancer and discuss with them the potential benefits and harms of mammography screening so they can make informed individual decisions about screening (Qaseem et al., 2007).
- The Cochrane Collaboration published a systematic review of mammography screening in 2006 and concluded that while such screening likely reduces breast cancer mortality, the magnitude of the effect is uncertain, and the screening also results in some women receiving a cancer diagnosis even though their cancer would not have led to death or sickness (Gøtzsche and Nielsen, 2006).
- The American Academy of Family Physicians recommended that mammography screening begin in average-risk women at age 50, and that all women aged 40–49 be counseled about the risks and benefits of mammography before making a decision to undergo screening (AAFP, 2003).
- The Annals of Internal Medicine conducted a readers’ survey and found that among clinician respondents, 67 percent reported that they will stop offering routine mammograms to women in their 40s (Annals of Internal Medicine, 2010).
While this is but one case study, it received a significant amount of attention. An atmosphere was created that undermined the trust between the American people and public health officials.
Lessons Learned
We need to be honest. We would all prefer that the correct message be simple and certain. However, it most often is not. We need better policies to ensure that the public, the media, healthcare providers, and policy
makers all have the tools they need to understand and explain uncertainty, understand evidence, and keep the needs of patients paramount.
Professional associations need stronger oversight because many clinical practice guidelines are issued by professional societies. Often, the developers of guidelines have a financial conflict of interest in the use of the interventions highlighted in the guidelines. For example, the American College of Radiology issues statements on breast cancer screening, although its members are not public health experts and have a financial interest in the outcome of such recommendations. The American Society for Clinical Oncology still recommends and has actively lobbied the federal government on coverage issues for the use of erythropoiesis-stimulating agents as supportive therapy for cancer patients, despite evidence that they actually stimulate tumor progression (FDA, 2010; Rizzo et al., 2008).
The public can receive particularly confusing messages when professional societies differ publicly in their assessment of the evidence, as the American College of Radiology and the American College of Physicians did on breast cancer screening for women aged 40–49. What ethical responsibilities do these trade associations have in making these pronouncements public? How do we communicate to the public who the experts are?
Campaigns to better educate the public, policy makers, and the media about the importance of evidence are crucial. We should not underestimate the public’s ability to understand and accept evidence. In the 2009 NBCC consumer survey, for example, consumers identified comparative effectiveness research as more likely than other healthcare reforms to improve quality of care for breast cancer patients.
Projects such as NBCC’s Project LEAD® (Leadership, Education, and Advocacy Development) training courses are important. Such courses on critically evaluating research and evidence need to be made more broadly available to the general public and journalists. Much work on this front is also being done by others, including Gary Schwitzer with his popular HealthNewsReview blog3; the Dartmouth Institute for Health Policy and Clinical Practice’s Center for Medicine and the Media4; and the Foundation for Informed Medical Decision Making.5
____________
3 For more information see http://www.healthnewsreview.org/blog/ (accessed October 15, 2010).
4 For more information see http://tdi.dartmouth.edu/centers/medicine-and-the-media/ (accessed October 15, 2010).
5 For more information see http://www.informedmedicaldecisions.org/index.html (accessed October 15, 2010).
REFERENCES
AAFP (American Academy of Family Physicians). 2003. Summary of policy recommendations for periodic health examinations, rev 5.4. Leawood, KS: American Academy of Family Physicians.
American Cancer Society. 2009. American Cancer Society responds to changes in USPSTF mammography guidelines. http://www.cancer.org/docroot/med/content/med_2_1x_american_cancer_society_responds_to_changes_to_uspstf_mammography_guidelines.asp (accessed October 15, 2010).
American College of Radiology. 2009. USPSTF mammography recommendations will result in countless unnecessary breast cancer deaths each year. http://www.acr.org/MainMenuCategories/media_room/FeaturedCategories/PressReleases/USPSTFMammoRecs.aspx (accessed October 14, 2010).
Annals of Internal Medicine. 2010. Editorial: When evidence collides with anecdote, politics, and emotion: Breast cancer screening. Annals of Internal Medicine 152(8):531-532.
Atkins, D., P. Briss, M. Eccles, S. Flottorp, G. Guyatt, R. Harbour, S. Hill, R. Jaeschke, A. Liberati, N. Magrini, J. Mason, D. O’Connell, A. Oxman, B. Phillips, H. Schunemann, T. Edejer, G. Vist, J. Williams, and T. G. W. Group. 2005. Systems for grading the quality of evidence and the strength of recommendations II: Pilot study of a new system. BMC Health Services Research 5(1):25.
Charles, C., A. Gafni, and T. Whelan. 1997. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Social Science & Medicine 44(5):681-692.
Cox, L. 2009. Stop annual mammograms, govt. panel tells women under 50. ABC Medical News Unit.
Deyo, R., D. Cherkin, J. Weinstein, J. Howe, M. Ciol, and A. J. Mulle. 2000. Involving patients in clinical decisions: Impact of an interactive video program on use of back surgery. Medical Care 38(9):959-969.
Ebell, M. H., J. Siwek, B. D. Weiss, S. H. Woolf, J. Susman, B. Ewigman, and M. Bowman. 2004. Strength of Recommendation Taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. American Family Physician 69(3):548-556.
Edwards, A., and G. Elwyn. 2009. Shared decision making in health care: Achieving evidence based patient choice. Oxford: Oxford University Press.
FCC (Federal Coordinating Council for Comparative Effectiveness Research). 2009. Report to the President and Congress. http://www.hhs.gov/recovery/programs/cer/cerannualrpt.pdf (accessed October 11, 2010).
FDA (Food and Drug Administration). 2010. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen) darbepoetin alfa (marketed as Aranesp). Safety announcement. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109375.htm (accessed October 15, 2010).
Fisher, E. S., D. E. Wennberg, T. A. Stukel, D. J. Gottlieb, F. L. Lucas, and E. L. Pinder. 2003. The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Annals of Internal Medicine 138(4):273-287.
Fox News. 2009. Transcript: Dr. Bernadine Healy on “FNS.” http://www.foxnews.com/story/0,2933,576291,00.html (accessed October 15, 2010).
Fox, S., and S. Jones. 2009. The social life of health information. http://www.pewinternet.org/Reports/2009/8-the-social-life-of-health-information (accessed October 14, 2010).
Goldberg, P. 2009. HHS secretary rebukes task force guidelines on breast cancer screening. The Cancer Letter 35(43).
Goodman, C. 2009. Comparative effectiveness research and personalized medicine: From contradiction to synergy. http://www.lewin.com/content/publications/Lewin_CER-PM.pdf (accessed October 14, 2010).
Gøtzsche, P. C., and M. Nielsen. 2006. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews 4.
Guirguis-Blake, J., E. Calonge, T. Miller, A. Siu, S. Teutsch, and E. Whitlock. 2007. Current processes of the U.S. Preventive Services Task Force: Refining evidence-based recommendation development. Annals of Internal Medicine 147(2):117-122.
Harbour, R., and J. Miller. 2001. A new system for grading recommendations in evidence based guidelines. BMJ 323(7308):334-336.
Holmboe, E., D. Fiellin, E. Cusanelli, M. Remetz, and H. Krumholz. 2000. Perceptions of benefit and risk of patients undergoing first-time elective percutaneous coronary revascularization. Journal of General Internal Medicine 15(9):632-637.
Howick, J., P. Glasziou, and J. K. Aronson. 2009. The evolution of evidence hierarchies: What can Bradford Hill’s ‘guidelines for causation’ contribute? Journal of the Royal Society of Medicine 102(5):186-194.
IOM (Institute of Medicine). 2001. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press.
———. 2009. Initial national priorities for comparative effectiveness research. Washington, DC: The National Academies Press.
Kassirer, J. 1997. Practicing medicine without a license—the new instructions by Congress. New England Journal of Medicine 336:1747.
Kopans, D. 2010. The Annals is ignoring and denigrating fundamental scientific issues that make the USPSTF guidelines on mammography screening, scientifically, unsupportable. http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00210.short/reply (accessed June 20, 2011).
Krumholz, H. M. 2010. Informed consent to promote patient-centered care. Journal of the American Medical Association 303(12):1190-1191.
McAlister, F. A., A. Laupacis, G. A. Wells, D. L. Sackett, and for the Evidence-Based Medicine Working Group. 1999. Users’ guides to the medical literature. XIX. Applying clinical trial results. B. Guidelines for determining whether a drug is exerting (more than) a class effect. Journal of the American Medical Association 282(14):1371-1377.
O’Connor, A. M., J. E. Wennberg, F. Legare, H. A. Llewellyn-Thomas, B. W. Moulton, K. R. Sepucha, A. G. Sodano, and J. S. King. 2007. Toward the “tipping point”: Decision aids and informed patient choice. Health Affairs 26(3):716-725.
Orloff, J., F. Douglas, J. Pinheiro, S. Levinson, M. Branson, P. Chaturvedi, E. Ette, P. Gallo, G. Hirsch, C. Mehta, N. Patel, S. Sabir, S. Springs, D. Stanski, M. R. Evers, E. Fleming, N. Singh, T. Tramontin, and H. Golub. 2009. The future of drug development: Advancing clinical trial design. Nature Review of Drug Discovery 8(12):949-957.
Pearson, S.-A., A. Moxey, J. Robertson, I. Hains, M. Williamson, J. Reeve, and D. Newby. 2009. Do computerised clinical decision support systems for prescribing change practice? A systematic review of the literature (1990-2007). BMC Health Services Research 9(1):154.
Qaseem, A., V. Snow, K. Sherif, et al. 2007. Screening mammography for women 40 to 49 years of age: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 146:511-515.
Rawlins, M. D. 2008. De testimonio: On the evidence for decisions about the use of therapeutic interventions. The Harveian Oration delivered before the Fellows of the Royal College of Physicians of London on 16 October 2008. London: Royal College of Physicians.
Rizzo, J., M. Somerfield, and K. Hagerty. 2008. Use of Epoetin and Darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. Journal of Clinical Oncology 26(1):132-149.
Sackett, D. L. 1989. Rules of evidence and clinical recommendations on the use of anti-thrombotic agents. Chest 95(2 suppl):2S-4S.
Sepucha, K. R., F. J. Fowler, Jr., and A. G. Mulley, Jr. 2004. Policy support for patient-centered care: The need for measurable improvements in decision quality. Health Affairs Suppl Web Exclusives:VAR54-62.
Szabo, L. 2008. Study: Do breast tumors go away on their own. USA Today, http://www.usatoday.com/news/health/2008-11-24-breast-cancer_N.htm (accessed June 20, 2011).
Teutsch, S. M., L. A. Bradley, G. E. Palomaki, J. E. Haddow, M. Piper, N. Calonge, W. D. Dotson, M. P. Douglas, and A. O. Berg. 2009. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: Methods of the EGAPP working group. Genetics in Medicine 11(1):3-14
Tu, H. T., and G. R. Cohen. 2008. Striking jump in consumers seeking health care information. http://www.hschange.com/CONTENT/1006/# (accessed October 14, 2010).
USPSTF (U.S. Preventive Services Task Force). 2008. Procedure manual. http://www.uspreventiveservicestaskforce.org/uspstf08/methods/procmanual.htm (accessed October 14, 2010).
———. 2009. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine 151(10):716-726, W-236.
Walach, H., T. Falkenberg, V. Fonnebo, G. Lewith, and W. B. Jonas. 2006. Circular instead of hierarchical: Methodological principles for the evaluation of complex interventions. BMC Medical Research Methodology 6:29.
Weinstein, J., D. Goodman, and J. Wennberg. 1998. The orthopaedic workforce: Which rate is right? Journal of Bone and Joint Surgery 80(3):327-330.
Wennberg, J. E. 2002. Unwarranted variations in healthcare delivery: Implications for academic medical centres. BMJ 325(7370):961-964.
Zahl, P., J. Maehlen, and H. Welch. 2008. The natural history of invasive breast cancers detected by screening mammography. Archives of Internal Medicine 168(21):2311-2316.




























