B
Workshop Papers
Specifying Optimal Nutrient Composition for Military Assault Rations
Andrew J. Young, US Army Research Institute of Environmental Medicine Gerald A. Darsch, US Army Soldier Systems Center
INTRODUCTION
This report summarizes expert panel deliberations during a workshop organized by the Committee on Military Nutrition Research (CMNR) of the Food and Nutrition Board of the Institute of Medicine (IOM). The CMNR organized the workshop to address questions raised by the US Army Research Institute of Environmental Medicine (USARIEM) regarding optimal nutritional content for a new individual combat ration, First Strike Ration (FSR), being developed by Department of Defense (DoD) Combat Feeding Directorate at the US Army Natick Soldier Center. This new restricted ration was developed for highly mobile soldiers in high-intensity conflict by providing foods that can be eaten “on the move.” As a result, the FSR is smaller and lighter than the main individual operational ration, the Meal, Ready-to-Eat (MRE). In addition to their practicality, the FSR received high customer acceptance during recent deployments (Operation Enduring Freedom, Operation Iraqi Freedom). However, constraints imposed by ration design factors on the range of food components suitable for inclusion in an FSR might limit intake of certain nutrients needed to maintain soldier performance during sustained combat operations. The IOM ad hoc committee, charged with evaluating those concerns and recommending optimal nutrient content for
future versions of the ration, convened a workshop to gather information regarding the nutritional needs of individuals doing high-intensity activities for a short term while under stress.
OPERATIONAL RATIONS AND MILITARY OPERATIONS
US Army Field Feeding Doctrine (i.e., fundamental principles by which the military forces guide their actions) calls for supporting soldiers by providing them with “the right meal at the right place at the right time” (US Department of the Army, 1996). To accomplish this, Natick Soldier Center’s DoD Combat Feeding Directorate conducts research, development, testing, evaluation and engineering support for combat rations, field food service equipment and total combat feeding systems for the military services and the Defense Logistics Agency. To that end, the Combat Feeding Directorate has developed an appropriate range of combat rations that are available for requisition through the military supply system. Each of these rations is designed to meet the Military Reference Dietary Intakes (MDRIs) as established in AR 40-25 (US Departments of the Army, Navy, and Air Force, 2001). Detailed descriptions, including menus, nutrient content, and packaging information for the entire spectrum of these operational rations, are documented elsewhere (US Department of Defense, 2004). Table B-1 briefly summarizes key features for several of the most widely used rations.
In those instances when operational conditions preclude serving hot, cafeteria-style rations, military personnel are provided individual operational rations, of which the MRE is the flagship ration. The nutrient requirements of most deployed soldiers can be satisfied when they are provided MREs or other appropriate rations listed in Table B-1. However, due to several factors explained below, the current rations may not completely satisfy nutritional requirements of soldiers in some situations (i.e., during the assault phase of combat operations, certain Special Operations Forces missions, and missions anticipated to be conducted by Future Force Soldiers).
First, daily energy expenditures of personnel engaged in these types of military missions are so high (Tharion et al., 2005) that these soldiers will not maintain an energy balance even when they consume three complete MREs per day, which could lead to adverse physiological effects and detriments in health and performance (see also next section on “Physiological Demands of Combat Operations”). Moreover, in some cases during combat operations, soldiers receive only two MREs, which further exacerbates the situation.
Second, soldiers who must carry heavy loads or engage in strenuous military duties (e.g., light infantry, US Marine Corps, and Special Operation Forces) compound their energy balance problem by “field-stripping” the MREs. To lighten their heavy loads, soldiers often open the individual meal packages, select certain components based on individual preference, and discard the remainder.
TABLE B-1 Principle Types of Operational Rations Fielded by the US Department of Defense
|
Ration |
Purpose |
Nutrition (Average Meal) |
Key Characteristics |
|
Unit Group Ration/Heat & Serve |
Group Feeding (50 meal/module) |
1,450 kcal 14% protein 32% fat 54% carbohydrate |
21 menus (14 lunch/dinner, 7 breakfast) Includes semi perishables 18 month shelf-life 113 lb, 5 ft3/module Organized food service required |
|
Unitized Group Ration, A |
Group Feeding (50 meal/module) |
1,450 kcal 14% protein 32% fat 54% carbohydrate |
21 menus (same as above) Includes perishables 3 month shelf life (made-to-order) 113 lb, 5 ft3/module Organized food service required |
|
Meal, Ready-to-Eat (MRE) |
Individual Feeding (3 MREs/day) |
1,300 kcal 13% protein 34% fat 53% carbohydrate |
24 menus Heat processed foods in flexible retort pouches 36 month shelf-life 1.5 lb, 0.052 ft3/meal Ready-to-Eat |
|
Meal, Cold-Weather/Food Packet, Long Range Patrol (MCW/LRP) |
Individual Feeding (1 to 3 MCW/LRP per day) during cold-weather or special operation forces and marine corps |
1,540 kcal 15% protein 35% fat 50% carbohydrate |
12 menus Dehydrated and dried food components, 28–40 oz water needed to fully rehydrate 36 month shelf life 1 lb/meal, 0.04 ft3/meal Individually prepared |
|
SOURCE: US Department of Defense (2004). |
|||
Because MREs and other rations were designed to meet the nutritional needs of deployed soldiers who consume the entire daily ration (in which the nutrients are not evenly distributed), such selective consumption will further decrease total intake of energy and/or will compromise adequate intake of specific nutrients. Although field-stripping does, in fact, lighten a soldier’s load, the cost of the discarded components can approach $34 over a three-day period. More importantly, studies at USARIEM and elsewhere have documented a variety of adverse biomedical and performance consequences (Lieberman, 2003; Montain and Young,
2003) when such semistarvation is coupled with other physiological stressors encountered during sustained combat operations (e.g., sleep deprivation, anxiety, dehydration).
The need for a specialized ration for high-tempo, assault-type missions has been recognized since the end of World War II (Samuels et al., 1947) when military planners recommended development of an “Assault Candy Ration.” To better meet the individual’s nutritional needs during these high-intensity operations, Natick Soldier Center’s Combat Feeding Directorate have developed a new smaller, lighter, individual operational ration comprised of eat-on-the-move food components, the FSR. The FSR is intended for use during specific missions such as those described above when soldiers must operate for periods of three days, or possibly longer, with minimal resupply. This ration will serve to “bridge the gap” until operational tempo abates and more nutritionally complete rations can provided. Because of the relevance of the operations for which FSRs are envisioned, their full implementation in the field may occur in an accelerated fashion; in fact, it is estimated that the FSR, pending the military services’ approval, will enter the procurement system in the first part of 2007. Through the use of spiral development generated under an Army Technology Objective (ATO) entitled “Nutritionally Optimized FSR” (IV.MD.2005.02), jointly sponsored by USARIEM and the Natick Soldier Center Combat Feeding Program, science and technology innovations are planned for insertion as part of a preplanned product improvement program. The objective of this ATO is to develop and utilize novel nutrient delivery systems, food formulations, and field feeding strategies to provide on-demand access to specific nutrients to best sustain performance. The specific strategies, when identified, will improve overall energy and nutrient intake by 20 percent and enhance cognitive and physical performance by 20 percent. The DOD asked the IOM committee to provide recommendations that will guide the design of the ration for sustained combat operations and will identify nutritional research to accomplish this objective.
WORKSHOP TASKING: RECOMMENDATIONS FOR NUTRITIONAL OPTIMIZATION OF THE FIRST-STRIKE RATION
Overall Approach
The Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations asked the workshop participants to consider the specific requirements for the FSR (size, volume, weight) that constrain the ration’s design such that the ration is unlikely to provide enough energy to match the daily energy expenditures of the soldiers. Table B-2 lists important design features and performance objectives for the FSR development effort. The workshop’s primary objective was to gather information and provide recommendations regarding nutritional strategies suitable for implementation to
TABLE B-2 Field Feeding Approaches During Assault Phase Operations
make an FSR that would best sustain health and performance despite semistarvation arising from high daily energy expenditure and constrained energy intake. Box B-1 details several specific questions that the workshop was directed to address. The overall question was the following: Given the fact that weight and size limitations preclude the FSR from completely preventing negative energy balance in soldiers subsisting on the ration, would health and performance best be preserved by a nutrient composition that maximizes energy density (i.e., minimize energy deficit) or, alternatively, by a micro- and macronutrient composition that specifically optimizes health status and performance, potentially at the expense of a less than maximum energy density?
Besides addressing nutrient content, the speakers at the workshop were also asked to consider ration design approaches to enhance overall consumption of the ration. The problem of ration underconsumption during field training and operational deployments is well documented and was the focus of an earlier report (IOM, 1995). The CMNR previously concluded that five broad categories of factors contributed to ration under consumption.
The first four categories of factors related to the environment and to behavior that potentially impairs appetite and/or limits food consumption were identified as the following: exposure to harsh climate and danger (environment); social interactions during meals; appropriateness of the meal to time of day (eating situation); and the attitudes toward field-feeding systems held by soldiers and
|
BOX B-1
|
their leaders (individual). Based on the CMNR recommendations, technical reports and commanders’ guides have been prepared and distributed providing guidance to minimize the effect of those negative factors; however, from a practical standpoint, commanders conducting combat operations are permitted little flexibility to mitigate those kinds of stressors. The fifth category is underconsumption factors, related to a ration’s characteristics that influence customer acceptability and for which the committee offered more practical recommendations to improve consumption. These recommendations included the following: to enhance menu variety; to improve food sensory features, packaging, and ease of use for the rations and components; and to provide smaller snacking or “nibbling” food items as well as energy- and nutrient-rich beverages that could be carried in pockets and consumed quickly while on the move.
Clearly, those recommendations should be the driving focus behind the FSR. All of those approaches have already been incorporated into the newest versions of the MRE and other operational rations during the continuous product improvement programs that Combat Feeding Directorate conducts and they have been recognized by troops who consume combat rations. For this workshop, the panel
of experts was asked to consider how similar, practical approaches could be used to further enhance consumption of the FSR, thereby better satisfying the nutritional needs of soldiers during assault type operations.
Workshop Overview
Under the auspices of the CMNR, the Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations and the IOM organized a workshop to address the questions posed by USARIEM. The workshop was hosted by USARIEM and the Natick Soldier Center in Natick, MA, in August 2004. USARIEM scientists provided the workshop participants with their tasks, overviews of military ration systems, and background briefings on the physiological and medical consequences of combat operations on military personnel. For the remainder of the workshop, invited scientific experts presented information on topics organized into five major categories: optimization of macronutrient composition; optimization of micronutrients and other bioactive compounds; nutritional optimization of the immune system; nutritional preventive medicine; and food product development. Those presentations are the basis for the remainder of this report.
REFERENCES
IOM (Institute of Medicine). 1995. Not Eating Enough. Washington, DC: National Academy Press.
Lieberman HR. 2003. Nutrition, brain function and cognitive performance. Appetite 40(3):245–254.
Montain SJ, Young AJ. 2003. Diet and physical performance. Appetite 40(3):255–267.
Samuels JP, McDevitt RP, Bollman MC, Maclinn W, Richardson LM, Voss LG. Conway HA. 1947. In: Meyer AI, eds. Ration Development. Operational Studies 1(12). Fort Lee, VA: Office of the Quartermaster General.
Tharion WJ, Lieberman HR, Montain SJ, Young AJ, Baker-Fulco CJ, Delany JP, Hoyt RW. 2005. Energy requirements of military personnel. Appetite 44(1):47–65.
US Department of the Army. 1996. Basic Doctrine for Army Field Feeding and Class 1 Operations Management. FM 10-23. Washington, DC: Department of the Army.
US Department of Defense. 2004. Operational Rations of the Department of Defense. Natick PAM 30-25. Natick, MA: US Army Natick Soldier Center.
US Departments of Army, Navy, and Air Force. 2001. Nutrition Standards and Education. AR 40-25/BUMEDINST 10110.6/AFI 44-141. Washington, DC: US Department of Defense Headquarters.
Physiological Demands of Combat Operations
Scott J. Montain, US Army Research Institute of Environmental Medicine
INTRODUCTION
The combat foot soldiers within the light infantry and special operations units are the military populations targeted for use of next-generation assault rations. These soldiers are required to carry or transport all of the supplies, sometimes exceeding 50 kg, they will need for the operation. Missions often are of the continuous type termed “sustained operations” (SUSOPS) lasting from two to seven days or longer that consist of near-continuous physical work, restricted sleep, and limited breaks for meals. While the energy cost of any single task is not necessarily high, total daily energy expenditures can reach extremely high levels because of long hours of wakefulness. Thus, these soldiers are faced with sustained environmental exposure, exertional fatigue, sleep deprivation, and energy deficits.
The lightweight and small assault ration being developed specifically for these soldiers must be capable of sustaining their performance while in repeated SUSOPS missions lasting for three days when energy expenditures exceed 18 MJ (4,300 kcal)/day, and up to seven days during which daily energy expenditures are expected to be less. This chapter describes the physiological challenges facing combat foot soldiers to facilitate defining their nutritional requirements. This topic has been discussed in greater depth in other publications (Friedl, 2003; Tharion et al., 2005), and the reader is referred to these papers for additional information.
ENERGY COST OF SOLDIER ACTIVITIES
The total daily energy expenditures of combat units during training exercises has ranged from 15.5 to 29.8 MJ (3,700 to 7,120 kcal)/day (Figure B-1); with the highest values occurring during cold-weather operations. The tasks performed typically have included long, sustained periods of low to moderate intensity work (expected metabolic rates of 250 to 450 Watts), with short periods of relatively high-intensity work (expected metabolic rates in excess of 600 Watts). Factors contributing to these high daily energy expenditures have been the relatively long periods of work (> 15 h/day), traversing rough terrain or soft surfaces (e.g., snow, mud, or sand), and carrying heavy loads.
Much of what we know regarding the energy cost of soldier activities comes from investigations of soldiers performing simulated combat missions as part of training courses. In these scenarios, total daily energy expenditures (TDEE) are often quite high. For example, airmen participating in the US Air Force Combat Survival Course averaged 19.7 MJ (4,700 kcal)/day of TDEE (Jones et al., 1992),
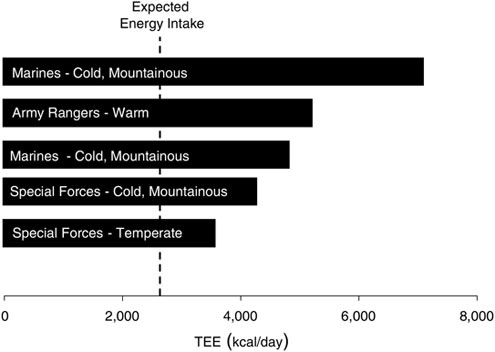
FIGURE B-1 Measured troop energy expenditures during military field operations.
NOTE: TEE = total energy expenditure.
SOURCE: Tharion et al. (2005).
soldiers attending the US Army Special Forces Assessment School averaged 21.7 MJ (5,180 kcal)/day (Fairbrother et al., 1995), and marines at the US Marine Corps Infantry Officer Course averaged 22.5 MJ (4,700 kcal)/day of TDEE during cold-weather operations (Hoyt et al., 2001) and 16 to17 MJ (3,820 to 4,060 kcal)/day during hot weather (author’s unpublished results; personal communication, R. Hoyt, US Army Research Institute of Environmental Medicine, 1999). The US Air Force Combat Survival Course is a 5-day course that trains aircrew members in parachuting and survival, evasion, resistance, and escape procedures, as well as simulated prisoner of war interrogations. The US Army Special Forces Assessment School conducts 20-day training and includes activities such as physical fitness tests, battle marches, and long-range movements carrying backpacks, weapons, and other field equipment. In the Marine Corps Infantry Officer Course, marines perform a series of simulated combat operations using dismounted infantry, mounted infantry, and amphibious tactics. In each of these courses, night operations are included, leading to near-continuous physical work, with marines active 16 to 22 h/day.
Students enrolled in the US Army Ranger training course perform a series of physically demanding field exercises intermittently over a 60-day period. While total energy expenditures averaged over the 60-day course are lower than those for many of the shorter, more intense military training courses, they are still quite high, averaging 16.8 MJ (4,010 kcal)/day (Moore et al., 1992) and 17.1 MJ (4,090 kal)/day (Shippee et al., 1994) for more than two months. Combat fundamentals taught during the course include patrolling; squad reconnaissance and ambush; mountaineering; small boat operations; and attack, ambush, and raid drills. The unique aspect of this course is repeated, periodic food restriction and sleep deprivation as one of the intentional stressors—making it a model for studying the physiological strain likely to be imposed on soldiers performing repeated SUSOPS missions during combat, which is the subject of interest in this workshop.
As noted in the introduction, combat foot soldiers carry their own supplies at high energy expenditure costs. The loads they carry can be very heavy depending on what phase of a mission they are performing. A recent study (Dean and Dupont, 2003) in which soldier loads were measured during actual operations in Afghanistan revealed that soldiers in the traveling phase of a mission carried an average of 59.3 kg (131 lb). Their approach load averaged 45.7 kg (101 lb), and their fighting load averaged 28.5 kg (63 lb). When normalized to body weight, these loads were equivalent to carrying 77 percent, 57 percent, and 35 percent of body mass, respectively. Current soldier development efforts are exploring methods to dramatically lower the soldier load, but soldier load remains a major physical stressor during combat execution.
PHYSIOLOGICAL CONSEQUENCES OF COMBAT OPERATIONS
The massive load each soldier carries limits the amount of space and weight that soldiers are willing to reserve for food. The nature of SUSOPS missions also means that food is consumed on the go. These two factors lead to soldiers stripping their personal rations of items that they don’t like or will be unlikely to eat. When voluntary intake has been measured, it typically averages between 10 and 12 MJ (2,390 to 2,870 kcal) (Baker-Fulco, 1995). Since energy expenditure is often much higher than intake, there is demand placed on endogenous energy stores to meet energy demands of the mission.
Metabolic Status and Body Composition Changes
Blood glucose levels typically decrease over SUSOPS missions, but the average values generally only fall 10 mg/dL (0.5 to 0.6 mM) from baseline values. There are soldiers, however, who demonstrate much higher reductions. In a recent investigation (author’s unpublished results), during which marines consumed 1,600 kcal/day and 210 g of carbohydrate/day while expending
3,850 kcal/day, it was observed that 12 to 23 percent of volunteers had blood glucose values below 76 mg/dL (< 4.2 mM) after four to eight days of SUSOPS. Concomitant with these metabolic changes were substantial increases in blood ketone and free fatty acid concentrations.
The underfeeding accompanying SUSOPS is accompanied by reductions of both fat and lean body mass. For example, Nindl and colleagues (2002) reported fat and lean tissues losses of 1.2 and 1.5 kg, respectively, over a 72 h SUSOPS. Similarly, Moore and colleagues (1992) reported that 38 percent of the 12 kg body mass loss that occurred during Ranger training (when severely underfed) was attributable to nonfat tissue loss.
Cognitive Performance
Opstad and colleagues (1978) found that visual vigilance decreased 4 to 28 percent after three to four days of sustained operations activity. Reaction time and coding declined 12 to 30 percent over the same time period, while prone marksmanship declined 10 percent. Inclusion of three to six hours of continuous sleep once during the five-day operation partially or fully reversed these declines. There was large interindividual variability, but general deterioration occurred due to omissions, not mistakes. Similar results have been reported by others, as Bugge and colleagues (1979) observed, decreased logical reasoning (46 percent), letter cancellation attempts (40 percent), and code test scores (45 percent) after four days of sustained operations. More recently, Tharion and colleagues (1997) reported a reduction in visual vigilance (44 percent), slower processing (21 percent), and fewer correct responses (17 percent) on a four-choice reaction test, and compromised performance on a match to sample task after 73 to 74 h of sleep deprivation and operational stress. Associated with the impaired cognitive performance were increased levels of fatigue, confusion, tension, and depression. Shippee and colleagues (1994) evaluated decoding, memory, reasoning, and pattern recognition during the eight-week US Army Ranger Course. Soldiers maintained near perfect accuracy at decoding and reasoning at the expense of speed (7 to 10 percent fewer attempts). Memory accuracy declined over time. Both speed and accuracy were impaired on the pattern recognition task—particularly during the desert and jungle phases.
Physical Performance
Prolonged operations lasting several weeks’ duration and associated with substantial losses of lean body mass (Moore et al., 1992; Shippee et al., 1994) have resulted in reductions (20 percent) in maximal lifting capacity and vertical jump height (15 to 16 percent). Shorter duration studies with minimal lean body mass loss generally showed little or no decrement in muscle strength, power, or fatigability (Bulbulian et al., 1996; Guezennec et al., 1994; Vanhelder and
Radomski, 1989); however, this is not universal (Legg and Patton, 1987). Sustained operations scenarios lasting less than one week have resulted in reduced maximal aerobic power and endurance (Guezennec et al., 1994) as have sleep-deprivation studies (Vanhelder and Radomski, 1989).
Performance of simple and well-learned motor tasks (e.g., weapon handling) do not appear to be compromised by sustained operational stress (Haslam, 1984). However, endurance time is frequently impaired during aerobic exercise tasks (Vanhelder and Radomski, 1989), and there is an increased perception of effort to perform the same task. Nindl and colleagues (2002) recently reported 25 percent lower work productivity on a physical persistence task; in agreement with the hypothesis that SUSOPS compromises prolonged and monotonous tasks (Figure B-2). Independent of energy intake (Rognum et al., 1986), operational effectiveness is also affected if sleep is inadequate.
Marksmanship can be compromised by sustained operation activities particularly when very little restorative sleep is obtained. Tharion and colleagues
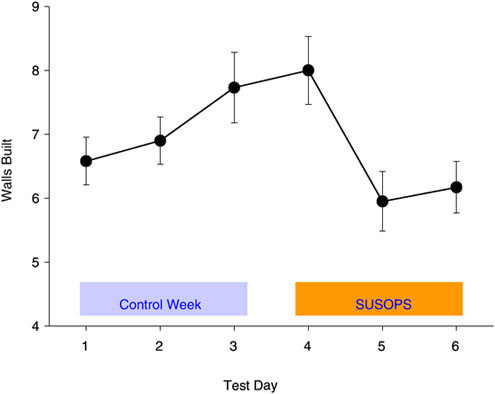
FIGURE B-2 Work productivity on a physical persistence task over 72 h of simulated military sustained operations (SUSOPS) training as well as a control week without near-continuous work and underfeeding.
SOURCE: Nindl et al. (2002).
(2003) reported significantly impaired marksmanship after 73 to 74 hours of total sleep deprivation during Navy Seal training. Specifically, they observed greater distance from center of mass (38 percent) increased dispersion of shot groups (235 percent), as well more missed targets (37 percent), all indicative of reduced marksmanship. These adverse findings occurred despite a 53 percent slower sighting time. In contrast, Johnson and colleagues (2001) reported that despite soldiers rating the marksmanship task as more difficult to perform after 48 and 72 h of SUSOPS (2 h of sleep per night), there was no reduction in the number of randomly appearing targets hit during the test. Haslam (1984) reported no negative results during nine days of sustained operations and sleeping either 1.5 or 3 h daily on shot group clustering when troops fired in the prone supported position. However, their ability to acquire and accurately hit a randomly appearing target declined during the course. The group receiving only 1.5 h of daily sleep performed 50 percent below baseline from days 5–9 of the field exercise, whereas the group receiving 3 h of daily sleep had a more modest reduction (the number of hits fell from 6–7 to 4–5).
Similarly, the caloric deficit associated with sustained operations scenarios appears to have inconsistent effects on soldier performance (Montain and Young, 2003). Rognum and colleagues (1986) reported no difference either in time to complete an assault course or in prone marksmanship performance when students were provided 1,500 or 8,000 kcal/day during a five-day scenario. However, Guezennec and colleagues (1994) observed 8 percent reductions in maximal aerobic power when soldiers were restricted to 1,800 kcal/day but no reduction in maximal aerobic power when soldiers were fed 3,200 or 4,200 kcal/day. Similarly, Tharion and Moore (1993) reported reductions in shot group tightness on a marksmanship task after sustained road marching soldiers were fed 250 g of carbohydrate per day, but no change when soldiers consumed a diet with 400 or 550 g of carbohydrate.
Time and content of the previous meal may be an explanation for the divergent results. Montain and colleagues (1997) reported a relationship in that an increase in energy (or carbohydrate) intake sustains uphill run time over three days of physically demanding field training. Moreover, 11 of 13 soldiers who best sustained uphill-run performance had eaten 70 to 378 g of carbohydrate during the four h preceding the uphill run, while 10 of the 13 soldiers with the greatest decrement had eaten none of their rations since the previous night’s meal. All participants in this study were provided with two Meals, Ready-to-Eat each day or approximately 2,600 kcal and 300 g of carbohydrate per day. These data suggest that soldiers subsisting on diets with these energy and carbohydrate levels during high-tempo operations are receiving near the minimum energy necessary to sustain performance, necessitating good food discipline (i.e., eating the food provided), and good food choices (i.e., eat the carbohydrate-containing foods) to preserve physical performance.
Endocrine Changes
Dramatic changes in the blood hormonal milieu arise during sustained operations protocols and persist during both rest and exercise. In the five day Norwegian Ranger Course, in which food and sleep are kept to a minimum, there was a progressive increase in catecholamine concentration (Rognum et al., 1981) as well as cortisol, growth hormone, and aldosterone levels (Opstad, 1992; Opstad and Aakvaag, 1981) and a reciprocal fall in testosterone and prolactin (Opstad and Aakvaag, 1982) as well as other adrenal and testicular androgens (Opstad, 1992). The increase in catecholamine concentration is associated with a downregulation of adrenergic receptors on white blood cells (Opstad et al., 1994). Similar observations have been reported for soldiers participating in the US Army Ranger Selection Course (Moore et al., 1992; Shippee et al., 1994).
The decline in testosterone (up to 70 percent from baseline) and other anabolic hormones may be due in part to the caloric and/or protein restriction imposed during the courses. Short periods of refeeding quickly reverse declines in insulin-like growth factor-1 (IGF-1) (Friedl et al., 2000) (Figure B-3). When additional energy of a mixed diet of protein, fat, and carbohydrate have been provided (400 and 1,400 kcal), reductions in IGF-1 and testosterone were attenuated (Friedl et al., 2000; Guezennec et al., 1994). However, when additional calories (4,900 kcal) have been provided predominately by carbohydrate alone, testosterone reductions were only modestly lowered (Opstad and Aakvaag, 1982), suggesting that the testosterone and IGF-1 changes may have been consequent to energy deficit and possibly insufficient amino acid intake (Sanchez-Gomez et al., 1999).
Immune Function
The effects of sustained operations on immune function remain poorly understood. The outbreak of infectious disease among soldiers participating in US Army Ranger and Special Forces Assessment Schools suggests that multistress environments can compromise immune defense mechanisms (Moore et al., 1992). Changes in immune cell parameters and in vitro responses to stress have varied from study to study, but they appear related to the duration and severity of the sustained operations stress.
The five day Norwegian Ranger Course produced a general leukocytosis, predominantly due to two- to threefold increase in neutrophils and monocytes (Boyum et al., 1996). Lymphocyte numbers decreased as did CD4 T cells, CD8 T cells, CD16 natural killer cells, and CD19 B cells. Neutrophil chemotaxis and oxidative burst capacity increased during the course before returning to baseline levels (or below) after five days of training (Wiik et al., 1996). These changes have been attributed to sleep deprivation and appear relatively insensitive to changes in caloric (carbohydrate) intake (Wiik et al., 1996). Immunoglobulin M
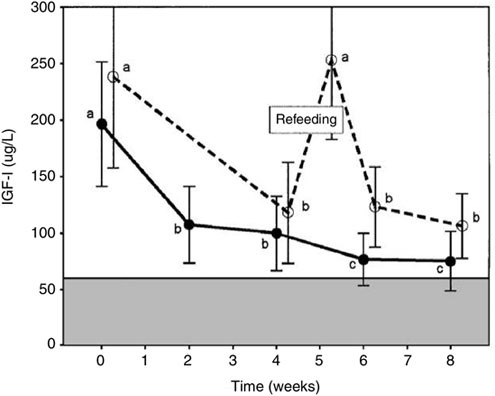
FIGURE B-3 Insulin-like growth factor-1 (IGF-1) response to US Army Ranger Training under two levels of underfeeding and response to several days of refeeding during the course. Letters indicate means that are not significantly different (Scheffe’s test).
NOTE: Different letters indicate a significant difference in IGF-1 response (e.g., a versus b).
SOURCE: Friedl et al. (2000).
and immunoglobulin A decrease 20 to 30 percent during the course, while mitogenic responses to antigen exposure have not been consistent from course to course (Boyum et al., 1996).
The activities of the eight week US Army Ranger School appear to modify immune function, although the magnitude varies among the type of response. For example, Moore and colleagues (1992) found a similar percentage of positive delayed hypersensitivity responses to streptococcus and tetanus over the course despite relatively severe sleep deprivation and a 16 percent drop in body mass. There was no change in immunoglobulin concentrations during the course. However, as lymphocyte proliferation to mitogen stimulation declined during the course, an increasing number of students became pneumonia carriers, and 25 percent of the students sought medical attention for infections during the final portion of the course (swamp and desert phases).
Additional ranger studies (Bernton et al., 1995) revealed reductions in cell mediated immunity responses among the trainees. Shippee and colleagues (1994) examined immunological responses after students were provided additional calories during the course. Only 2 to 8 percent of the students required medical attention during the mountain and swamp phases. Immunoglobulin levels did not change, and neutrophil oxidative burst capability increased during the course. There was a general leukocytosis, due primarily to increased granulocytes, but lymphocyte concentrations fell. The ability of leukocytes to proliferate in response to mitogen stimulation was suppressed but was less than reported in previous courses when underfeeding was more severe (Moore et al., 1992). These latter responses suggest a possible shift in proportion of lymphocytes of the T-helper type 1 (TH1) phenotype (cell-mediated) to the T-helper type 2 (TH2) phenotype (antibody-mediated). This immune system adaptation occurs after trauma (Decker et al., 1996; Mack et al., 1997; O’Sullivan et al., 1995) and with disease (Raziuddin et al., 1998) and can immunocompromise the host to certain types of infectious agents (Mack et al., 1997; O’Sullivan et al., 1995).
SUMMARY
The goal of the US military developmental assault ration, currently called the First Strike Ration, is to sustain troop health and performance for at least 96 h of unsupported military operations. To accomplish this objective, the nutritional components must sustain a soldier commonly expending in excess of 18 MJ (4,300 kcal) of energy per day and carrying loads in excess of 45 to 50 kg during while exposed to environmental extremes. It is well documented that the mission stress can challenge immune and neuroendocrine homeostasis and if sutained too long or repeated too frequently, that troop health and performance can be compromised. Likewise, there is evidence that nutritional support can reduce the physiological strain. The challenge is in defining the proper mix of macro- and micro-nutrients to sustain the soldier within a ration system the soldiers will choose to carry and consume during mission execution.
ACKNOWLEDGMENTS
The views, opinions and/or findings in this report are those of the authors, and should not be construed as an official Department of the Army position, policy, or decision, unless so designated by other official documentation.
REFERENCES
Baker-Fulco CJ. 1995. Overview of dietary intakes during military exercises. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 121–149.
Bernton E, Hoover D, Galloway R, Popp K. 1995. Adaptation to chronic stress in military trainees. Adrenal androgens, testosterone, glucocorticoids, IGF-1, and immune function. Ann N Y Acad Sci 774:217–231.
Boyum A, Wiik P, Gustavsson E, Veiby OP, Reseland J, Haugen AH, Opstad PK. 1996. The effect of strenuous exercise, calorie deficiency and sleep deprivation on white blood cells, plasma immunoglobulins and cytokines. Scand J Immunol 43(2):228–235.
Bugge JF, Opstad PK, Magnus PM. 1979. Changes in the circadian rhythm of performance and mood in healthy young men exposed to prolonged, heavy physical work, sleep deprivation, and caloric deficit. Aviat Space Environ Med 50(7):663–668.
Bulbulian R, Heaney JH, Leake CN, Sucec AA, Sjoholm NT. 1996. The effect of sleep deprivation and exercise load on isokinetic leg strength and endurance. Eur J Appl Physiol Occup Physiol 73(3–4):273–277.
Dean CE, Dupont F. 2003. Modern Warrior’s Combat Load. Dismounted Operations in Afghanistan. April–May 2003. Letter Report. Ft. Leavenworth, KS: US Army Center for Army Lessons Learned.
Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA. 1996. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 119(3):316–325.
Fairbrother B, Kramer T, Mays M, Kramer M, Tulley R, Delany J, Marchitelli L, Tessicini M, Shippee RL, Askew S, Popp K, Hoyt R, Rood J, Frykman P, Arsenault J, Jezior D. 1995. Nutritional and Immunological Assessment of Soldiers During the Special Forces Assessment and Selection Course. Technical Report No. T95-22. Natick, MA: US Army.
Friedl KE. 2003. Military studies and nutritional immunology: Undernutrition and susceptability to illness. In: Hughes DA, Darlington LG, Bendich A, eds. Diet and Human Immune Function. Towtown, NJ: Humana Press, Inc. Pp. 381–396.
Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW. 2000. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol 88(5):1820–1830.
Guezennec CY, Satabin P, Legrand H, Bigard AX. 1994. Physical performance and metabolic changes induced by combined prolonged exercise and different energy intakes in humans. Eur J Appl Physiol Occup Physiol 68(6):525–530.
Haslam DR. 1984. The military performance of soldiers in sustained operations. Aviat Space Environ Med 55(3): 216-221.
Hoyt RW, Buller MJ, DeLany JP, Stultz D, Warren K, Hamlet MP, Shantz D, Matthew WT, Tharion WJ, Smith P, Smith B. 2001. Warfighter Physiological Status Monitoring (WPSM): Energy Balance and Thermal Status during a 10-day Cold Weather US Marine Corps Infantry Officer Course Field Exercise. Technical Report No. T02-02. Natick, MA: US Army Research Institute of Environmental Medicine.
Johnson RF, Merullo DJ, Montain SJ, Castellani JW. 2001. Marksmanship during simulated sustained operations. Proceedings of the Human Factors and Ergonomics Society. 45th Annual Meeting. 45:1382–1385.
Jones TE, Mutter SH, Aylward JM, DeLany JP, Stephens RL, Caretti DM, Jezior DA, Cheema B, Lester LS, Askew EW. 1992. Nutrition and Hydration Status of Aircrew Members Consuming the Food Packet, Survival, General Purpose, Improved During a Simulated Survival Scenario. Technical Report No. T1-93. Natick, MA: US Army Research Institute of Environmental Medicine.
Legg SJ, Patton JF. 1987. Effects of sustained manual work and partial sleep deprivation on muscular strength and endurance. Eur J Appl Physiol Occup Physiol 56(1):64–68.
Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. 1997. Candida infection following severe trauma exacerbates Th2 cytokines and increases mortality. J Surg Res 69(2):399–407.
Montain SJ, Young AJ. 2003. Diet and physical performance. Appetite 40(3):255–267.
Montain SJ, Shippee RL, Tharion WJ. 1997. Carbohydrate-electrolyte solution effects on physical performance of military tasks. Aviat Space Environ Med 68(5):384–391.
Moore RJ, Friedl KE, Kramer TR, Martinez-Lopez LE, Hoyt RW, Tulley RE, DeLany JP, Askew EW, Vogel JA. 1992. Changes in Soldier Nutritional Status & Immune Function During the Ranger Training Course. Technical Report No. T13-92. Natick, MA: US Army Medical Research & Development Command.
Nindl BC, Leone CD, Tharion WJ, Johnson RF, Castellani JW, Patton JF, Montain SJ. 2002. Physical performance responses during 72 h of military operational stress. Med Sci Sports Exerc 34(11):1814–1822.
O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. 1995. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 222(4):482–490.
Opstad PK. 1992. Androgenic hormones during prolonged physical stress, sleep, and energy deficiency. J Clin Endocrinol Metab 74(5):1176–1183.
Opstad PK, Aakvaag A. 1981. The effect of a high calory diet on hormonal changes in young men during prolonged physical strain and sleep deprivation. Eur J Appl Physiol Occup Physiol 46(1):31–39.
Opstad PK, Aakvaag A. 1982. Decreased serum levels of oestradiol, testosterone and prolactin during prolonged physical strain and sleep deprivation, and the influence of a high calorie diet. Eur J Appl Physiol Occup Physiol 49(3):343–348.
Opstad PK, Ekanger R, Nummestad M, Raabe N. 1978. Performance, mood, and clinical symptoms in men exposed to prolonged, severe physical work and sleep deprivation. Aviat Space Environ Med 49(9):1065–1073.
Opstad PK, Haugen AH, Sejersted OM, Bahr R, Skrede KK. 1994. Atrial natriuretic peptide in plasma after prolonged physical strain, energy deficiency and sleep deprivation. Eur J Appl Physiol Occup Physiol 68(2):122–126.
Raziuddin S, al-Dalaan A, Bahabri S, Siraj AK, al-Sedairy S. 1998. Divergent cytokine production profile in Behcet’s disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatol 25(2):329–333.
Rognum TO, Vaage O, Hostmark A, Opstad PK. 1981. Metabolic responses to bicycle exercise after several days of physical work and energy deficiency. Scand J Clin Lab Invest 41(6):565–571.
Rognum TO, Vartdal F, Rodahl K, Opstad PK, Knudsen-Baas O, Kindt E, Withey WR. 1986. Physical and mental performance of soldiers on high- and low-energy diets during prolonged heavy exercise combined with sleep deprivation. Ergonomics 29(7):859–867.
Sanchez-Gomez M, Malmlof K, Mejia W, Bermudez A, Ochoa MT, Carrasco-Rodriguez S, Skottner A. 1999. Insulin-like growth factor-I, but not growth hormone, is dependent on a high protein intake to increase nitrogen balance in the rat. Br J Nutr 81(2):145–152.
Shippee R, Friedl K, Kramer T, Mays M, Popp K, Askew E, Fairbrother B, Hoyt R, Vogel J, Marchitelli L, Frykman P, Martinez-Lopez L, Bernton E, Kramer M, Tulley R, Rood J, Delany J, Jezior D, Arsenault J. 1994. Nutritional and Immunological Assessment of Ranger Students with Increased Caloric Intake. Technical Report No. T95-5. Fort Detrick, MD: US Army Medical Research and Materiel Command.
Tharion WJ, Moore RJ. 1993. Effects of Carbohydrate Intake and Load Bearing Exercise on Rifle Marksmanship Performance. Technical Report No. T5-93. Natick, MA: US Army Medical Research and Development Command.
Tharion WJ, Lieberman HR, Montain SJ, Young AJ, Baker-Fulco CJ, Delany JP, Hoyt RW. 2005. Energy requirements of military personnel. Appetite 44(1):47–65.
Tharion WJ, Shukitt-Hale B, Coffey B, Desai M, Strowman SR, Tulley R, Lieberman HR. 1997. The Use of Caffeine to Enhance Cognitive Performance, Reaction Time, Vigilance, Rifle Marksmanship, and Mood States in Sleep-Deprived Navy SEAL (BUD/S) Trainees. T98-4. Natick, MA: US Army Research Institute of Environmental Medicine.
Tharion WJ, Shukitt-Hale B, Lieberman HR. 2003. Caffeine effects on marksmanship during high-stress military training with 72 hour sleep deprivation. Aviat Space Environ Med 74(4):309–314.
VanHelder T, Radomski MW. 1989. Sleep deprivation and the effect on exercise performance. Sports Med 7(4):235–247.
Wiik P, Opstad PK, Boyum A. 1996. Granulocyte chemiluminescence response to serum opsonized zymosan particles ex vivo during long-term strenuous exercise, energy and sleep deprivation in humans. Eur J Appl Physiol Occup Physiol 73(3–4):251–258.
Carbohydrate and Fat Intake: What is the Optimal Balance?
Jørn Wulff Helge, Copenhagen Muscle Research Center
INTRODUCTION
This paper addresses the optimal distribution of fat and carbohydrate to be included in the macronutrient composition of military assault rations as well as the specific types of fat to be used in these rations, and describes possible related performance and health issues. The purpose of this paper is to provide guidance regarding questions that may be used to make recommendations for an optimal ration composition, the task of the Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations. To provide practical guidance for the complex and broad issues when improving fat and carbohydrate composition of assault rations, this paper focuses on these specific questions:
What is the optimal fat–carbohydrate balance in the ration?
-
Is it possible to perform strenuous physical tasks/training on a high-fat diet?
-
Is there a performance enhancement under short-term exposure to a fat-rich diet?
-
What do we know about the effects of energy deficit and heavy physical demand on the carbohydrate/fat balance–substrate stores?
Are there specific types of fat that are optimal for the ration?
-
Do specific fatty acids affect performance?
-
Do specific fatty acids affect health issues?
These questions are addressed using the assumptions specified for the military assault situation: The ration is targeted for well-trained male soldiers, with an average weight of 80 kg, undergoing average daily energy expenditures of
4,000 to 4,500 kcal, and deployed to repeatable three- to seven-day missions with recovery periods of one to three days. During these missions, they are carrying a heavy load while engaged in prolonged, low- to moderate-intensity activity (up to 20 h/day) interspersed with brief, high-intensity activity.
OPTIMAL FAT–CARBOHYDRATE BALANCE IN THE RATION
Strenuous Physical Tasks and Training on a High-Fat Diet
It is well known that work performed at higher exercise intensities requires a large contribution of carbohydrates (Brooks and Mercier, 1994). Furthermore, there is evidence that short-term, high-fat diet adaptation can be used to spare muscle glycogen and decrease carbohydrate oxidation during exercise and, hypothetically, increase high-intensity exercise capacity (Burke and Hawley, 2002; Helge, 2000). Unfortunately, such carbohydrate sparing after short-term, high-fat diet adaptation is often accompanied by a reduced muscle (Phinney et al., 1983) and liver (Hultman and Nilsson, 1971) glycogen storage in comparison with the results from consumption of a short-term, high-carbohydrate diet. It is, therefore, pertinent to know whether high-intensity exercise capacity can be upheld when dietary fat contribution is high.
Stepto and colleagues (2002) studied seven elite trained endurance athletes who underwent two four-day dietary periods consuming either a high-carbohydrate diet (70 to 75 percent carbohydrate) or a high-fat diet (> 65 percent fat) in a crossover design with an 18-day washout period in between. During the dietary adaptation period, subjects performed two controlled exercise sessions on days one and four, during which they exercised for 20 minutes at 65 percent of VO2max and subsequently performed eight 5-minute exercise bouts at 86 percent of VO2max interspersed with 60-second breaks. In addition to the training performed in the laboratory, the subjects also undertook training outside, but no difference in duration of training was apparent between dietary adaptation periods. The subjects were capable of performing the training, except during one high-intensity exercise bout, while consuming the fat-rich diet. Despite these results, the subjective rating of perceived exertion was higher on day four in the last few bouts after a fat-rich diet when compared with the results of a carbohydrate-rich diet (Table B-3). These results indicate that, for at least four days, elite athletes are capable of performing training at a reasonably high intensity while consuming a fat-rich diet but with a higher subjective rate of perceived exertion.
Consistent with these data, we noted that in our studies involving moderately untrained male subjects during long-term training and high-fat diet adaptation, the subjects on a high-fat diet were able to train and exercise at moderate and high intensities but perceived increased mental effort as compared with subjects on a high-carbohydrate diet (Helge, 2002). There is currently no explanation for this higher mental effort, but previous studies of subjects on a short-
TABLE B-3 Comparing the Effects of a High-Carbohydrate Diet versus a High-Fat Diet on Elite Trained Athletes
|
Day 4 |
High-Carbohydrate Diet |
High-Fat Diet |
|
VO2 (L/minute) |
||
|
Bout 1 |
4.3 ± 0.4 |
4.3 ± 0.4 |
|
Bout 4 |
4.3 ± 0.4 |
4.4 ± 0.3 |
|
Bout 8 |
4.3 ± 0.3 |
4.5 ± 0.2 |
|
RER |
||
|
Bout 1 |
0.94 ± 0.03 |
0.86 ± 0.03 |
|
Bout 4 |
0.91 ± 0.03a |
0.85 ± 0.03 |
|
Bout 8 |
0.90 ± 0.04a |
0.85 ± 0.02 |
|
RPE |
13.8 ± 1.8 |
16.00 ± 1.3a |
|
NOTE: Values are mean ± standard error of the means. RER = respiratory exchange ratio; RPE = rate of perceived exertion; VO2 = oxygen uptake during exercise at 80 percent of peak power output (86 percent VO2max). a(p < 0.05) high carbohydrate versus high fat. SOURCE: Stepto et al. (2002). |
||
term adaptation to a fat-rich diet (Galbo et al., 1979; Jansson et al., 1982) found a higher catecholamine response and heart rate during submaximal exercise as compared with subjects on a carbohydrate-rich diet. The possible influence of a high-fat diet adaptation on the sympathetic nervous system response during exercise might cause mental strain; however, the mechanistic coupling between the increase in sympathetic response induced by a high-fat diet and the increased perception of exertion during submaximal exercise remains to be explained.
Effects of Short-Term Consumption of a Fat-Rich Diet on Performance
The coupling between muscle glycogen storage and endurance exercise capacity, which was demonstrated by Bergstrom and colleagues (1967), spurred researchers to investigate means to manipulate glycogen storage and use or both. One approach was a fat-rich diet adaptation, which induces markedly higher fat oxidation during exercise and reduced carbohydrate use (Christensen and Hansen, 1939; Phinney et al., 1983). This higher fat oxidation, however, occurs at the expense of a muscle glycogen concentration that is, at best, maintained and, in many cases, lower than that in those consuming a carbohydrate-rich diet (Helge et al., 1998b; Phinney et al., 1983). Despite these conflicting adaptations, a number of studies have manipulated the dietary fat content to achieve an improved performance capacity. In this context, short-term exposure to fat-rich diets is defined as adaptation periods lasting less than eight days and having a fat content contributing more than 40 percent of total energy in the diet. The studies are
listed in Table B-4, and overall, only one study shows an increased capacity to perform exercise after short exposure to a fat-rich diet. This study, by Muoio and colleagues (1994), has been extensively criticized in the literature due to a nonrandomized use of diet adaptation. The remaining ten studies found either an unchanged (three studies) or decreased (seven studies) exercise capacity after a short-term, fat-rich diet, indicating a lack of performance enhancement after such consumption and, in the worst case, a decreased performance. However, because of the number of variables influencing these findings (such as the content of fat:carbohydrate in the diet, the subjects’ training background, the exercise intensity, and the type of exercise test applied to test performance enhancement [Helge et al., 1998b]), caution should be used when drawing final conclusions. A more detailed discussion on the effects of short-term, high-fat adaptation can be found elsewhere (Burke and Hawley, 2002; Helge, 2000).
Effects of Energy Deficit and Intense Physical Activity on the Carbohydrate–Fat Balance and Substrate Stores
An essential goal for the assault ration is to enable soldiers to perform prolonged, low-intensity activity interspersed with high-intensity activity, with a daily energy deficit estimated to be approximately 2,000 kcal.
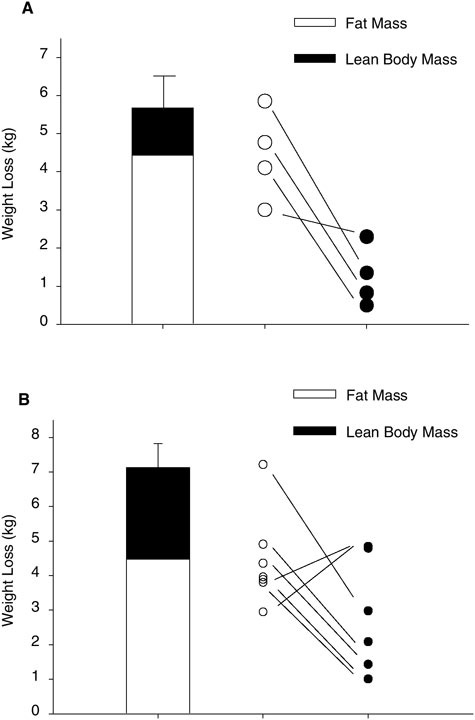
Only few studies are available in which the conditions mimicked those experienced by the soldiers. At the Copenhagen Muscle Research Centre, we have studied two groups of moderately to well-trained men who, fully self-supported and on cross-country skis pulling heavy sledges, traversed the Greenland ice cap (Helge et al., 2003; unpublished results). The dietary macronutrient compositions and basic subject characteristics are given in Table B-5. The two groups crossed the ice cap in 42 and 32 days and experienced a weight loss of approximately 6 and 7 kg, respectively, of which the majority was fat and the remainder, lean body mass (Figure B-4). Based on standard calculations, these losses are equivalent to a daily energy deficit of approximately 1,000 to 1,500 kcal. Albeit the differences in study design (e.g., the two interventions are for longer terms than those for the assault rations), the results may provide useful information for developing assault rations. The skiers’ maximal oxygen uptake remained unchanged in upper-body exercise (arm cranking), but it decreased in lower-body exercise (normal bicycle) (Helge et al., 2003; unpublished results). Accordingly, the muscle biopsy data, enzyme activities, and capillarization data indicated that the arm muscle response tended to be positive, whereas the leg muscle response was neutral or negative. Overall, this would suggest that despite the energy deficit and the type and large amount of physical work, the macronutrient compositions did provide sufficient substrate to almost fully fuel and retain the capacity for physical activity. Based on the available evidence, the fat content could probably be increased up to 50 percent without causing adverse effects. Unfortunately, due to the study design and the timing of muscle biopsies,
TABLE B-4 Short-Term Adaptation for High-Fat Diets and High-Carbohydrate Diets in Humans: Effects on Endurance Performance
|
|
Duration (days) |
Dietary Content |
Exercise Intensity |
||
|
Fat (% of energy) |
Carbohydrate (% of energy) |
% of VO2max |
Performance Reference measure† (min) |
||
|
Christensen and Hansen, 1939 |
3 |
94 |
4 |
176 Watta |
88 ± 4 |
|
3 |
3 |
83 |
176 Watt |
240 (n = 2) |
|
|
Bergstrom et al., 1967 |
3 |
46 |
5 |
75 |
57 ± 2* |
|
3 |
0 |
82 |
75 |
167 ± 18* |
|
|
Martin et al., 1978 |
3 |
— |
< 10 |
72 |
33 ± 3 |
|
3 |
— |
> 75 |
72 |
78 ± 5* |
|
|
Galbo et al., 1979 |
4 |
76 |
10, 5 |
70 |
64 ± 6 |
|
4 |
76 |
10, 5 |
70 |
59 ± 6 |
|
|
4 |
9, 5 |
77 |
68 |
106 ± 5* |
|
|
O’Keeffee et al., 1989 |
7 |
59 |
13 |
80 |
60 ± 5 |
|
7 |
— |
72 |
80 |
113 ± 12* |
|
|
Williams et al., 1992 |
7 |
48 |
37 |
71 |
135 ± 5‡ |
|
7 |
35 |
56 |
71 |
127 ± 5‡ |
|
|
Muoio et al., 1994 |
7 |
38 |
50 |
75–80 |
91 ± 10* |
|
7 |
24 |
61 |
75–80 |
69 ± 7 |
|
|
7 |
15 |
73 |
75–80 |
76 ± 8 |
|
|
Starling et al., 1997 |
1 |
68 |
16 |
75b |
139 ± 7‡ |
|
1 |
5 |
83 |
75 |
||
|
Pitsiladis and Maughan, 1999 |
3 |
65 |
9 |
70 (10°C)c |
89.2 [78–130]d |
|
3 |
9 |
82 |
70 (10°C) |
158 [117–166]* |
|
|
3 |
65 |
9 |
70 (10°C) |
44 [32–51] |
|
|
3 |
6 |
82 |
70 (10°C) |
53 [50–82] * |
|
|
Burke et al., 2000 |
5 + 1e |
68 |
19 |
TTf |
31 ± 1‡ |
|
5 + 1 |
13 |
74 |
TT |
34 ± 3‡ |
|
|
Carey et al., 2001 |
6 + 1e |
69 |
16 |
TTg |
44 ± 1 km |
|
6 + 1 |
15 |
70 |
TT |
42 ± 1 km |
|
|
NOTE: Data are mean values; however, for exercise performance mean ± standard error of the means; n = number in sample. *p < 0.05 different from other diets (same exercise intensity). †Performance measure is time (min) to exhaustion unless otherwise noted. ‡Performance mesure is time (min) to complete task. aExercise performance at 1,080 kJ (VO2 during exercise was 2.6 L). bPerformance was measured as a 1,600 kJ, self-paced cycling bout. cRoom temperature. dMean and range. eHigh-fat diet followed by one day of high-carbohydrate diet. fTime trial (TT) (7 kJ/kg body mass). gTime trial (TT) (60 min). |
|||||
TABLE B-5 Subject Characteristics and Dietary Composition
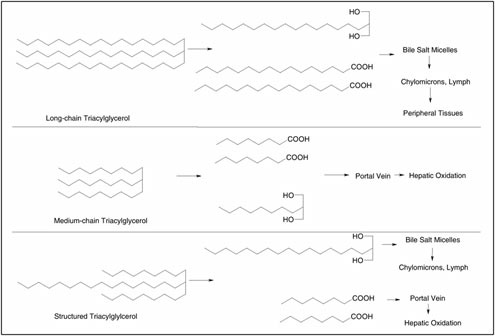
muscle substrates during the traverse are unavailable. Of particular interest in this context, however, is whether the muscle triacylglycerol stores, a main substrate source during prolonged, low- to moderate-intensity exercise in well-trained soldiers, are replenished by the ration. Several research groups recently have suggested that repletion of muscle triacylglycerol stores after physical activity could be important to optimize muscle recovery and exercise capacity (Berggren et al., 2004; Johnson et al., 2004; Spriet and Gibala, 2004). Decombaz and colleagues (2001) suggested that daily consumption of fat at a level of 2 g/kg of body mass would be sufficient to fully restore muscle triacylglycerol levels. This would imply that an average soldier at 80 kg would need to consume fat at a level of 160 g/day, which is achieved if the ration contains approximately 55 percent of total energy as fat. The macronutrient composition in the ration must be optimized such that the replenishment of both muscle glycogen and triacylglycerol is sufficiently increased within the given energy limitation of the ration.
SPECIFIC FATS FOR THE ASSAULT RATION
Fatty Acids and Optimal Performance
In the literature, animal studies have demonstrated preferential mobilization (Raclot and Groscolas, 1993) and oxidation (Jones et al., 1992; Leyton et al., 1987; Shimomura et al., 1990) of unsaturated versus saturated fatty acids. As mentioned, interventions that spare carbohydrate use may benefit performance capacity. The available evidence showing that a difference in dietary fatty acid composition will affect endurance is, however, sparse. In rats, endurance performance, measured in vitro in rat extensor digitorum longus muscle after intermit-
tent, low-frequency stimulation, was lower after nine weeks’ adaptation to a diet rich in n-6 fatty acids as compared with adaption to a diet rich in n-3 fatty acids; both diets contained 10 percent fat (w/w) (Ayre and Hulbert, 1996). Another study in rats found no effect of dietary fatty acid composition on endurance performance in trained or sedentary animals after high-fat diet adaptation, but substrate oxidation was affected (higher oxidation) by the dietary fatty acid composition (Helge et al., 1998a, see Figure B-5). Studies in salmon (McKenzie et al., 1998; Wagner et al., 2004) have also addressed the effects of dietary fatty acid composition on performance capacity, but the data show no consistent trends. In humans, the evidence is limited: A study (n = 3) found that endurance performance measured at 70 to 75 percent VO2max decreased after consumption
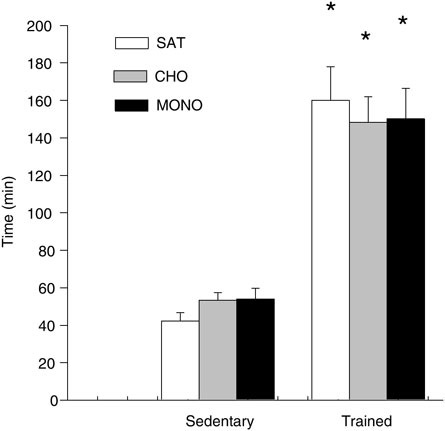
FIGURE B-5 Endurance time to exhaustion in sedentary and trained male rats after four weeks’ adaptation to one of two fat-rich diets containing either unsaturated or saturated fatty acids or to a carbohydrate-rich diet.
NOTE: Values are means ± standard error (n = 7–11). CHO = carbohydrate; MONO = monounsaturated fat; n = number in sample; SAT = saturated fat. * = significantly different from the sedentary groups (p < 0.05).
SOURCE: Helge et al. (1998a).
of a polyunsaturated fat-rich diet as compared with a saturated-fat diet (Lukaski et al., 2001). The author’s unpublished observations found that the consumption of a saturated-fat diet, versus a polyunsaturated-fat diet, had no effect on endurance performance. Thus, insufficient data are available to support a role for dietary fatty acid composition on exercise performance.
Fatty Acids and Health Considerations
Are there adverse or positive effects of including different fatty acids in the ration? This question includes a number of complex issues that are incompletely resolved; space limitations prevent their being addressed here, but the following list of potential roles for dietary fatty acids should be considerd for further research:
-
The influence of dietary fatty acid composition on membrane function (Pan et al., 1994).
-
The influence of dietary fatty acid composition (particularly the content of n-3 and n-6 fatty acids) on immune function (Venkatraman et al., 2000).
-
The potential hypolipedemic effects of n-3 fatty acids (Harris, 1997).
-
The effects of saturated fatty acids on decreasing insulin sensitivity (Pan et al., 1994).
-
The interactions with and effects of specific fatty acids on gene expression in muscle (Spriet and Gibala, 2004; Venkatraman et al., 2000).
The amount of physical activity performed by these soldiers as well as their good training status suggests that shorter term modifications of dietary fatty acid composition and dietary fat content would have insignificant effects on their general health and function.
SUMMARY
The following arguments confer positive and negative aspects of adding increased amounts of fat to the ration. The positive effects of adding more fat include an increased energy content of the ration and a faster repletion of muscle triacylglycerol stores. The negative effects of adding more fat include increased rates of perceived exertion and mental strain during physical activity and a potential lowering of muscle glycogen stores (possibly liver glycogen). There may be an additional increased risk of inducing higher ketone levels but, in my opinion, such a risk does not pose a problem in this situation.
Insufficient evidence exists to verify the effects of specific dietary fatty acids on performance capacity. Some data suggest health and metabolic benefits from the consumption of specific fatty acids. However, in fit and healthy younger men exposed to intense physical activity, these effects, if indeed present, would have more influence on long-term health status rather than on short-term exer-
cise capacity and health. Therefore, the fatty acid composition in the ration should adhere to the requirements established by the Institute of Medicine (IOM, 2002).
Based on the issues addressed above the recommendations for the ration are the following:
-
The fat content in the ration can be between 30 to 50 percent (of total energy) without reducing physical capacity; however, increasing the fat content may lead to slightly higher mental strain.
-
When protein requirements are met and sufficient carbohydrate for high-intensity tasks is given, fat can be used to increase the energy content of the ration.
-
While no specific fatty acid types improve performance, adequate essential fatty acids must be included in the ration at the current recommended levels (IOM, 2002).
ACKNOWLEDGMENTS
The author is affiliated with the Copenhagen Muscle Research Centre, which is supported by grants from the University of Copenhagen, from the faculties of science and of health sciences at this university, and from the Copenhagen Hospital Corporation.
REFERENCES
Ayre KJ, Hulbert AJ. 1996. Effects of changes in dietary fatty acids on isolated skeletal muscle functions in rats. J Appl Physiol 80(2):464–471.
Berggren JR, Hulver MW, Dohm GL, Houmard JA. 2004. Weight loss and exercise: Implications for muscle lipid metabolism and insulin action. Med Sci Sports Exerc 36(7):1191–1195.
Bergstrom J, Hermansen L, Hultman E, Saltin B. 1967. Diet, muscle glycogen and physical performance. Acta Physiol Scand 71(2):140–150.
Brooks GA, Mercier J. 1994. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J Appl Physiol 76(6):2253–2261.
Burke LM, Hawley JA. 2002. Effects of short-term fat adaptation on metabolism and performance of prolonged exercise. Med Sci Sports Exerc 34(9):1492–1498.
Burke LM, Angus DJ, Cox GR, Cummings NK, Febbraio MA, Gawthorn K, Hawley JA, Minehan M, Martin DT, Hargreaves M. 2000. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J Appl Physiol 89(6):2413–2421.
Carey AL, Staudacher HM, Cummings NK, Stepto NK, Nikolopoulos V, Burke LM, Hawley JA. 2001. Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. J Appl Physiol 91(1):115–122.
Christensen EH, Hansen O. 1939. Arbeitsfähigkeit und ernärung. Skand Archiv Physiol 81:160–171.
Decombaz J, Schmitt B, Ith M, Decarli B, Diem P, Kreis R, Hoppeler H, Boesch C. 2001. Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am J Physiol Regul Integr Comp Physiol 281(3):R760–R769.
Galbo H, Holst JJ, Christensen NJ. 1979. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand 107(1):19–32.
Harris WS. 1997. n-3 Fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr 65(5 Suppl):1645S–1654S.
Helge JW. 2000. Adaptation to a fat-rich diet. Effects on endurance performance in humans. Sports Med 30(5):347–357.
Helge JW. 2002. Long-term fat diet adaptation effects on performance, training capacity, and fat utilization. Med Sci Sports Exerc 34(9):1499–1504.
Helge JW, Ayre K, Chaunchaiyakul S, Hulbert AJ, Kiens B, Storlien LH. 1998a. Endurance in high-fat-fed rats: Effects of carbohydrate content and fatty acid profile. J Appl Physiol 85(4):1342–1348.
Helge JW, Lundby C, Christensen DL, Langfort J, Messonnier L, Zacho M, Andersen JL, Saltin B. 2003. Skiing across Greenland icecap: Divergent effects on limb muscle adaptations and substrate oxidation. J Exp Biol 206(Pt 6):1075–1083.
Helge JW, Wulff B, Kiens B. 1998b. Impact of a fat-rich diet on endurance in man: Role of the dietary period. Med Sci Sports Exerc 30(3):456–461.
Hultman E, Nilsson L. 1971. Liver glycogen in man. Effect of different diets and muscular exercise. In: Pernow B, Saltin B, eds. Muscle Metabolism During Exercise. Proceedings of a Karolinska Institutet Symposium held in Stockholm, Sweden, September 6–9, 1970. New York: Plenum. Pp. 143–151.
IOM (Institute of Medicine). 2002. Dietary Reference Intakes. Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press.
Jansson E, Hjemdahl P, Kaijser L. 1982. Diet induced changes in sympatho-adrenal activity during submaximal exercise in relation to substrate utilization in man. Acta Physiol Scand 114(2):171–178.
Johnson NA, Stannard SR, Thompson MW. 2004. Muscle triglyceride and glycogen in endurance exercise: Implications for performance. Sports Med 34(3):151–164.
Jones PJ, Ridgen JE, Phang PT, Birmingham CL. 1992. Influence of dietary fat polyunsaturated to saturated ratio on energy substrate utilization in obesity. Metabolism 41(4):396–401.
Leyton J, Drury PJ, Crawford MA. 1987. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr 57(3):383–393.
Lukaski HC, Bolonchuk WW, Klevay LM, Milne DB, Sandstead HH. 2001. Interactions among dietary fat, mineral status, and performance of endurance athletes: A case study. Int J Sport Nutr Exerc Metab 11(2):186–198.
Martin B, Robinson S, Robertshaw D. 1978. Influence of diet on leg uptake of glucose during heavy exercise. Am J Clin Nutr 31(1):62–67.
McKenzie DJ, Higgs DA, Dosanjh BS, Deacon G, Randall DJ. 1998. Dietary fatty acid composition influences swimming performance in Atlantic salmon (Salmo salar) in seawater. Fish Physiol Biochem 19(2):111–122.
Muoio DM, Leddy JJ, Horvath PJ, Awad AB, Pendergast DR. 1994. Effect of dietary fat on metabolic adjustments to maximal VO2 and endurance in runners. Med Sci Sports Exerc 26(1):81–88.
O’Keeffe KA, Keith RE, Wilson GD, Blessing DL. 1989. Dietary carbohydrate intake and endurance exercise performance of trained female cyclists. Nutr Res 9:819–830.
Pan DA, Hulbert AJ, Storlien LH. 1994. Dietary fats, membrane phospholipids and obesity. J Nutr 124(9):1555–1565.
Phinney SD, Bistrian BR, Evans WJ, Gervino E, Blackburn GL. 1983. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32(8):769–776.
Pitsiladis YP, Maughan RJ. 1999. The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans. J Physiol 517(Pt 3):919–930.
Raclot T, Groscolas R. 1993. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J Lipid Res 34(9):1515–1526.
Shimomura Y, Tamura T, Suzuki M. 1990. Less body fat accumulation in rats fed a safflower oil diet than in rats fed a beef tallow diet. J Nutr 120(11):1291–1296.
Spriet LL, Gibala MJ. 2004. Nutritional strategies to influence adaptations to training. J Sports Sci 22(1):127–141.
Starling RD, Trappe TA, Parcell AC, Kerr CG, Fink WJ, Costill DL. 1997. Effects of diet on muscle triglyceride and endurance performance. J Appl Physiol 82(4):1185–1189.
Stepto NK, Carey AL, Staudacher HM, Cummings NK, Burke LM, Hawley JA. 2002. Effect of short-term fat adaptation on high-intensity training. Med Sci Sports Exerc 34(3):449–455.
Venkatraman JT, Leddy J, Pendergast D. 2000. Dietary fats and immune status in athletes: Clinical implications. Med Sci Sports Exerc 32(7 Suppl):S389–S395.
Wagner GN, Balfry SK, Higgs DA, Lall SP, Farrell AP. 2004. Dietary fatty acid composition affects the repeat swimming performance of Atlantic salmon in seawater. Comp Biochem Physiol A Mol Integr Physiol 137(3):567–576.
Williams C, Brewer J, Walker M. 1992. The effect of a high carbohydrate diet on running performance during a 30-km treadmill time trial. Eur J Appl Physiol Occup Physiol 65(1):18–24.
Carbohydrate–Protein Balance for Physical Performance
Kevin D. Tipton, University of Birmingham, UK
INTRODUCTION
Proper nutrition for military personnel has long been an important consideration for military planners. Nutrition may be especially important for military combat personnel performing duties that entail short-term, strenuous physical tasks in high-stress situations. Stress during military operations results from a combination of increased energy expenditure, decreased energy intake, and a lack of sleep for extended periods. These multiple stressors on mental and physical capabilities may decrease muscle performance enough to compromise both lives and military success. Sufficiently effective nutrients in combat rations would enhance the soldiers’capabilities and reduce both the loss of combat effectiveness and the number of casualties. Protein and carbohydrate consumption may contribute to optimal muscle performance during these situations.
The unique circumstances in the field make it difficult for soldiers to consume sufficient protein and carbohydrate as well as overall energy during their missions. For example, weight and size that soldiers can carry is limited and evidence shows that high-stress combat can suppress appetite (Popper et al., 1989). These factors lead to large energy deficits and macronutrient deficiencies for the three- to seven-day periods of these operations. These energy and macronutrient deficits could lead to metabolic disturbances that impair muscular performance during demanding and intense military operations.
The combination of stressors—decreased energy and protein intake, sleep deprivation, extreme physical activity, and high stress levels—that soldiers face
during these missions represents a complex and unique situation. Unfortunately, little, if any, direct information is available about muscular metabolic response to these stressors and about the appropriate nutrient intake that would be optimal for these capabilities.
This review uses data collected during periods of physiological stress to develop strategies to enhance protein and carbohydrate intake during sustained military operations. Because of a paucity of such data, however, this review provides a speculative guideline for recommendations. The following are specific questions that this review addresses:
-
What would be the optimal macronutrient balance between protein and carbohydrate for an assault ration to enhance muscle performance during combat missions? Does the intensity of activity (i.e., high-tempo, stressful, repetitive combat missions) alter the optimal balance?
-
What protein intake is recommended to best sustain homeostasis while people are eating reduced calories and exercising?
-
What are the types and levels of macronutrients (e.g., complex versus simple carbohydrates, proteins with specific amino acid profiles, other sources of nitrogen, etc.) that would optimize such an assault ration to enhance muscle performance during combat missions?
ENERGY INTAKE AND PHYSICAL ACTIVITY
Energy intake plays a major role in body protein metabolism. Operational requirements, as noted above, limit the intake of available energy in rations during short-term, high-stress missions. Such limitations in energy intake, combined with high levels of physical activity, ensure that participants are in an energy deficit for the three- to seven-day duration of a mission. Measurements made during simulated sustained operations do not clearly demonstrate muscle loss (Montain and Young, 2003; Nindl et al., 2002); however, body composition measures may not have the sensitivity to detect small changes over a short time. Nevertheless, the evidence provided below suggests that energy deficits that occur during these missions will lead to muscle loss.
Many classic studies from the laboratory of Calloway and Butterfield have demonstrated the importance of energy balance to maintain body nitrogen balance. Todd and colleagues (1984) clearly demonstrated that nitrogen balance is better maintained when energy balance is positive or, at least, zero (Figure B-6). Nitrogen balance could not be maintained when energy intake was 15 percent less than energy output. Presumably, the body’s predominant and accessible source, muscle protein, would also be the major site of this nitrogen loss. Energy restriction over longer periods (e.g., 10 weeks) results in the loss of lean mass during very low calorie dieting (Layman et al., 2003); however, the metabolic mechanisms for this loss remain unexplained.
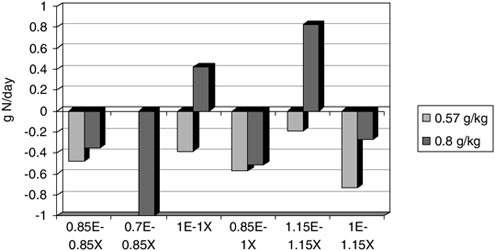
FIGURE B-6 Nitrogen (N) balance during differing levels of energy intake (E) and physical activity (X) at two different levels of protein intake. Each bar is at either energy balance or 15 percent deficit due to increased exercise. These data illustrate that nitrogen balance is negative during energy deficits and improved by low-intensity exercise. Nitrogen balance is not positive in any combination of energy intake and exercise on the lowest (0.57 g·kg–1·d–1) level.
SOURCE: Adapted from Todd et al. (1984).
Muscle protein synthesis is reduced by 65 percent in food-deprived rats (Anthony et al., 2000), and perturbations in muscle metabolism from energy deficits could occur very rapidly. Recently, Tipton et al. (2003) demonstrated that the release of amino acids from leg muscle in healthy volunteers occurred in the first 24 h of energy restriction (80 percent of their weight maintenance levels). In that study, the volunteers were resting and consumed their habitual level of dietary protein. It seems that reducing energy intake by only 20 percent immediately stimulates a catabolic situation in muscle, suggesting that soldiers participating in missions where their energy intake is half of their energy output would be losing nitrogen, most likely from muscle. Unfortunately, it is not clear how the energy restriction and negative energy balance inherent for these sustained operations reduces muscle performance (Friedl, 1995).
A portion of the uncertainty about the effects of energy balance on muscle performance in short-term military missions with concomitant stressors, such as high-physical activity, sleep loss, and dietary restrictions, can be attributed to the complexity of the situation and the lack of information from studies. Protein use can be improved by physical activity (Butterfield and Calloway, 1984; Todd et al., 1984) (Figure B-6). Increased energy intake to support increased physical
activity improves the nitrogen balance, and the physical activity improves nitrogen retention (Butterfield and Calloway, 1984; Todd et al., 1984). Following an acute bout of resistance exercise, muscle protein synthesis and net muscle protein balance are increased, even in trained individuals (Biolo et al., 1995; Phillips et al., 1997, 1999). Muscle protein synthesis was increased following four hours of walking (Carraro et al., 1990), but no balance data are available for that type of activity.
Whereas these data could be interpreted to suggest that the physical activity common during military maneuvers may improve protein balance and have a good effect on muscle protein, there are reasons to believe otherwise. There is typically a period of adaptation to physical activity in which nitrogen balance is diminished (Butterfield, 1987; Butterfield and Calloway, 1984; Gontzea et al., 1975). This transient increase in protein loss peaks at four to ten days following initiation of the exercise program; easily encompassing the time typically used for these missions. However, these data are from untrained individuals initiating physical activity, so the applicability of these data to the situations being discussed for soldiers is unclear. It is easy to accept that the habitual level of training before participating in difficult missions could be protective, but there are no data to support this contention. Furthermore, the data supporting a benefit to nitrogen balance from activity were obtained during an energy balance (Todd et al., 1984). Positive nitrogen balance could not be maintained when energy balance was negative, despite increased physical activity (Figure B-6). Thus, it seems that the conditions intrinsic to the sustained operations missions will result in negative nitrogen balance and loss of muscle protein.
The intensity of the activity necessary for successful completion of the missions may increase the loss of muscle protein and, thus, exacerbate the effects of the negative energy balance. Given that the logistical limitations to the rations for these missions preclude carrying additional rations to accommodate the increased activity, any increase in activity will result in increased energy deficits. Furthermore, there is evidence that high-intensity exercise is debilitating for muscle protein metabolism. Whereas muscle protein synthesis has been demonstrated to increase following both resistance (Biolo et al., 1995; Phillips et al., 1997, 1999) and endurance (Carraro et al., 1990) exercise in humans, it is depressed following prolonged, high-intensity exercise in rats (Anthony et al., 1999; Gautsch et al., 1998). It is possible that species-specific differences in the response of muscle to exercise may explain the differential. On the other hand, the response of muscle protein synthesis to resistance exercise in rats (Farrell et al., 1999; Hernandez et al., 2000) is similar to that for humans (Biolo et al., 1995; Phillips et al., 1997, 1999). It is more likely that the intensity of the exercise during the treadmill running was severe and resulted in decreased muscle protein synthesis. The rats ran at approximately 80 percent of VO2max for approximately two hours, roughly equivalent to the effort that only an elite runner could maintain for a marathon. Furthermore, if relative life span is taken into
account, two hours on a treadmill for a rat could be considered equivalent to the effort of several days for humans. Thus, it is conceivable that this intense physical exercise would also result in reduced muscle protein synthesis in humans. The evidence presented suggests that, rather than increasing protein use, the physical activity involved in prolonged military missions may be detrimental to muscle protein metabolism. The energy deficit, combined with high-tempo, stressful physical activity, suggests that muscle protein metabolism will be altered during missions of this type.
Protein Intake
As with energy intake, protein intake during these missions can be limited by operational factors. There is a great deal of controversy in the sports science community about the protein requirements for athletes and active individuals; however, little question remains that increased protein intake would be advantageous in an energy deficit made worse by high levels of physical activity. Todd and colleagues (1984) demonstrated that, at low levels of protein intake (0.57 g/kg per day), nitrogen balance was negative during both energy balance and energy deficit. Increasing protein intake from 0.57 to 0.85 g/kg per day improved nitrogen balance when energy intake was 15 percent less than energy output (Figure B-6). Either way, nitrogen balance was negative during both intakes. At this time, there is no way to determine if the loss of body protein could be ameliorated by higher protein intakes and, if so, what would constitute the optimal level of protein intake. A question also remains of a ceiling effect whereby no further improvement is possible with increased protein intake. Unknown as well is whether positive balance is feasible during the conditions inherent to military operations. In addition, it is impossible to determine whether eliminating negative nitrogen balance is necessary to improve the performance and health of the military. A recent study demonstrated that a greater proportion of weight lost during energy restriction comes from fat, rather than lean, mass when protein intake is increased (Layman et al., 2003). Data from this study suggest that increasing protein intake spares body protein during periods of negative energy balance. However, the applicability to soldiers during sustained operations is limited because this study was conducted on obese female subjects in weight-loss situations and did not involve exercise.
Loss of nitrogen likely is exacerbated by the combination of higher protein intakes before missions and lower protein intake during missions. Millward and colleagues demonstrated that body protein is lost when dietary protein intake is decreased (Pacy et al., 1994; Price et al., 1994; Quevedo et al., 1994). Data from these studies suggest an adaptation period that takes several days (often the time expected for most of these missions) (Figure B-7). These data also suggest that the sudden decline in protein intake expected when soldiers undertake these missions would contribute to a loss of body proteins, most likely from muscle.
However, in these studies, the subjects were in energy balance and did not exercise, so it is unclear how applicable these data are to sustained operations.
The available information suggests that decreasing protein intake during these missions will be detrimental to muscle protein metabolism. The recommendation to maximize protein intake during the missions seems prudent; however, there are sufficient questions to prevent a specific recommendation from being made with any confidence.
Carbohydrate and Lipid Intake
There is no question that carbohydrate intake is critical for optimal muscle performance during strenuous activity. Maintenance of blood glucose and glycogen for muscle fuel are important for muscular performance (Burke et al., 2004; Coyle, 2004; Hargreaves et al., 2004). Several studies have demonstrated that increased carbohydrate intake improved performance in military tasks (Montain and Young, 2003; Montain et al., 1997). Severe muscle glycogen depletion has been shown during four- to five-day field operations (Jacobs et al., 1983). Although provision of extra carbohydrates did not increase the glycogen levels in these soldiers (Jacobs et al., 1983) sufficient carbohydrate intake seems important for performance maintenance during sustained military operations (Montain and Young, 2003).
Carbohydrate intake may also enhance muscle protein metabolism. Acute provision of carbohydrates increases net muscle protein balance following exercise,
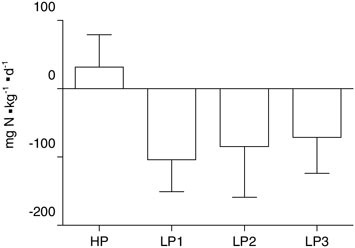
FIGURE B-7 Change in nitrogen (N) balance due to change from the first three days of high-protein (HP) to low-protein (LP) intake.
SOURCE: Quevedo et al. (1994).
presumably through the action of insulin (Borsheim et al., 2004b). Nevertheless, any increase in net muscle protein balance from ingestion of carbohydrate is much less than that from ingestion of an amino acid source (Borsheim et al., 2004a; Miller et al., 2003). On a whole body level, Gaudichon and colleagues (1999) demonstrated that carbohydrate ingestion increased postprandial use of dietary protein to a greater extent than did lipids. It is likely that increased use of available nutrients by the body is especially important in situations of limited nutrient availability. Replacing carbohydrate with fat has been demonstrated to increase nitrogen balance during hypocaloric situations (Richardson et al., 1979). On the other hand, Boirie and colleagues have presented a series of studies (Boirie et al., 1997; Dangin et al., 2003) suggesting that, after protein ingestion, amino acids released slowly into the blood provide a superior anabolic response. Given that lipids do slow the digestion of nutrients, it could be argued that lipids may improve the anabolic response in the muscle. We recently tested this notion in our laboratory, and despite equivalent protein ingestion during two small, separate meals, net muscle protein balance was greater from proteins when fat was included in the meal (Elliot et al., unpublished data). Thus, including both carbohydrates and lipids in the ration should maximize the accretion of ingested protein into muscle when nutrients are limited. The exact composition of such nutrients remains unknown.
Intake of Amino Acids
Amino acids provided by protein ingestion stimulate the accretion of muscle protein. Evidence from acute metabolic studies suggests that increased muscle protein synthesis and net muscle protein synthesis result only from the provision of essential amino acids; that is, nonessential amino acids are unnecessary to stimulate muscle protein accretion (Borsheim et al., 2002; Tipton and Wolfe, 2001; Tipton et al., 1999, 2003). Furthermore, the response of muscle protein balance to essential amino acids seems dose dependent (Borsheim et al., 2002). Aside from increasing amino acid availability for protein synthesis, essential amino acids may act as signals for stimulating protein synthesis. Essential amino acids, particularly leucine, activate translational signaling (Anthony et al., 2000; Kimball and Jefferson, 2001; Kimball et al., 2002). In rats, leucine stimulates muscle protein synthesis that was diminished by severe exercise (Anthony et al., 1999), but feeding carbohydrates only did not (Gautsch et al., 1998). Essential amino acids may be particularly effective during sustained operations when physical activity is extreme.
More evidence for the potential efficacy of essential amino acids during sustained military operations comes from acute metabolic studies performed in our laboratory. Following resistance exercise, ingesting 12 g of essential amino acids resulted in an amino acid uptake that was more than double that from ingesting 20 g of whole proteins (Borsheim et al., 2002; Tipton et al., 2004)
(Figure B-8). Consuming only 60 percent of the volume more than doubled the anabolic response, which means a superior response was obtained for less mass/volume. Furthermore, essential amino acids, combined with exercise, ameliorated the amino acid release from muscle in response to a 20 percent energy deficit (Tipton et al., 2003). These factors suggest that essential amino acids could be important for ration design.
SUMMARY
The metabolic demands endured by soldiers on sustained military operations are severe and unique. Very little research exists that specifically examines the results from missions that involve underfeeding and high-activity levels as well as high stress on muscle metabolism and performance. The indirectly available information suggests that optimal ration development should include maximizing the energy and protein content within the operational limits. Carbohydrate and fat are clearly important, but it is unclear what quantity each should represent in the overall composition. Recent evidence from acute metabolic studies suggests that essential amino acids may be an important component of a ration for sustained missions. The anabolic response to essential amino acids seems to be superior to a larger amount of intact protein, thus offering higher efficiency. Further research is necessary to determine the effects of these various nutrients on muscle metabolism and performance in specific situations experienced during sustained military operations.
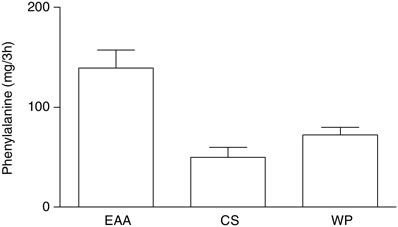
FIGURE B-8 Phenylalanine uptake during three hours (h) following ingestion of 12 g of free essential amino acids (EAA), 20 g of casein (CS), or 20 g of whey proteins (WP).
SOURCE: Borsheim et al. (2002); Tipton et al. (2004).
REFERENCES
Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. 2000. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130(2):139–145.
Anthony JC, Anthony TG, Layman DK. 1999. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr 129(6):1102–1106.
Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. 1995. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 268(3 Pt 1):E514–E520.
Boirie Y, Danglin M, Gachon P, Vasson M, Maubois JL, Beaufrere B. 1997. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94(26):14930–14935.
Borsheim E, Tipton KD, Wolf SE, Wolfe RR. 2002. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metabol 283(4):E648–E657.
Borsheim E, Aarsland A, Wolfe RR. 2004a. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab 14(3):255–271.
Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. 2004b. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol 96(2):674–678.
Burke LM, Kiens B, Ivy JL. 2004. Carbohydrates and fat for training and recovery. J Sports Sci 22(1):15–30.
Butterfield GE. 1987. Whole-body protein utilization in humans. Med Sci Sports Exerc 19(5 Suppl):S157–S165.
Butterfield GE, Calloway DH. 1984. Physical activity improves protein utilization in young men. Br J Nutr 51(2):171–184.
Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. 1990. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol 259(4 Pt 1):E470–E476.
Coyle EF. 2004. Fluid and fuel intake during exercise. J Sports Sci 22(1):39–55.
Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballevre O, Beaufrere B. 2003. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol 549(Pt 2):635–644.
Farrell PA, Fedele MJ, Vary TC, Kimball SR, Lang CH, Jefferson LS. 1999. Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am J Physiol 276(4 Pt 1):E721–E727.
Friedl KE. 1995. When does energy deficit affect soldier physical performance? In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 253–283.
Gaudichon C, Mahe S, Benamouzig R, Luengo C, Fouillet H, Sare S, van Oycke M, Ferriere F, Rautureau J, Tome D. 1999. Net postprandial utilization of [15N]-labeled mild protein nitrogen is influenced by diet composition in humans. J Nutr 129(4):890–895.
Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS. 1998. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol 274(2 Pt 1):C406–C414.
Gontzea I, Sutzescu P, Dumitrache S. 1975. The influence of adaptation to physical effort on nitrogen balance in man. Nutr Rept Intern 11:231–236.
Hargreaves M, Hawley JA, Jeukendrup A. 2004. Pre-exercise carbohydrate and fat ingestion: Effects on metabolism and performance. J Sports Sci 22(1):31–38.
Hernandez JM, Fedele MJ, Farrell PA. 2000. Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats. J Appl Physiol 88(3):1142–1149.
Jacobs I, Anderberg A, Schele R, Lithell H. 1983. Muscle glycogen in soldiers on different diets during military field manoeuvres. Aviat Space Environ Med 54(10):898–900.
Kimball SR, Jefferson LS. 2001. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care 4(1):39–43.
Kimball SR, Farrell PA, Jefferson LS. 2002. Invited review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 93(3):1168–1180.
Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. 2003. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr 133(2):411–417.
Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. 2003. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc 35(3):449–455.
Montain SJ, Young AJ. 2003. Diet and physical performance. Appetite 40(3):255–267.
Montain SJ, Shippee RL, Tharion WJ. 1997. Carbohydrate-electrolyte solution effects on physical performance of military tasks. Aviat Space Environ Med 68(5):384–391.
Nindl BC, Leone CD, Tharion WJ, Johnson RF, Castellani JW, Patton JF, Montain SJ. 2002. Physical performance responses during 72 h of military operational stress. Med Sci Sports Exerc 34(11):1814–1822.
Pacy PJ, Price GM, Halliday D, Quevedo MR, Millward DJ. 1994. Nitrogen homeostasis in man: The diurnal responses of protein synthesis and degradation and amino acid oxidation to diets with increasing protein intakes. Clin Sci 86(1):103–118.
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. 1997. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273(1 Pt 1):E99–E107.
Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. 1999. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol 276(1 Pt 1):E118–E124.
Popper R, Smits G, Meiselman HL, Hirsch E. 1989. Eating in combat: A survey of US Marines. Mil Med 154(12):619–623.
Price GM, Halliday D, Pacy PJ, Quevedo MR, Millward DJ. 1994. Nitrogen homeostasis in man: Influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clin Sci 86(1):91–102.
Quevedo MR, Price GM, Halliday D, Pacy PJ, Millward DJ. 1994. Nitrogen homoeostasis in man: Diurnal changes in nitrogen excretion, leucine oxidation and whole body leucine kinetics during a reduction from a high to a moderate protein intake. Clin Sci 86(2):185–193.
Richardson DP, Wayler AH, Scrimshaw NS, Young VR. 1979. Quantitative effect of an isoenergetic exchange of fat for carbohydrate on dietary protein utilization in healthy young men. Am J Clin Nutr 32(11):2217–2226.
Tipton KD, Wolfe RR. 2001. Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab 11(1):109–132.
Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. 2003. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metabol 284(1):E76–E89.
Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. 2004. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 36(12):2073–2081.
Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. 1999. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 276(4 Pt 1):E628–E634.
Todd KS, Butterfield GE, Calloway DH. 1984. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr 114(11):2107–2118.
Carbohydrate Ingestion During Intense Activity
Edward F. Coyle, University of Texas at Austin
INTRODUCTION
The Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations was charged with making recommendations on the composition of a food ration (assault ration) that will best sustain physical and cognitive performance for short-term use by highly-trained soldiers during high-tempo, stressful, repetitive combat missions; in addition, the food ration will also prevent possible adverse health consequences under the conditions of a hypocaloric diet. Stress may be due to high physical and cognitive workloads such as exercise, extreme environmental temperature, dehydration, heat exhaustion, threat to personal safety, sleep deprivation, and other operational demands. Important health concerns for soldiers during combat missions are the optimization of gastrointestinal processes and prevention of diarrhea, dehydration, hyperthermia, kidney stones; optimization of the function of immune system; and prevention of infections. The expected daily expenditure of these soldiers is approximately 4,000 to 4,500 kcal/day. The assault ration will provide approximately 2,400 kcal/day for three to seven days with one to three days of recovery.
This paper focuses on several aspects of the design of such a ration. Although a lot of the literature presented is from the sports and exercise community, their conclusions can be used to support recommendations for the assault ration described above. This paper attempts to answer the following questions:
-
What would be the optimal amount of carbohydrates for an assault ration to enhance performance during combat missions?
-
What are the types and levels of macronutrients (e.g., complex verses simple carbohydrates) that would optimize an assault ration to enhance performance during combat missions?
-
How much is performance going to decline when there is a reduction in both carbohydrates and calories?
CARBOHYDRATE INTAKE DURING EXERCISE
Despite long-ago evidence that carbohydrate ingestion during exercise improved athletic performance, the discovery in the 1960s and 1970s of muscle glycogen’s importance as a source of carbohydrate energy for athletes (Bergstrom et al., 1967) seemed to have overshadowed, until the mid-1980s, the potential energy contribution from carbohydrate ingested during the period of exercise (Coggan and Coyle, 1991; Coyle et al., 1986; Hargreaves, 1996). Costill and
Miller (1980) emphasized the need for fluid intake during exercise but recommend against ingesting very much carbohydrate. This recommendation is understandable for that period of time, given that the physiological benefits of fluid replacement were beginning to be established, as reflected in the 1975 American College of Sports Medicine (ACSM) position-statement, whereas the physiological benefits of carbohydrate ingestion for blood glucose supplementation as well as the physiological mechanisms explaining this benefit were not yet established (Hargreaves, 1996). In the 1980s, the observation that adding carbohydrate to water temporarily slowed the gastric emptying rate was interpreted to suggest that fluid replacement solutions should not contain much carbohydrate (Coyle et al., 1978). It was also thought, albeit mistakenly, that “ingested glucose contributes very little to the total energy utilized during exercise” (Costill and Miller, 1980). Therefore, the prevailing recommendation in 1980 was that “under conditions that threaten the endurance athlete with dehydration and hyperthermia, fluid replacement solutions should contain little sugar (> 25 g/L or > 2.5 percent) and electrolytes” (Costill and Miller, 1980).
Later in the 1980s, it was established that ingested carbohydrate and blood glucose can indeed be oxidized at rates of approximately 1 g/minute and that this exogenous carbohydrate becomes the predominant source of energy late in a bout of prolonged, continuous exercise (Convertino et al., 1996). Carbohydrate ingestion can delay muscle fatigue during prolonged cycling and running, and it also improves the power output that can be maintained (Hargreaves, 1996; Millard-Stafford, 1992; Millard-Stafford et al., 1997). It is now understood that the slight slowing of gastric emptying caused by solutions containing up to 8 percent of carbohydrate is a relatively minor factor in fluid replacement rate compared with the large influence of increased fluid volume for increasing gastric emptying and fluid replacement rate (Coyle and Montain, 1992a, b; Maughan, 1991; Maughan and Noakes, 1991; Maughan et al., 1993). Therefore, it is generally recommended that endurance athletes ingest carbohydrate at a rate of 30 to 60 g/h (Casa et al., 2000; Convertino et al., 1996). The type of carbohydrate can be glucose, sucrose, maltodextrins, or some high-glycemic starches. Fructose intake should be limited to amounts that do not cause gastrointestinal discomfort (Casa et al., 2000; Convertino et al., 1996). This rate of carbohydrate ingestion can be met by drinking solutions containing 4 to 8 percent of carbohydrates (4 to 8 g/100 mL) (Casa et al., 2000; Convertino et al., 1996; Rehrer, 1994; Rehrer et al., 1993).
Performance During Short-Term, Intermittent High-Intensity Exercise
Benefits of carbohydrate ingestion during performance of high-intensity, intermittent exercise attempted after at least 60 minutes of continuous moderate-intensity exercise (i.e., 65 to 80 percent VO2max) was the focus of study beginning in the late 1980s. Power output measured over 5 to 15 minutes of high-
intensity and predominantly aerobic exercise has been generally observed to increase by ingesting carbohydrate (Coggan and Coyle, 1988; Mitchell et al., 1989; Murray et al., 1987; Sugiura and Kobayashi, 1998) In the 1996 ACSM position statement on Exercise and Fluid Replacement, it was concluded that “During intense exercise lasting longer than 1 h, it is recommended that carbohydrates be ingested at a rate of 30–60 g/h to maintain oxidation of carbohydrates and delay fatigue” (Convertino et al., 1996).
During the past decade, attention has focused on determining if carbohydrate intake during sporting events such as soccer and tennis improves various indices of performance. As discussed below, carbohydrate ingestion appears to frequently benefit performance as demonstrated in tests of “shuttle-running” ability, which simulates the stop-and-start nature of many sports requiring bursts of speed and some fatigue resistance (Nicholas et al., 1995). Physiological mechanisms for this ergogenic effect of carbohydrate ingestion are not clear and have been theorized to involve more than simply skeletal muscle metabolism, implying a neuromuscular component. The challenges now are identifying the types of physical activity and sporting situations during which carbohydrate ingestion is advisable and those during which such a recommendation is not effective or even counterproductive.
Conditions During Which Carbohydrate Ingestion During Exercise Does Not Appear to Improve Performance
Performance or fatigue resistance can be governed by numerous physiological factors involving, primarily, the skeletal muscle, the cardiovascular system, and the nervous system. Some primary causes of fatigue are not influenced by carbohydrate ingestion during exercise; for example, the negative effects of hyperthermia on performing prolonged exercise in a hot environment [e.g., 33°C to 35°C (91.4°F to 96°F)] do not appear to be lessened by carbohydrate ingestion (Febbraio et al., 1996; Fritzsche et al., 2000). However, during exercise in a cool environment [i.e., 5°C (41°F)] (Febbraio et al., 1996) that is not limited by hyperthermia, or when subjects drink fluids during exercise in a hot environment and also do not become hyperthermic (Fritzsche et al., 2000), carbohydrate ingestion improves performance. Under conditions not eliciting hyperthermia, the most important factor for performing prolonged, intense exercise appears to be maintaining carbohydrate availability and thus carbohydrate oxidation, especially from blood glucose oxidation as muscle glycogen concentration declines. This goal was better achieved by ingesting 7 percent carbohydrate solutions as compared with a 14 percent solution (Febbraio et al., 1996).
Another example of a condition when carbohydrate ingestion during exercise would not be expected to improve performance is when the cause of fatigue is the accumulation of hydrogen ion in skeletal muscle (e.g., low muscle pH), as occurs during a single bout of intense exercise performed continuously for 2 to
30 minutes. Performing exercise that is not sufficiently stressful to cause fatigue, as evidenced by reduced power production, or that does not require high levels of effort to maintain power, as reflected, for example, by high levels of various stress hormones, would not benefit from carbohydrate ingestion. Furthermore, carbohydrate intake is not generally recommended during events, performed either continuously or intermittently, that are completed in 30 to 45 minutes or less. Although this last concept has not been extensively studied to date, it is a valid assumption based on the practices of athletes competing in events lasting only 30 to 45 minutes. As discussed, carbohydrate ingestion does not appear to lessen fatigue from hyperthermia or dehydration-induced hyperthermia, even when the durations of exercise are prolonged (e.g., one to three hours) (Febbraio et al., 1996; Fritzsche et al., 2000). Thus, there does not appear to be any benefit of adding carbohydrate to fluid replacement solution under these conditions. Accordingly, people who exercise at moderate intensity for less than one hour and do not experience fatigue do not appear to benefit from carbohydrate ingestion during exercise. Yet ingesting carbohydrate at a rate of 30 to 60 g/h does not appear to present a general physiological risk to people who do not experience gastrointestinal discomfort.
Conditions During Which Carbohydrate Ingestion Improves Performance through Unexplained Physiological Mechanisms
Carbohydrate ingestion during prolonged exercise can benefit performance if inadequate carbohydrate energy from blood glucose is the cause of fatigue (Coggan and Coyle, 1991; Febbraio et al., 1996). This effect on performance is a well-documented physiological mechanism by which the ergogenic benefit of carbohydrate ingestion during exercise can be explained. However, carbohydrate ingestion has been observed to improve performance under conditions in which fatigue is not clearly caused by a lack of aerobic or anaerobic carbohydrate energy. For example, when the duration of continuous exercise is extended to approximately 60 minutes and thus the intensity is 80 to 90 percent VO2max, carbohydrate ingestion during exercise has been shown to improve power output by 6 percent during the 50- to 60-minute period (Below et al., 1995).
Other recent studies have also reported a performance benefit of carbohydrate feeding when the total duration of the performance bout is approximately 60 minutes or more by breaking the time into shorter exercise durations, thereby simulating the demands of many sports (basketball, soccer, hockey) in which high-intensity exercise is interspersed with periods of recovery (Mitchell et al., 1989; Murray et al., 1987). Carbohydrate ingestion is ergogenic during 15-minute bouts of intermittent “shuttle running,” performed numerous times (e.g., five times) as well as during repeated high-intensity intervals of one minute’s duration and a three-minute recovery (Davis et al., 1997, 1999, 2000; Nicholas et al., 1995; Welsh et al., 2002). The total duration of these work–rest bouts was more
than 60 minutes. The physiological mechanisms responsible for these performance benefits from carbohydrate ingestion are not clear and have been theorized to involve the central nervous system, skeletal muscle, and the cardiovascular system. It is likely that carbohydrate feeding influences the interactions of all three systems, possibly through the actions of neurotransmitters, hormones, and peptides that are already known (e.g., insulin, catecholamines, and serotonin), that are newly recognized (e.g., interluken-6), or that have yet to be discovered. Regardless, sufficient evidence is accumulating to recommend carbohydrate ingestion during exercise bouts of continuous or intermittent exercise that lasts for 60 minutes or longer and cause fatigue from factors other than hyperthermia.
Can Carbohydrate Ingestion During Exercise Be Counterproductive?
It is recommended that carbohydrate be ingested at a rate of 30 to 60 g/h during exercise (Convertino et al., 1996), recognizing that ingesting more does not increase oxidation rate but can produce gastrointestinal discomfort in many people (Rehrer et al., 1992; Wagenmakers et al., 1993). In the latter case, carbohydrate feeding can be counterproductive when ingested in amounts (> 60 to 90 g/h) or concentrations (> 7 to 8 percent) that are too large (Febbraio et al., 1996; Galloway and Maughan, 2000).
If it produced gastrointestinal discomfort, carbohydrate ingestion at 30 to 60 g/h during exercise can impair performance as compared with no carbohydrate ingestion, a factor that is likely to vary from sport to sport and athlete to athlete. Carbohydrate ingestion should be used with caution during events lasting approximately 15 to 45 minutes and requiring repeated bouts of intense exercise lasting several minutes followed by several minutes of rest because these events may cause large swings in blood glucose and insulin concentration. Feeding plans must be specific to the varied intensity and time demands of the event. Those feeding schedules might be more than those described below, yet without data or experience to make more specific recommendations; all that can be done at present, besides recognizing these limitations, is to encourage systematic trial and error.
From a practical perspective, the recommendation of ingesting 30 to 60 g/h of carbohydrate during exercise should emphasize that this be accomplished by taking feedings every 10 to 30 minutes, as allowed by the event. The goal of the feeding schedule should be to create a steady flow of carbohydrate into the blood stream, which will then provide a steady flow of exogenous glucose into the blood. In other words, if carbohydrate feeding is begun during an event, it should be continued throughout the event in a manner that allows for a steady flow of exogenous glucose into the blood with minimal gastrointestinal discomfort. Avoid giving a large bolus of carbohydrate (i.e., more than 30 to 60 g) early in an event and then discontinuing carbohydrate feeding. This practice will prime the body for glucose metabolism, reduce fat oxidation, and then deprive the body of the fuel it has been primed to metabolize.
Summary and Recommendations for Carbohydrate Intake During Exercise
During exercise that lasts longer than one hour and that causes fatigue, physically active people are advised to ingest 30 to 60 g/h of carbohydrate that can be rapidly converted to blood glucose because doing so generally improves performance. There is not a clear physiological need to consume any fluid or fuel when beginning exercise while reasonably hydrated and proceeding to exercise at low or moderate intensity for less than one hour without experiencing undo fatigue. However, there is no apparent reason for people to avoid fluid or carbohydrate intake if this is their preference and is well tolerated.
ACKNOWLEDGMENTS
The author is a member of the Sports Medicine Review Board of the Gatorade Sports Science Institute. Portions of this manuscript are similar to a recent review by the author published in 2004. Coyle EF. 2004. Fluid and fuel intake during exercise. Journal of Sports Sciences 22:39–55.
REFERENCES
Below PR, Mora-Rodriguez R, Gonzalez-Alonso J, Coyle EF. 1995. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc 27(2):200–210.
Bergstrom J, Hermansen L, Hultman E, Saltin B. 1967. Diet, muscle glycogen and physical performance. Acta Physiol Scand 71(2):140–150.
Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BSE, Roberts WO, Stone JA. 2000. National Athletic Trainers’ Association Position Statement: Fluid replacement for athletes. J Athlet Train 35(2):212–224.
Coggan AR, Coyle EF. 1988. Effect of carbohydrate feedings during high-intensity exercise. J Appl Physiol 65(4):1703–1709.
Coggan AR, Coyle EF. 1991. Carbohydrate ingestion during prolonged exercise: Effects on metabolism and performance. Exerc Sport Sci Rev 19:1–40.
Convertino VA, Armstrong LE, Coyle EF, Mack GW, Sawka MN, Senay LC, Sherman WM. 1996. American College of Sports Medicine Position Stand on exercise and fluid replacement. Med Sci Sports Exerc 28(1):i–vii.
Costill D, Miller J. 1980. Nutrition for edurance sports. Carbohydrate and fluid balance. Int J Sports Med 1:2–14.
Coyle EF, Montain SJ. 1992a. Benefits of fluid replacement with carbohydrate during exercise. Med Sci Sports Exerc 24(9 Suppl):S324–S330.
Coyle EF, Montain SJ. 1992b. Carbohydrate and fluid ingestion during exercise: Are there trade-offs? Med Sci Sports Exerc 24(6):671–678.
Coyle EF, Coggan AR, Hemmert MK, Ivy JL. 1986. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol 61(1):165–172.
Coyle EF, Costill DL, Fink WJ, Hoopes DG. 1978. Gastric emptying rates for selected athletic drinks. Res Q 49(2):119–124.
Davis JM, Jackson DA, Broadwell MS, Queary JL, Lambert CL. 1997. Carbohydrate drinks delay fatigue during intermittent, high-intensity cycling in active men and women. Int J Sport Nutr 7(4):261–273.
Davis JM, Welsh RS, Alerson NA. 2000. Effects of carbohydrate and chromium ingestion during intermittent high-intensity exercise to fatigue. Int J Sport Nutr Exerc Metab 10(4):476–485.
Davis JM, Welsh RS, De Volve KL, Alderson NA. 1999. Effects of branched-chain amino acids and carbohydrate on fatigue during intermittent, high-intensity running. Int J Sports Med 20(5):309–314.
Febbraio MA, Murton P, Selig SE, Clark SA, Lambert DL, Angus DJ, Carey MF. 1996. Effect of CHO ingestion on exercise metabolism and performance in different ambient temperatures. Med Sci Sports Exerc 28(11):1380–1387.
Fritzsche RG, Switzer TW, Hodgkinson BJ, Lee SH, Martin JC, Coyle EF. 2000. Water and carbohydrate ingestion during prolonged exercise increase maximal neuromuscular power. J Appl Physiol 88(2):730–737.
Galloway SD, Maughan RJ. 2000. The effects of substrate and fluid provision on thermoregulatory and metabolic responses to prolonged exercise in a hot environment. J Sports Sci 18(5):339–351.
Hargreaves M. 1996. Carbohydrates and exercise performance. Nutr Rev 54(4 Pt 2):S136–S139.
Maughan RJ. 1991. Fluid and electrolyte loss and replacement in exercise. J Sports Sci 9 (Spec No):117–142.
Maughan RJ, Noakes TD. 1991. Fluid replacement and exercise stress. A brief review of studies on fluid replacement and some guidelines for the athlete. Sports Med 12(1):16–31.
Maughan RJ, Goodburn R, Griffin J, Irani M, Kirwan JP, Leiper JB, MacLaren DP, McLatchie G, Tsintsas K, Williams C, Wellington P, Wilson WM, Wootton S. 1993. Fluid replacement in sport and exercise—A consensus statement. Br J Sport Med 27(1):34.
Millard-Stafford M. 1992. Fluid replacement during exercise in the heat. Review and recommendations. Sports Med 13(4):223–233.
Millard-Stafford M, Rosskopf LB, Snow TK, Hinson BT. 1997. Water versus carbohydrate-electrolyte ingestion before and during a 15-km run in the heat. Int J Sport Nutr 7(1):26–38.
Mitchell JB, Costill DL, Houmard JA, Fink WJ, Pascoe DD, Pearson DR. 1989. Influence of carbohydrate dosage on exercise performance and glycogen metabolism. J Appl Physiol 67(5):1843–1849.
Murray R, Eddy DE, Murray TW, Seifert JG, Paul GL, Halaby GA. 1987. The effect of fluid and carbohydrate feedings during intermittent cycling exercise. Med Sci Sports Exerc 19(6):597–604.
Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. 1995. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci 13(4):283–290.
Rehrer NJ. 1994. The maintenance of fluid balance during exercise. Int J Sports Med 15(3):122–125.
Rehrer NJ, Beckers EJ, Brouns F, Saris WH, Ten Hoor F. 1993. Effects of electrolytes in carbohydrate beverages on gastric emptying and secretion. Med Sci Sports Exerc 25(1):42–51.
Rehrer NJ, Wagenmakers AJ, Beckers EJ, Halliday D, Leiper JB, Brouns F, Maughan RJ, Westerterp K, Saris WH. 1992. Gastric emptying, absorption, and carbohydrate oxidation during prolonged exercise. J Appl Physiol 72(2):468–475.
Sugiura K, Kobayashi K. 1998. Effect of carbohydrate ingestion on sprint performance following continuous and intermittent exercise. Med Sci Sports Exerc 30(11):1624–1630.
Wagenmakers AJ, Brouns F, Saris WH, Halliday D. 1993. Oxidation rates of orally ingested carbohydrates during prolonged exercise in men. J Appl Physiol 75(6):2774–2280.
Welsh RS, Davis JM, Burke JR, Williams HG. 2002. Carbohydrates and physical/mental performance during intermittent exercise to fatigue. Med Sci Sports Exerc 34(4):723–731.
Macronutrient Composition of Military Rations for Cognitive Performance in Short-Term, High-Stress Situations
Randall J. Kaplan, Canadian Sugar Institute
INTRODUCTION
The importance of nutrition for cognitive performance in military settings has long been recognized. The Committee on Military Nutrition Research (CMNR) of the Food and Nutrition Board, Institute of Medicine (IOM) of the National Academies has published several volumes on this topic, including reports on the cognitive effects of performance-enhancing food components (IOM, 1994); inadequate energy intakes (IOM, 1995); protein and amino acids (IOM, 1999); and caffeine (IOM, 2001).
Cognitive performance refers to intellectual behaviors such as memory, reasoning, attention, vigilance, and choice reaction time. Mood (e.g., happy, sad, calm, tense) and psychomotor performance (e.g., sensation, perception, agility) are distinct from cognitive performance, but they can have a considerable influence on it (Mays, 1995; Spring et al., 1994) and are therefore relevant to this discussion. The aspects of cognition that are important in combat settings include the ability to perceive, attend to, and respond appropriately to cues; make prompt decisions; and sustain vigilance (IOM, 1994; Mays, 1995).
The Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations, an ad hoc committee of the CMNR, has been given the task of recommending the nutrient composition of a ration for combat missions to optimize physical and cognitive performance and to prevent adverse health consequences.
This daily ration is intended for repeated short-term use (three to seven days followed by one to three days of ad libitum recovery) by fit male soldiers during high-tempo, stressful combat missions. Stress may be caused by physical and cognitive workloads, extreme temperature, threats to safety, and sleep deprivation, all of which can interfere with cognitive performance (Lieberman et al., 2002b; Owen et al., 2004).
The purpose of this report is to briefly review relevant evidence on the effects of energy and macronutrients (i.e., carbohydrate, protein, and fat) on cognitive performance and mood, and to provide recommendations for the macronutrient composition of the assault ration, including types of each macronutrient, to enhance cognitive performance. Because factors associated with combat operations are likely to lead to cognitive deficits relative to normal functioning, the realistic goal in optimizing the nutrient composition of the ration should be to decrease these deficits, rather than to enhance performance beyond normal bounds.
To address this task this paper discusses the following specific questions:
-
Should the energy content of the ration (energy density) be maximized so as to minimize the energy debt, or is there a more optimal “mix” of macronutrients and micronutrients, not necessarily producing maximal energy density?
-
What would be the optimal macronutrient balance between carbohydrate, protein, and fat for such an assault ration to enhance cognitive performance during combat missions?
-
What are the types and levels of macronutrients (e.g., complex verses simple carbohydrates, proteins with specific amino acid profiles, type of fat, etc.) that would optimize such an assault ration to enhance cognitive performance during combat missions?
For the purpose of this paper, it has been assumed that other nutrional requirements of the soldiers (e.g., water, micronutrients) will be met. If these other requirements are not met, the results could override the importance of macronutrient requirements. For instance, it is well established that hypohydration (Wilson and Morley, 2003) and iron deficiency (Sandstead, 2000) present significant detriments to cognitive performance. In the combat situation, particularly during extreme temperatures, the highest priority for reducing cognitive impairment is adequate hydration (IOM, 1995).
The maximum weight of the macronutrient component of a 1,360 gram-ration is approximately 500 g [calculated from a standard, approximately 2,400-kcal ration, comprising 50 percent of energy as carbohydrate (300 g), 30 percent as fat (80 g), and 20 percent as protein (120 g)], with the remaining 860 g comprised of noncaloric components, including moisture, fiber, and micronutrients. Theoretically, 500 g could provide between 2,000 kcal (i.e., entirely protein and carbohydrate) and 4,500 kcal (i.e., entirely fat), in which case the energy needs of the soldiers (4,000 to 4,500 kcal/day) could be met within the weight limitation of the ration if energy density were increased as much as possible. The question is: Should the energy content of the ration be maximized, or is there a more optimal ratio of macronutrients for cognitive performance?
ENERGY INGESTION AND COGNITIVE PERFORMANCE
Acute Effects
The acute effects (hours) of energy ingestion on cognitive performance have been examined in a number of studies in healthy, nonstressed subjects. Several reviews of the literature have concluded that the provision of energy in the morning (breakfast) generally improves cognitive performance over the next 30 minutes to 2 h, compared with no energy provision, with more robust effects on tests of memory and less consistent effects on tests of attention or vigilance (Bellisle et al., 1998; Dye and Blundell, 2002; Kanarek, 1997; Leigh Gibson and
Green, 2002; Pollitt and Matthews, 1998). The mechanism for these results has not been elucidated, but likely both gut-mediated and centrally acting postabsorptive signals are involved (Kaplan et al., 2001).
By contrast to the breakfast studies, large meals provided at mid-day (lunch) consistently impair cognitive performance (e.g., memory, vigilance, and reaction time) and mood (e.g., alertness and fatigue) over the next one to two hours, compared with the effects of those consuming no lunch or a light lunch. This phenomenon is known as the “post-lunch dip” and is likely related to normal changes in daily circadian rhythm as well as changes to habitual intake patterns (Bellisle et al., 1998; Dye et al., 2000; Kanarek, 1997; Leigh Gibson and Green, 2002).
A few studies have examined the short-term effects of energy intake on cognitive performance and mood under stressful conditions, showing the benefits of increased energy intake. One study found no changes in mood after low-energy intake during breakfast and lunch (264 kcal) as compared with a higher energy intake (1,723 kcal; consistent with energy needs of the subjects) during a nonstressful condition (Macht, 1996). However, when participants were subjected to emotionally stressful white noise, those with a low-energy intake experienced a degradation in mood (i.e., more irritability and less relaxation), whereas those with a higher energy intake did not.
Another study strongly supports a beneficial effect of increased energy ingestion on cognitive performance and mood under stressful conditions (Lieberman et al., 2002a). In this study, 143 male subjects from an elite combat unit were tested during periods of intense physical activity over 10 h, during which energy needs were not met with regular meals (approximately 765 kcal) (Lieberman et al., 2002a). Energy supplementation (carbohydrate-containing drink) throughout the day improved vigilance and mood (i.e., increased vigor, decreased confusion) in a dose-dependent manner compared with the effects of a noncaloric placebo drink. The strongest effects on performance resulted from the highest energy drink (approximately 1,309 kcal), followed by the medium-energy drink (approximately 654 kcal), and the placebo (0 kcal) (Figure B-9). It is impossible to determine from this study whether the beneficial effects were caused by an increase in energy ingestion or an increase in carbohydrate ingestion.
Medium-Term Effects
The medium-term effects (days) of dieting to lose weight consistently impair cognitive performance and mood (Leigh Gibson and Green, 2002). The effects are attributed to the act of dieting, rather than to inherent differences between dieters and nondieters because the impedence on performance is not observed when the same individuals are not dieting (Green and Rogers, 1995). It has been suggested that this impairment is caused more by a psychological pre-occupation with food and feelings of hunger than by a physiologic effect of low
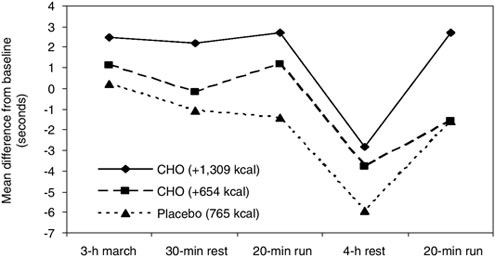
FIGURE B-9 Vigilance performance of soldiers who received carbohydrate (CHO) beverages or placebo in addition to regular meals (providing approximately 765 kcal) while engaging in various activities over 10 h.
NOTE: A higher number on the y-axis represents improved performance.
SOURCE: Adapted from Lieberman et al. (2002a) with permission by the American Journal of Clinical Nutrition © Am J Clin Nutr. American Society for Clinical Nutrition.
energy intake (Leigh Gibson and Green, 2002). This conclusion is based on evidence that (1) individuals perform worse on cognitive tests when they are dieting, even when no actual weight is lost; (2) the magnitude and structure of the deficits is comparable to those caused by anxiety and depression; and (3) performance is not clearly affected by weight loss in the absence of other stress. It has been hypothesized that task-irrelevant feelings of hunger and thoughts of food may impair performance by interfering with normal working memory function. Working memory can be conceptualized as “the fundamental cognitive processing system, in that it serves to allocate limited cognitive processing capacity to other ongoing cognitive operations in order of their relevance or importance to an individual” (Leigh Gibson and Green, 2002).
A study using a similar eating pattern as would be consumed by combat soldiers (several days of hypocaloric intake followed by ad libitum intake) supports the hypothesis that energy restriction impedes performance in the absence of significant weight loss (Laessle et al., 1996). In this study, healthy female subjects consumed a low-calorie diet (approximately 651 kcal/day) for four days, followed by ad libitum intake (approximately 2,876 kcal/day) for three days each week for four weeks. During the low-calorie periods, subjects reported stronger feelings of hunger and thoughts about food as well as demonstrating
worse moods, more irritability, difficulties concentrating, and greater fatigue than during the ad libitum periods even though weight loss was minimal. Repeated episodes of low energy intakes over the 4 weeks did not reduce feelings of hunger. The implication of this study is that the negative effects of hunger are unlikely to be reduced during periods of low energy intake when soldiers repeatedly eat hypocaloric diets followed by ad libitum recovery periods. In other words, it may be very difficult to train soldiers to ignore their hunger feelings. It should be noted, however, that this study was performed with women and not under the unique added stressors of combat missions.
The hypothesis presented suggests that psychological feelings of hunger contribute to cognitive deficits during periods of low energy intake; however, the physiologic consequences of low energy intake and negative energy balance cannot be ruled out as contributing factors because other acute evidence (presented earlier) and longer term evidence under the stressful conditions presented below do not clearly indicate the mechanism involved. Most likely, both psychological and physiological factors associated with low energy intake, particularly under stressful conditions, contribute to the performance deficit.
The evidence that increased appetite interferes with the ability to perform optimally suggests that reducing hunger may be beneficial for mood and cognitive performance. Indeed, declining hunger sensations have been associated with being more energetic, lively, calm, and relaxed (Fischer et al., 2004). The macronutrient composition of foods can play an important role in minimizing hunger. Protein consistently and robustly induces greater satiety than carbohydrate or fat on a per calorie basis (Westerterp-Plantenga, 2003) and has a greater effect on satiety than do substantially higher energy intakes from the other macronutrients (Stubbs and Whybrow, 2004; Stubbs et al., 2000) (Figure B-10). Carbohydrate appears to induce greater satiety than fat does on a per calorie basis (Stubbs et al., 2000), but the differences are minimal when palatability and energy density of foods are matched (Rolls and Bell, 1999). Thus, to reduce the negative effects of hunger on cognitive performance and mood, it may be beneficial to increase the protein content of hypocaloric military rations and to reduce the fat content.
Various types of each macronutrient also have different effects on satiety (Stubbs et al., 2000). A review concluded that high glycemic index carbohydrates (i.e., rapidly raise blood glucose concentration) have a greater effect on satiety than low glycemic index carbohydrates over the short term (up to one hour after carbohydrate ingestion), whereas lower glycemic index carbohydrates have a stronger effect on satiety over the longer term (i.e., up to 6 h after carbohydrate ingestion) (Anderson and Woodend, 2003). However, these effects are not likely caused entirely by changes in blood glucose (Kaplan and Greenwood, 2002). The data implicating different effects of fat type on satiety are mixed, with some suggesting that polyunsaturated fatty acids have a stronger effect on increasing satiety than monounsaturated or saturated fatty acids do (French and Robinson, 2003; Lawton et al., 2000), whereas others have found no differences between
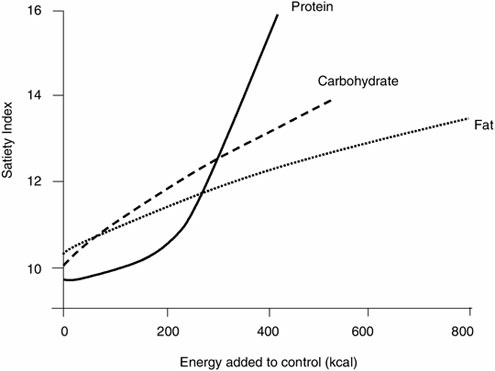
FIGURE B-10 Effect of increasing energy content of macronutrient loads on satiety over 3.25 h.
NOTE: Data were collated from a number of studies.
SOURCE: Adapted from Stubbs and Whybrow (2004).
the two (Alfenas and Mattes, 2003; Flint et al., 2003). Medium-chain triglycerides, which are rapidly metabolized, have been shown to exert a stronger effect on satiety than long-chain triglycerides do; however, only a few human studies are available and the results are inconclusive (French and Robinson, 2003; St-Onge and Jones, 2002). Research on the effects of protein type on satiety is also limited because evidence in human studies suggesting that some proteins might suppress appetite more than others is inconsistent (Anderson and Moore, 2004; Westerterp-Plantenga, 2003).
It is important to note that, although it is argued here that reducing hunger in soldiers consuming hypocaloric rations may be beneficial for cognitive performance, this may not be relevant if appetite is suppressed by hypohydration, physical activity, or stress to the extent that feelings of hunger and thoughts of food are eliminated. It is well documented that soldiers tend to underconsume foods, and this may be partially due to a suppression of appetite (IOM, 1995). Thus, it is important to determine whether the expected increase in appetite
associated with the hypocaloric intake of combat soldiers is stronger or weaker than the suppression of appetite that will be caused by other factors. There is evidence, however, that when military rations provide only 60 percent of energy needs (i.e., energy intake and energy expenditure was 1,946 kcal and 3,200–3,300 kcal, respectively), the entire rations are consumed (Shukitt-Hale et al., 1997), suggesting that soldiers’ appetites are strong under these circumstances. These data would support the argument that strong feelings of hunger will be present in combat soldiers consuming assault rations containing approximately 2,400 kcal/day when their needs are > 4,000 kcal/day.
Long-Term Effects
The long-term effects (weeks or months) of energy intake on cognitive performance are not relevant if soldiers are able to consume enough excess energy during the one- to- three-day ad libitum recovery period to make up for the negative energy balance during the three to seven days, such that they do not lose weight. By contrast, if soldiers are in a state of negative energy balance over weeks and months because of repeated consumption of hypocaloric diets and inadequate recovery periods, then the long-term effects of low energy intake are relevant.
Evidence on the long-term effects of low energy intake on cognitive performance shows that those effects are minimal in the absence of significant stress but are significant if stress is present. In a review of the effects of underconsumption of military rations on cognitive performance, Mays (1995) concluded that underconsumption leading to weight loss up to 6 percent over 10 to 45 days does not affect performance in the absence of significant stress. A more recent study over a 30-day trial supports this conclusion (Shukitt-Hale et al., 1997). However, consistent with the long-term dieting data (e.g., Kretsch et al., 1997), underconsumption combined with stress during military operations (e.g., exercise, sleep deprivation, and danger) significantly degrades performance within a few days (Mays, 1995). Based on the available data, Mays proposed a relationship between long-term negative energy balance and cognitive performance (Figure B-11). These data suggest that consumption levels of 75 to 90 percent of requirements may actually enhance performance in the first 3 to 15 days, whereas consumption of 50 percent or less of requirements degrades performance, particularly under stressful conditions. Over the longer term, performance continues to degrade along with negative energy balance. Thus, if repeated short-term periods of low energy intake during combat operations lead to long-term negative energy balance, deficits in cognitive performance can be expected.

FIGURE B-11 Proposed relationship between energy intake as a percentage of energy expenditure and cognitive performance over two months, based on the results of several studies.
SOURCE: Adapted from Mays (1995).
CARBOHYDRATE INGESTION AND COGNITIVE PERFORMANCE
Glucose Compared with a Noncaloric Placebo
For the purposes of this paper, “carbohydrate” refers to digestible or glycemic carbohydrates that provide energy (primarily sugars and starches). It does not include dietary fiber, resistant starch, or other nondigestible carbohydrates that do not provide energy as a carbohydrate.
A large number of studies conducted over the past 20 years have shown beneficial effects of glucose ingestion on cognitive performance as compared with noncaloric sweetened placebos (e.g., aspartame, saccharin) in animals and humans over the short term—from 15 minutes to 3 h after ingestion (Greenwood, 2003; Korol, 2002; Korol and Gold, 1998; Messier, 2004). Cognitive impairments associated with hypoglycemic episodes have also been a consistent finding
(McCall, 1992). Early studies suggested that the cognitive-enhancing effects of glucose were only evident when an existing deficit was present, such as in elderly subjects and in patients with Alzheimer’s disease, schizophrenia, and Down syndrome, but not in healthy young adults (Korol and Gold, 1998). Since that time, others have shown that consuming glucose can improve performance in young adults if cognitive tasks are sufficiently demanding (Benton, 2001); however, the magnitude of the effects depends on glucose regulation and baseline cognitive abilities (Awad et al., 2002; Greenwood, 2003; Kaplan et al., 2000; Messier, 2004).
The beneficial effects of consuming glucose are dependent on the type of cognitive test, task difficulty, and glucose dose (Greenwood, 2003; Messier, 2004). In contrast with the data showing the benefits of eating breakfast and the detriments of eating lunch, the effects of consuming glucose appear to be positive in both the morning and the afternoon (Sunram-Lea et al., 2001). The effects of glucose are strongest on functions mediated by the medial temporal lobe and surrounding areas, including long-term verbal memory, and less robust for other tasks, including short-term memory, attention, and reaction time. Glucose consumption can, however, improve performance on a wide range of tasks as long as the tasks are of sufficient difficulty. For instance, Kennedy and Scholey (2000) found glucose ingestion improved performance in young adults on a difficult test (serial sevens, which requires subjects to count backwards by sevens), but not on two easier tests (Figure B-12). These findings suggest that an increased brain requirement for glucose is needed before benefits of glucose ingestion can be observed. Conditions for which glucose could reverse deficits in performance caused by increased brain requirements for glucose include those of aging (McNay and Gold, 2001), increased cognitive demand (Fairclough and Houston, 2004), and physical activity (Brun et al., 2001; Grego et al., 2004; Nybo and Secher, 2004). Thus, carbohydrate ingestion is likely to benefit cognitive performance during combat operations for which brain glucose requirements would likely be increased because of the demanding nature of the tasks and high levels of physical activity.
The dose of glucose that enhances cognitive performance follows an inverted U-shaped response in humans and animals. Consistently, low doses have no effect on performance, intermediate doses (25 to 75 g) improve performance, and high doses have no effect or impair performance (Greenwood, 2003; Greenwood et al., 2003; Messier, 2004; Parsons and Gold, 1992) (Figure B-13). Taken together, these findings suggest that a moderate amount of glucose improves performance on a range of demanding cognitive tests in young adults, or on tasks in demanding situations (e.g., stress, physical activity), but glucose is unlikely to improve performance on simpler tasks under nonstressful conditions.
There is no consensus on the mechanism that explains the effects of glucose on cognitive performance, but several hypotheses have been proposed. The following effects of glucose ingestion have been most commonly suggested as
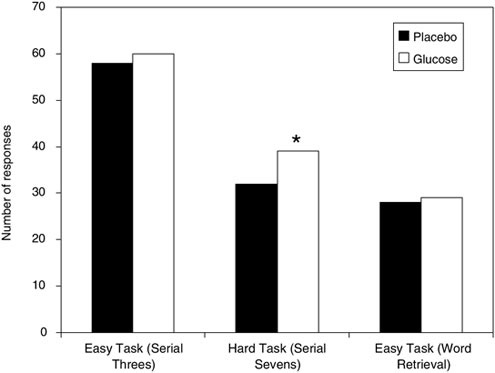
FIGURE B-12 Mean number of responses (subtractions or words produced) made on each of three tasks after ingestion of noncaloric saccharin-sweetened placebo or of 25 g glucose drinks.
*p < 0.01 compared with corresponding placebo group.
SOURCE: Adapted from Kennedy and Scholey (2000).
possible mechanisms: replenishes brain extracellular glucose that declines during difficult tasks; increases synthesis of the brain neurotransmitter acetylcholine; increases central insulin; or influences peripheral signals that are relayed to the brain via the vagus nerve (Greenwood, 2003; Messier, 2004; Park, 2001).
Carbohydrate Foods
As noted earlier, one study found that a carbohydrate-containing beverage provided in addition to regular hypocaloric meals benefited vigilance and mood during combat training (Figure B-9), but it is impossible to determine whether the benefits were due to increased energy intake or to carbohydrate intake (Lieberman et al., 2002a). The authors suggested that the benefits could have been due to an increased supply of glucose to the brain, which could have helped
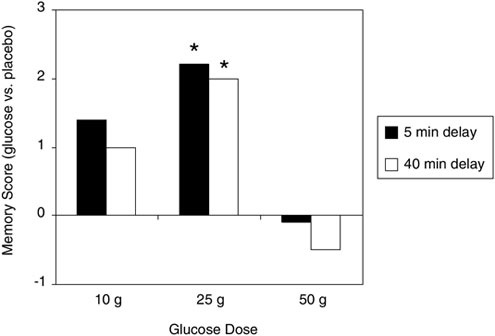
FIGURE B-13 Logical memory scores in elderly subjects 5 and 40 minutes after glucose ingestion, presented as differences from placebo (saccharin). Significant memory enhancement was observed after 25 g glucose, but not at the higher or lower doses.
* p < 0.05 versus placebo.
SOURCE: Adapted from Parsons and Gold (1992), used with permission from Elsevier.
prevent a reduction in the synthesis of neurotransmitters protecting the brain from the consequences of an energy deficit.
Other studies suggest that carbohydrate, independent of energy intake, benefits performance under hypocaloric (Wing et al., 1995) and physically demanding (Achten et al., 2004) circumstances that are relevant for combat operations. Wing and colleagues (1995) found that a low-energy (549 kcal/day), low-carbohydrate–high-fat diet was associated with impaired performance on a test of general brain function after one week as compared with the results of an equal-energy, high-carbohydrate–low-fat diet. In addition, during 11 days of intense running training, Achten and colleagues (2004) found that higher carbohydrate–lower fat diets improved overall mood and reduced fatigue as compared with the results of equal-energy (approximately 3,900 kcal/day), low-carbohydrate–high-fat diets.
Under nonexercise, adequate energy situations, carbohydrate ingestion has been associated with both better and worse moods (Benton, 2002; Leigh Gibson and Green, 2002), which may be dependent on the testing time after ingestion. Carbohydrate may improve mood up to 30 minutes after ingestion, but it is
associated with a sedative effect after about 2 h (Benton, 2002). The sedating effect may be caused by a carbohydrate-induced increased tryptophan/large neutral amino acid ratio, which increases both the brain tryptophan levels and the synthesis of the neurotransmitter serotonin (Spring et al., 1994). However, the effect of carbohydrate on serotonin is abolished with the ingestion of as little as 4-percent protein (Benton, 2002; Benton and Donohoe, 1999; Benton and Nabb, 2003; Spring et al., 1994), so these findings are likely not relevant for military rations with mixed macronutrient composition. Another hypothesis suggests that beneficial effects of carbohydrate on mood are related to an increased release of endorphins relevant to the ingestion of any palatable food, rather than to carbohydrate per se (Benton, 2002).
Carbohydrate Type
The type of carbohydrate may be important for cognitive performance although research in this area is limited to two studies. In one study in healthy elderly subjects, 50 g of carbohydrate from glucose, potato (high glycemic index), or barley (low glycemic index) all improved performance on memory and a test of general brain function up to one hour after ingestion, particularly in subjects with poorer baseline memory and glucose regulation (Kaplan et al., 2000). The cognitive-enhancing effects of barley, which raised blood glucose to 6 mmol/L, were novel because it had previously been believed that blood glucose must be at a concentration of 8 to 10 mmol/L for a benefit to be observed. These findings were supported by a recent study that found that a high-carbohydrate meal with a lower glycemic index improved memory in young adults up to 3 h after ingestion, but a higher glycemic index meal did not (Benton et al., 2003). The findings could not be related to blood glucose levels, but these data, along with the evidence showing an inverted U-shaped dose response for glucose, suggest that a prolonged increase in blood glucose and insulin levels, rather than rapid fluctuations, may be beneficial.
It is noteworthy that virtually all studies examining the effects of carbohydrate on cognitive performance or mood have used glucose or glucose polymers (e.g., starches). A few studies have examined the effects of fructose which is metabolized differently from glucose, in rats, but the evidence is mixed (Messier, 2004). Until evidence to the contrary is available in humans, it should be assumed that the beneficial effects of carbohydrate are limited to sources of glucose or glucose polymers.
PROTEIN, FAT, MACRONUTRIENT BALANCE, AND COGNITIVE PERFORMANCE
Protein has the potential to influence cognitive performance because several amino acids, including tryptophan, tyrosine, phenylalanine, arginine, histadine,
and threonine, are precursors of neurotransmitters or neuromodulators and can influence their synthesis (Lieberman, 2003). Indeed, individual amino acids have been shown to influence cognitive performance; however, these effects are likely not relevant when examining the effects of protein consumed as part of a food ration (Greenwood, 1994). In particular, tyrosine benefits cognitive performance in sleep deprived, stressed subjects, likely by its role as a precursor for the neurotransmitters norepinephrine and dopamine (Lieberman, 2003). Tryptophan may be beneficial as a sleep aid, by way of its effects on increasing synthesis of serotonin, and it improves vigilance by way of increasing the release of melatonin (Lieberman, 2003). However, any potential benefits of these amino acids would likely be as between-meal supplements, rather than as part of normal meals (Greenwood, 1994).
As noted in the carbohydrate section above, several reports have concluded that high-carbohydrate–low-protein meals are sedating because they cause an increase in serotonin synthesis, whereas protein-rich meals are arousing and can improve reaction time and vigilance (Dye and Blundell, 2002; IOM, 1994; Leigh Gibson and Green, 2002). However, as noted, these findings are likely not relevant for mixed macronutrient military rations because as little as 4 percent protein prevents the effect on serotonin. Moreover, other data do not support a negative influence on mood by consumption of a high-carbohydrate–low-protein diet. A review by Benton and Donohoe (1999) concluded that diets higher in carbohydrate and lower in protein are associated with less depression, anger, and tension and with being more energetic.
Although the fat content of diets can have an effect on cognitive performance over the long term (i.e., months or years) (Greenwood and Young, 2001; Kaplan and Greenwood, 1998), fat likely only has limited effects over the short term (i.e., hours or days), possibly related to the fact that rates of fat and carbohydrate oxidation are not influenced by the fat content of a meal (Flatt et al., 1985). Nevertheless, some studies have found acute effects of fat on performance and mood, but the data are inconsistent. Recent reviews concluded that in general, high-fat–low-carbohydrate meals can lead to declines in alertness and reaction time, particularly when they differ from habitual fat intake (Dye and Blundell, 2002; Leigh Gibson and Green, 2002).
Few direct comparisons between pure macronutrients have been made. Kaplan and colleagues (2001) found that in healthy elderly persons, pure protein, carbohydrate, and fat could all improve memory performance 15 minutes after ingestion compared with a noncaloric placebo, suggesting a general benefit of energy ingestion, likely mediated by a peripheral mechanism, which could include gut peptides or signals to the brain by way of the vagus nerve. However, it was also found that each macronutrient had unique effects on performance. For instance, as shown in Figure B-14, only glucose tended to improve memory 60 minutes after ingestion, and performance on a visual-motor task; and protein was the only macronutrient to slow the rate of forgetting. Various peripheral and
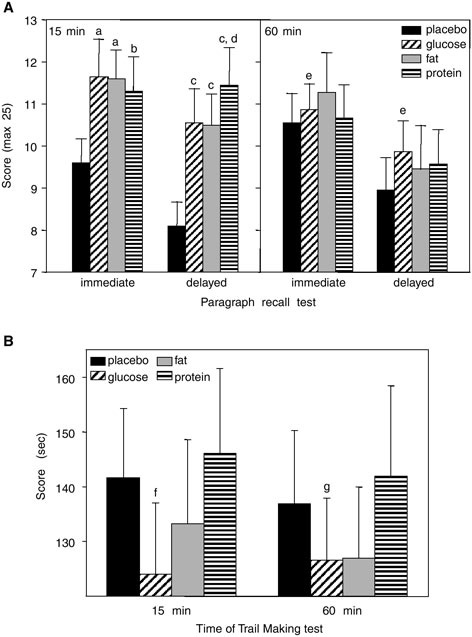
FIGURE B-14 (A) Mean (± standard error of means) scores on immediate and delayed paragraph recall 15 and 60 minutes after consumption of placebo, glucose, fat, and protein test drinks. (B) Mean (± standard error of means) scores on Trail Making Test (parts A + B) in men (n = 10) at 15 and 60 minutes after ingestion of placebo, glucose, fat, and protein test drinks.
NOTE: Lower scores represent better performance. (A) a, b, c, d, e = significantly different from placebo: a = p ≤ 0.02; b = p for trend = 0.04; c = p ≤ 0.001; d = p = 0.002 (rate of forgetting, immediate + delayed); e = p for trend = 0.09 (for composite score, immediate + delayed). (B) f, g = significantly different from placebo; f = p for trend = 0.04; g = p = 0.02.
SOURCE: Adapted from Kaplan et al. (2001) with permission by the American Journal of Clinical Nutrition © Am J Clin Nutr. American Society for Clinical Nutrition.
central mechanisms could account for these specific effects. Another study also found unique effects of each macronutrient in healthy young adults (Fischer et al., 2001). Carbohydrate improved choice reaction and short-term memory time after one hour; protein improved choice reaction time after two hours; fat improved performance on short-term memory and attention.
Taken together, several reviews have concluded that, although macronutrients have been shown to have different effects on cognitive performance, the effects have been inconsistent, and few overall conclusions can be made at this time about an optimal ratio of macronutrients for cognitive performance (Bellisle et al., 1998; Dye and Blundell, 2002; Dye et al., 2000; Kanarek, 1997; Lieberman, 2003).
CONCLUSIONS
Despite discrepancies in the literature, some conclusions can be made about the effects of energy ingestion on cognitive performance. (1) Energy ingestion approaching the level of energy needs generally improves cognitive performance and mood over the short term (several hours) compared with no energy or very low energy ingestion; the fact that benefits seem to be independent of weight loss suggest that feelings of hunger and thoughts of food contribute to the cognitive and mood deficits. Thus, minimizing hunger may minimize the negative effects on performance. Hunger can be minimized with higher protein and lower fat ingestion. (3) Over longer periods (weeks or months), there is a gradual impairment in cognitive performance associated with negative energy balance and weight loss, with greater and more rapid deficits associated with very low energy intakes (< 50 percent of energy requirements), particularly under stressful conditions.
The review of the research presented in this paper shows that the scientific literature in this area is inconsistant and contradictory; therefore, it is difficult to make definitive recommendations regarding the questions posed in the introduction. The research, although not conclusive, supports the following conclusions described below.
The following are desired characteristics of an assault ration designed for cognitive performance:
-
Adequate and sustained glucose supply to the brain. The provision of glucose or carbohydrate to the brain improves mood and performance when brain glucose requirements increase, which appears to occur during cognitively demanding tasks, particularly under hypocaloric, stressful, or physically demanding conditions.
-
Moderate fluctuations in blood glucose. Consistent evidence shows that both hypoglycemia and hyperglycemia impair cognitive performance; that intermediate, but not high or low, doses of glucose improve performance;
-
and that limited evidence shows that low glycemic index carbohydrates improve performance over a longer period than do higher glycemic index carbohydrates. Thus moderate, rather than extreme, fluctuations in blood glucose are desirable.
-
Minimize feelings of hunger. Hypocaloric intakes are associated with deficits in cognitive performance and mood, and this is caused, in part, by feelings of hunger and thoughts of food interfering with normal cognitive processes. Reducing feelings of hunger is desirable.
-
Minimize long-term negative energy balance. Sufficient data indicate that long-term energy deficits resulting in negative energy balance and weight loss are associated with deficits in cognitive performance and mood under stressful situations. Long-term negative energy balance should be minimized.
The optimal composition of the assault ration for cognitive performance would be characterized by the following:
-
A maximized carbohydrate content of the ration to provide an adequate and sustained supply of glucose to the brain, because of their hypocaloric nature and the fact that substantial carbohydrate will be required to sustain physical activity. The carbohydrate source should ideally provide glucose (e.g., glucose, sucrose, starch) rather than other monosaccharides (e.g., fructose) as the beneficial effects of carbohydrate have only been shown with glucose. The effect of the ration on blood glucose should be moderate for optimal and sustained cognitive performance and should also reduce feelings of hunger. Importantly, the carbohydrate source may have a high or low glycemic index to give the overall ration a moderate effect on blood glucose, because the other components of the ration could affect blood glucose. For instance, both protein (increases insulin release) and fiber can lower blood glucose. Higher protein and fiber content rations may necessitate higher glycemic index carbohydrates to obtain a ration with a moderate effect on blood glucose.
-
If feelings of hunger are strong and long-term energy balance is not a major problem (i.e., energy balance can be maintained by high energy intakes during the recovery periods between combat operations), then protein content of the ration should be increased and fat content should be decreased to increase satiety and reduce feelings of hunger. In this case, the energy density of the ration will not be maximized. Not enough evidence exists to recommend a specific amino acid or fatty acid profile that will optimize cognitive performance compared with any other profile. It seems prudent to supply minimum amino acid requirements.
-
If feelings of hunger are weak because of stress or hypohydration out-weighing the effects on appetite of the low calorie diet, and long-term
-
negative energy balance and weight loss are expected, then minimum protein requirements should be met for nitrogen balance, and fat content should be increased to maximize energy intake over the long term. In this case, additional protein to reduce hunger would be less important than increasing long-term energy intake by maximizing fat content and energy density of the ration.
-
To reduce long-term negative energy balance, high-energy intake should be encouraged during the recovery periods. Thus, intake during recovery should consist of energy-dense foods with minimal effects on satiety (i.e., high-fat, low-protein foods).
REFERENCES
Achten J, Halson SL, Moseley L, Rayson MP, Casey A, Jeukendrup AE. 2004. Higher dietary carbohydrate content during intensified running training results in better maintenance of performance and mood state. J Appl Physiol 96(4):1331–1340.
Alfenas RC, Mattes RD. 2003. Effect of fat sources on satiety. Obes Res 11(2):183–187.
Anderson GH, Moore SE. 2004. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr 134(4):974S–979S.
Anderson GH, Woodend D. 2003. Effect of glycemic carbohydrates on short-term satiety and food intake. Nutr Rev 61(5):S17–S26.
Awad N, Gagnon M, Desrochers A, Tsiakas M, Messier C. 2002. Impact of peripheral glucoregulation on memory. Behav Neurosci 116(4):691–702.
Bellisle F, Blundell JE, Dye L, Fantino M, Fern E, Fletcher RJ, Lambert J, Roberfroid M, Specter S, Westenhofer J, Westerterp-Plantenga MS. 1998. Functional food science and behaviour and psychological functions. Br J Nutr 80(Suppl 1):S173–S193.
Benton D. 2001. The impact of the supply of glucose to the brain on mood and memory. Nutr Rev 59(1 Pt 2):S20–S21.
Benton D. 2002. Carbohydrate ingestion, blood glucose and mood. Neurosci Biobehav Rev 26(3):293–308.
Benton D, Donohoe RT. 1999. The effects of nutrients on mood. Public Health Nutr 2(3A):403–409.
Benton D, Nabb S. 2003. Carbohydrate, memory, and mood. Nutr Rev 61(5):S61–S67.
Benton D, Ruffin MP, Lassel T, Nabb S, Messaoudi M, Vinoy S, Desor D, Lang V. 2003. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacology (Berl) 166(1):86–90.
Brun JF, Dumortier M, Fedou C, Mercier J. 2001. Exercise hypoglycemia in nondiabetic subjects. Diabetes Metab 27(2 Pt 1):92–106.
Dye L, Blundell J. 2002. Functional foods: Psychological and behavioural functions. Br J Nutr 88(Suppl 2):S187–S211.
Dye L, Lluch A, Blundell JE. 2000. Macronutrients and mental performance. Nutrition 16(10):1021–1034.
Fairclough SH, Houston K. 2004. A metabolic measure of mental effort. Biol Psychol 66(2):177–190.
Fischer K, Colombani PC, Langhans W, Wenk C. 2001. Cognitive performance and its relationship with postprandial metabolic changes after ingestion of different macronutrients in the morning. Br J Nutr 85(3):393–405.
Fischer K, Colombani PC, Wenk C. 2004. Metabolic and cognitive coefficients in the development of hunger sensations after pure macronutrient ingestion in the morning. Appetite 42(1):49–61.
Flatt JP, Ravussin E, Acheson KJ, Jequier E. 1985. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest 76(3):1019–1024.
Flint A, Helt B, Raben A, Toubro S, Astrup A. 2003. Effects of different dietary fat types on postprandial appetite and energy expenditure. Obes Res 11(12):1449–1455.
French S, Robinson T. 2003. Fats and food intake. Curr Opin Clin Nutr Metab Care 6(6):629–634.
Green MW, Rogers PJ. 1995. Impaired cognitive functioning during spontaneous dieting. Psychol Med 25(5):1003–1010.
Greenwood CE. 1994. Performance-enhancing effects of protein and amino acids. In: Marriott BM, ed. Food Components to Enhance Performance. Washington, DC: National Academy Press. Pp. 263–275.
Greenwood CE. 2003. Dietary carbohydrate, glucose regulation, and cognitive performance in elderly persons. Nutr Rev 61(5):S68–S74.
Greenwood CE, Young SN. 2001. Dietary fat intake and the brain: A developing frontier in biological psychiatry. J Psychiatry Neurosci 26(3):182–184.
Greenwood CE, Kaplan RJ, Hebblethwaite S, Jenkins DJ. 2003. Carbohydrate-induced memory impairment in adults with type 2 diabetes. Diabetes Care 26(7):1961–1966.
Grego F, Vallier JM, Collardeau M, Bermon S, Ferrari P, Candito M, Bayer P, Magnie MN, Brisswalter J. 2004. Effects of long duration exercise on cognitive function, blood glucose, and counterregulatory hormones in male cyclists. Neurosci Lett 364(2):76–80.
IOM (Institute of Medicine) 1994. Food Components to Enhance Performance. Washington, DC: National Academy Press.
IOM. 1995. Not Eating Enough. Washington, DC: National Academy Press.
IOM. 1999. The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington, DC: National Academy Press.
IOM. 2001. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations. Washington, DC: National Academy Press.
Kanarek R. 1997. Psychological effects of snacks and altered meal frequency. Br J Nutr 77(Suppl 1):S105–S118.
Kaplan RJ, Greenwood CE. 1998. Dietary saturated fatty acids and brain function. Neurochem Res 23(5):615–626.
Kaplan RJ, Greenwood CE. 2002. Influence of dietary carbohydrates and glycaemic response on subjective appetite and food intake in healthy elderly persons. Int J Food Sci Nutr 53(4):305–316.
Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. 2000. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr 72(3):825–836.
Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. 2001. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. Am J Clin Nutr 74:687–693.
Kennedy DO, Scholey AB. 2000. Glucose administration, heart rate and cognitive performance: Effects of increasing mental effort. Psychopharmacology (Berl) 149(1):63–71.
Korol DL. 2002. Enhancing cognitive function across the life span. Ann N Y Acad Sci 959:167–179.
Korol DL, Gold PE. 1998. Glucose, memory, and aging. Am J Clin Nutr 67(Suppl):764S–771S.
Kretsch MJ, Green MW, Fong AK, Elliman NA, Johnson HL. 1997. Cognitive effects of a long-term weight reducing diet. Int J Obes Relat Metab Disord 21(1):14–21.
Laessle RG, Platte P, Schweiger U, Pirke KM. 1996. Biological and psychological correlates of intermittent dieting behavior in young women. A model for bulimia nervosa. Physiol Behav 60(1):1–5.
Lawton CL, Delargy HJ, Brockman J, Smith FC, Blundell JE. 2000. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr 83(5):473–482.
Leigh Gibson E, Green MW. 2002. Nutritional influences on cognitive function: Mechanisms of susceptibility. Nutr Res Rev 15(1):169–206.
Lieberman HR. 2003. Nutrition, brain function and cognitive performance. Appetite 40(3):245–254.
Lieberman HR, Falco CM, Slade SS. 2002a. Carbohydrate administration during a day of sustained aerobic activity improves vigilance, as assessed by a novel ambulatory monitoring device, and mood. Am J Clin Nutr 76(1):120–127.
Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. 2002b. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during US Navy SEAL training. Psychopharmacology (Berl) 164:250–261.
Macht M. 1996. Effects of high- and low-energy meals on hunger, physiological processes and reactions to emotional stress. Appetite 26(1):71–88.
Mays MZ. 1995. Impact of underconsumption on cognitive performance. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 285–302.
McCall AL. 1992. The impact of diabetes on the CNS. Diabetes 41(5):557–570.
McNay EC, Gold PE. 2001. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Gerontol A Biol Sci Med Sci 56(2):B66–B71.
Messier C. 2004. Glucose improvement of memory: A review. Eur J Pharmacol 490(1–3):33–57.
Nybo L, Secher NH. 2004. Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol 72(4):223–261.
Owen G, Turley H, Casey A. 2004. The role of blood glucose availability and fatigue in the development of cognitive impairment during combat training. Aviat Space Environ Med 75(3): 240–246.
Park CR. 2001. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev 25(4):311–323.
Parsons MW, Gold PE. 1992. Glucose enhancement of memory in elderly humans: An inverted-U dose-response curve. Neurobiol Aging 13(3):401–404.
Pollitt E, Mathews R. 1998. Breakfast and cognition: An integrative summary. Am J Clin Nutr 67(Suppl):804S–813S.
Rolls BJ, Bell EA. 1999. Intake of fat and carbohydrate: Role of energy density. Eur J Clin Nutr 53(Suppl 1):S166–S173.
Sandstead HH. 2000. Causes of iron and zinc deficiencies and their effects on brain. J Nutr 130(2 Suppl):347S–349S.
Shukitt-Hale B, Askew EW, Lieberman HR. 1997. Effects of 30 days of undernutrition on reaction time, moods, and symptoms. Physiol Behav 62(4):783–789.
Spring BJ, Pingitore R, Schoenfeld J. 1994. Carbohydrates, protein and performance. In: Marriott BM, ed. Food Components to Enhance Performance. Washington, DC: National Academy Press. Pp. 321–350.
St-Onge MP, Jones PJ. 2002. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J Nutr 132(3):329–332.
Stubbs RJ, Whybrow S. 2004. Energy density, diet composition and palatability: Influences on overall food energy intake in humans. Physiol Behav 81(5):755–764.
Stubbs J, Ferres S, Horgan G. 2000. Energy density of foods: Effects on energy intake. Crit Rev Food Sci Nutr 40(6):481–515.
Sunram-Lea SI, Foster JK, Durlach P, Perez C. 2001. Glucose facilitation of cognitive performance in healthy young adults: Examination of the influence of fast-duration, time of day and pre-consumption plasma glucose levels. Psychopharmacology (Berl) 157(1):46–54.
Westerterp-Plantenga MS. 2003. The significance of protein in food intake and body weight regulation. Curr Opin Clin Nutr Metab Care 6(6):635–638.
Wilson MM, Morley JE. 2003. Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr 57(Suppl 2):S24–S29.
Wing RR, Vazquez JA, Ryan CM. 1995. Cognitive effects of ketogenic weight-reducing diets. Int J Obes Relat Metab Disord 19(11):811–816.
Do Structured Lipids Offer Advantages for Negative Energy Balance Stress Conditions?
R. J. Jandacek, University of Cincinnati
INTRODUCTION AND REVIEW
The objective of this paper is to address the question of whether structured lipids offer advantages as a source of energy for hypocaloric, high-energy expenditure stress conditions of short-term combat operations (repetitive three- to seven-day missions with recovery periods of one to three days). A review of structured lipids and their metabolism is followed by a discussion of the question provided and the recommendation.
Ingested Energy Sources
Virtually all of the fat that we consume is in the form of triacylglcyerols, three long-chain fatty acids bonded to glycerol. The chain length of most dietary fatty acids is 16 and 18 carbon atoms.
It was found by Rubner (1885) and Atwater and Bryant (1900) that the energy in food that is available to support metabolic and other activity is equivalent to that produced by oxidation of the carbon and hydrogen atoms of the compounds in food macronutrients. In a bomb calorimeter, the oxidation of carbohydrates generates 4 kcal/g (16.7 kJ/g). The oxidation of fatty acids with 18-carbon chains yields 9.5 kcal/g (39.7 kJ/g). Fatty acids of shorter chain length contain a higher fraction of carbon atoms bonded to oxygen and therefore give off less energy when oxidized. Heat produced by the oxidation of octanoic acid is 8 kcal/g (33.4 kJ/g).
Although we have understood this concept for a century, the equivalence of the bomb calorimeter and the human body is a remarkable discovery. The β-oxidation of palmitic acid that produces acetyl CoA and carbon dioxide (CO2) via the TCA (tricarboxylic acid) cycle provides the same amount of heat and work as the bomb calorimeter. The sum of the reactions in the body is the same as that in the bomb calorimeter: fat plus oxygen produces heat, CO2, and water.
The chemical energy contained in the C-C and C-H bonds of fatty acids is the densest source of energy available in foods, where C is carbon and H is hydrogen. The energy of ingested fat is stored if total caloric intake exceeds energy expenditure, or it is used to meet energy needs. The sections that follow briefly review the types of dietary fat and the unique properties of fats made with medium-chain fatty acids.
Types of Dietary Fat
Dietary fat comprises triacylglycerols, phospholipids, and sterols. Phospholipids and sterols are important in health, but triacylglycerols provide essentially all of the energy in dietary lipids.
A triacylglycerol molecule contains an asymmetric carbon atom (carbon 2 of the glycerol) if the fatty acids in the 1 and 3 positions are different. Fatty acid positions in the 1 and 3 positions are hydrolyzed by pancreatic lipase. The chemical structure of a triacylglycerol molecule (trilaurin) is shown in Figure B-15.
Figure B-16 shows dietary fatty acids in animal and vegetable triacylglycerols. The long-chain fatty acids account for a majority of the fat consumed by people. Docosahexaenoic acid is found in fatty fish such as salmon and tuna.
Medium-Chain Fatty Acids and Structured Lipids
In addition to these long-chain fatty acids in common foods, small quantities of shorter fatty acids are part of the triacylglycerols of butterfat. These fatty acids include butyric, hexanoic (caproic), octanoic (caprylic), decanoic (capric), and dodecanoic (lauric) acids. Octanoic and decanoic acids make up approximately 4 percent of butterfat fatty acids.
Octanoic and decanoic acids account for approximately 13 percent of the fatty acids in coconut oil. They became commercially available as byproducts of coconut oil fractionation to obtain lauric acid, an ingredient for detergent products. These byproducts made it possible to synthesize medium-chain fatty acid triacylglycerols (MCT, MCT oil).
FIGURE B-15 A triacylglycerol, trilaurin (C39H74O6).
FIGURE B-16 Principal long-chain fatty acids found in dietary fat. Octanoic acid is a medium-chain fatty acid that is a principal component of structured lipids.
Structure
Synthetic triacylglycerols were also made from mixtures of long-chain and medium-chain fatty acids; these esters were termed “structured lipids,” even though the fatty acid distribution in the three positions of the glycerol was completely random and unstructured. More recently, enzymatic techniques have made it possible to synthesize triacylglycerols with medium-chain fatty acids in the 1 and 3 positions, and long-chain fatty acids in the 2 position.
Absorption
MCT oil was found to have therapeutic advantages in cases of pancreatic insufficiency because its intestinal hydrolysis is more rapid than that of long-chain fats. MCT oil is absorbed from the intestine in subjects with subnormal pancreatic lipase, such as patients with cystic fibrosis. The hydrolysis products are transported via the portal vein rather than via the lymphatic route. MCT does not provide the essential fatty acids linoleic and α-linolenic acids.
Utilization
Structured lipids were proposed to have benefits in providing energy to patients who were not able to consume food through the gastrointestinal tract and therefore required intravenous nutritional support (i.e., total parenteral nutrition, TPN). The provision of intravenous energy has successfully used emulsions of fat in small particles. It was found that these particles in blood circulation acquire surface lipoproteins (e.g., apolipoprotein C-II, C-III, E) from high-density lipoproteins. The lipid is hydrolyzed by endothelial lipoprotein lipase and the lipolytic products are used by peripheral tissues.
Medium-chain fatty acids are rapidly hydrolyzed from triacylglycerol and do not require carnitine for tissue uptake; however, acidosis can result from this high rate of hydrolysis. For this reason, physical mixtures of MCTs or structured lipids with medium-chain fatty acids and long-chain fatty acids (including essential fatty acids) bonded to the same glycerol molecule may have advantages for TPN compared with long-chain triacylglycerols (Bellantone et al., 1999; Rubin et al., 2000). Some reported advantages of structured lipids in TPN were summarized previously (Jandacek, 1994).
In addition to use in TPN, another hypothetical nutritional advantage for medium-chain fatty acids (and therefore for structured lipids) was explored. This hypothesis suggests that the rapid absorption of medium-chain fatty acids via the portal vein to the liver results in rapid mitochondrial oxidation of these acids that can then reduce the body’s use of glycogen as a fuel. It was also suggested that the use of MCTs in place of carbohydrate would reduce insulin elevation and hypoglycemia. As reviewed previously (Jandacek, 1994), studies did not support these hypotheses in human exercise trials, and a more recent trial also found no performance improvement with a specific structured lipid (Vistisen et al., 2003).
A further refinement to structured lipids was the synthesis of “truly structured” lipids, in which the positions of the medium- and long-chain fatty acids were specific rather than random. The advantage of this kind of structure is that the placement of the medium-chain fatty acids in the exterior 1 and 3 positions would result in their rapid lipase-catalyzed hydrolysis (Jandacek et al., 1987). Specific position structured lipids would be capable of providing essential fatty acids (in the 2 position) with rapidly hydrolyzed octanoic or decanoic acid in the
1 and 3 positions. This kind of molecule could therefore be advantageous in providing fat in patients with pancreatic insufficiency.
The isomerization of monoacylglycerols may affect the metabolism of structured lipids. The preferred (low energy) form of monoacylglycerols is the 1(3)-monoacylglycerol. Isomerization of the 2-monoacylglycerol to the 1(3) isomer is accelerated by heat is rapid for short- and medium-chain fatty acids and for long-chain polyunsaturated fatty acids. It is likely that a significant fraction of 2-monoacylglycerols of medium-chain fatty acids formed in the intestine is isomerized (and then hydrolyzed) within the time frame of intestinal transit. A comparison of the absorption of long-chain fatty acid triacylglycerols, MCTs, and structured lipids is shown in Figure B-17. The structured lipid is shown as a “specific” triacylglycerol.
DISCUSSION OF QUESTIONS
Based on the physical properties and metabolism of structured fatty acids, three areas of discussion address the question of whether or not structured lipids have any of the following advantages in energy deficit status during high stress circunstances: (1) intestinal absorption, (2) energy content, (3) energy utilization, and (4) appetite.
Is There an Advantage in Intestinal Absorption?
Current data indicate that there is no advantage in the absorption of medium-chain fatty acids in structured lipids, as long as the subjects have normal levels of pancreatic lipase. There is no need to optimize the hydrolysis rate in the small intestine of normal healthy individuals. There is a 20-fold excess capacity for the absorption of fat, suggesting that there are more than adequate levels of lipase, bile salts, enterocyte capacity, and chylomicron formation. Evidence for this is supported by the work of Kinsell and colleagues (1953). They fed vegetable oil as the entire diet to subjects for a week and observed not only that there were no ill effects, but also that there were reductions in serum cholesterol. Work by Kasper (1970) found that when dietary fat was raised to three times the normal consumption at a level of 300 g/day, the body was able to compensate, and this level of fat was well absorbed. He then pushed the system further with a diet of 639 g fat/day. This amount also was well tolerated by a subject during a 20-day trial, further confirming that normal humans have an excess capacity for the absorption of fat. These results negate the need to optimize the hydrolysis rate in the small intestine of healthy individuals. Furthermore, there is no evidence that subjects stressed by several days of caloric deficit will absorb less than normal levels of dietary fat. The use of a fat that is hydrolyzed rapidly will therefore not enhance caloric use relative to normal, well-absorbed, long-chain fats in people with normal pancreatic lipase levels.
Is There an Advantage in Energy Content?
As discussed above, the maximum energy content of macronutrients available to a person is the heat of combustion. For long-chain fats this value is 9.5 kcal (39.7 kJ)/g. The caloric energy of the fatty acids is dependent on chain length, and the energy that can be used from octanoic acid is 8 kcal (33.5 kJ)/g. Although the difference in caloric density of long-chain and medium-chain fatty acids is small, it seems prudent to maximize the energy provided per unit weight of the food provided in anticipated caloric deprivation.
Is There an Advantage in Energy Utilization?
The energy provided by macronutrient metabolism results in storage, work, or heat. In the case of excessive caloric intake, a portion of the energy is directed toward storage, principally in the form of triacylglycerol fatty acids in adipose tissue. In negative caloric balance, ingested energy is converted to heat and work. In a situation of energy stress in a high-temperature environment, it would be desirable to maximize ingested energy as work (ATP production).
A study of high relevance to this topic was reported by Bendixen and coworkers (2002). They compared four types of triacylglycerol fats in 11 normal, healthy men ranging in age from 22 to 28 years. The fats included a conventional long-chain triacylglcyerol; a positionally specific structured fat (octanoic acid in the 1(3) position); a randomly structured lipid; and a mixture of MCT with long-chain triacylglycerols. All three fats that included medium-chain fatty acids resulted in higher postprandial energy expenditure than did the long-chain dietary fat. This result is consistent with rapid absorption of medium-chain fatty acids via the portal vein and, because elongation is not favored, they are directed toward mitochondrial oxidation. If this oxidation is not entirely coupled to the production of ATP (e.g., peroxisomal oxidation) then the result would be a negative energy balance. In this study there was no difference among fats in subjective appetite measures or ad libitum energy consumption. Total energy expenditure was smaller after the conventional fat meal, relative to the other fats. Diet-induced thermogenesis was lower after the conventional fat than after the random structured lipid. In the 5-hour postprandial period there was a (nonsignificant) trend toward lower oxidation of fat from the long-chain fat (1.65 g/5 h versus 3.1–3.5 g/5 h), and a trend toward higher carbohydrate oxidation in this group (13.9 versus 11.1–13.1 g/5 h). The authors concluded that “structured fats do not change short-term postprandial appetite sensations or ad libitum energy intakes, but do result in higher postprandial energy expenditure than do conventional fats and hence promote negative energy and fat balance” (Bendixen et al., 2002).
Is There an Advantage in Appetite?
During hypocaloric stress it is possible that a nutrient that provides satiety would be advantageous. There was no difference in the satiating effect of the different fats in the Bendixen study in terms of subjective tests and in terms of ad libitum caloric intake (Bendixen et al., 2002). However, in some reports long-chain fatty acids have been reported to be more satiating than medium-chain fatty acids (Cox et al., 2004; Feltrin et al., 2004).
CONCLUSION
There is no reason to substitute structured lipids for long-chain fatty acid triacylglycerols to meet the needs of stress resulting from high energy expenditure and low energy intake. Maximizing caloric density is the optimal approach. During a relatively short duration of five to ten days, essential fatty acids are not limiting factors. In terms of the fat composition, triacylglycerols that are oxidatively stable, high in energy density, and readily digested are recommended. Oils high in oleic acid and low in polyunsaturated acids (e.g., high oleic oils from cultivars of sunflower and safflower oils) meet these criteria.
REFERENCES
Atwater WO, Bryant AP. 1900. The availability and fuel value of food materials. Connecticut Storrs Agricultural Extension Station Annual Report, 1899. Pp. 73–110.
Bellantone R, Bossola M, Carriero C, Malerba M, Nucera P, Ratto C, Crucitti P, Pacelli F, Doglietto GB, Crucitti F. 1999. Structured versus long-chain triglycerides: A safety, tolerance, and efficacy randomized study in colorectal surgical patients. J Parenter Enteral Nutr 23(3):123–127.
Bendixen H, Flint A, Raben A, Hoy CE, Mu H, Xu X, Bartels EM, Astrup A. 2002. Effect of 3 modified fats and a convential fat on appetite, energy intake, energy expenditure, and substrate oxidation in healthy men. Am J Clin Nutr 75(1):47–56.
Cox JE, Kelm GR, Meller ST, Randich A. 2004. Suppression of food intake by GI fatty acid infusions: Roles of celiac vagal afferents and cholecystokinin. Physiol Behav 82(1):27–33.
Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C. 2004. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 287(3):R524–R533.
Jandacek RJ. 1994. Structured lipids: An overview and comments on performance enhancement potential. In: Marriott BM, ed. Food Components to Enhance Performance. Washington, DC: National Academy Press. Pp. 351–379.
Jandacek RJ, Whiteside JA, Holcombe BN, Volpenhein RA, Taulbee JD. 1987. The rapid hydrolysis and efficient absorption of triglycerides with octanoic acid in the 1 and 3 positions and long-chain fatty acid in the 2 position. Am J Clin Nutr 45(5):940–945.
Kasper H. 1970. Faecal fat excretion, diarrhea, and subjective complaints with highly dosed oral fat intake. Digestion 3(6):321–330.
Kinsell LW, Michaels GD, Partridge FW, Boling LA, Balch HE, Cochrane GC. 1953. Effect upon serum cholesterol and phospholipids of diets containing large amounts of vegetable fat. Am J Clin Nutr 1(3):224–231.
Rubin M, Moser A, Vaserberg N, Greig F, Levy Y, Spivak H, Ziv Y, Lelcuk S. 2000. Structured triacylglycerol emulsion, containing both medium- and long-chain fatty acids, in long-term home parenteral nutrition: A double-blind randomized cross-over study. Nutrition 16(2):95–100.
Rubner M. 1885. Calorimetrische Untersuchungen. Zeitsch Biol 21:377.
Vistisen B, Nybo L, Xu X, Hoy CE, Kiens B. 2003. Minor amounts of plasma medium-chain fatty acids and no improved time trial performance after consuming lipids. J Appl Physiol 95(6):2434–2443.
Optimum Protein Intake in Hypocaloric States
L. John Hoffer, Jewish General Hospital
INTRODUCTION
The goal of this workshop is to advise the military in developing a hypocaloric ration that maximizes physical and mental performance. As currently envisioned, rations of 2,400 kcal per day will be consumed by soldiers expending approximately 4,000 to 4,500 kcal per day for three to seven days followed by one to three days of recovery. What is the optimum protein content of such a ration? Should the energy deficit be minimized, or are other aspects of the ration more important?
The main concerns when a person’s energy expenditure consistently exceeds intake—that is, when he or she is starving—are depletion of the fat store and loss of muscle mass and function. The 32 young men who participated in the famous Minnesota human starvation experiment (Keys et al., 1950) consumed approximately 1,500 kcal per day and, during the subsequent 26 weeks, they lost approximately 25 percent of their lean tissue mass. This occurred despite their consumption of 50 g of protein per day, an amount that exceeded the average protein requirement of 0.6 g/kg of body weight and was close to the current Recommended Dietary Allowance (RDA) of 0.8 g/kg (IOM, 2002). By the 24th week of the study, physiologic adaptation had restored energy and nitrogen equilibrium despite continuing starvation. This adaptation is crucial for survival during starvation (Hoffer, 1999a, b).
The Minnesota experiment illustrates what has long been known (Elwyn et al., 1979; Munro, 1964; Pellett and Young, 1992) and recently reemphasized (IOM, 2002; Millward, 2004; Tipton and Wolfe, 2004): starvation induces body protein loss. For a variety of reasons analyzed earlier by the Committee on Military Nutrition Research (CMNR) (IOM, 1995), soldiers in a stressful, high-energy-expenditure environment typically fail to increase their energy intake enough to match their energy expenditure. This situation fits the definition of starvation, and in keeping with its cardinal features, these soldiers lose fat and lean tissue mass. Their physiologic state appears to be the same as that of starvation that is induced simply by low food consumption. Thus, despite ample carbo-
hydrate intake, circulating concentrations of insulin-like growth factor-1 and 3,5,3′-triiodothyronine are decreased as occurs in conventional starvation, and they increase when the energy gap is narrowed, even if there is no other change in the stressful environment (Friedl et al., 2000).
Carbon oxidation always equals energy expenditure, and when energy expenditure exceeds food energy consumption, the body draws on endogenous fat, its chief energy store. Fat loss is therefore an essential feature of starvation. But the situation with regard to protein loss is unclear. Why does it occur? How avoidable is it? Factors that affect its magnitude are enumerated below.
Micronutrient Status
Mineral deficiencies, particularly those of potassium (Rudman et al., 1975), phosphorus (Rudman et al., 1975), zinc (Khanum et al., 1988; Wolman et al., 1979), and, presumably, magnesium, impair the normal protein-sparing adaptation to starvation.
Absence of a Catabolic State
Tissue injury, inflammation, and systemic infection reverse the physiologic adaptation to starvation and increase body nitrogen loss (Hoffer, 1999b).
Magnitude of the Energy Deficit
This phenomenon is illustrated by a study in which male volunteers with a body mass index (BMI) of 24.5 kg/m2 participated in an 8-week US Army Ranger course (Friedl, 1997; Friedl et al., 2000). The program consisted of four periods of an initial several days of ad libitum feeding followed by seven to ten days of food restriction in a setting of daily energy expenditures of 4,000 to 4,500 kcal, thermal stress, and sleep deprivation. In the first of two studies, food intake was restricted to 1,400 kcal, with 52 g of protein per day (similar to the Minnesota study). In the second study, supplemental food provided an additional 400 kcal and 15 g of protein. In both experiences food intake during the brief recovery periods did not fully compensate for the deficits incurred during the restriction periods. The greater intake of energy (but also of protein; see below) by the second group produced marked benefits. In particular, the better-fed group experienced less weight loss and their percentage of fat-free mass (FFM) was significantly greater at the end of the study (Friedl et al., 2000).
Fat Store
Some experts claim that very obese, starving people lose body nitrogen more slowly (and have a slower rate of nitroten loss as a fraction of total weight
loss) than starving people of normal weight (Elia et al., 1999; Van Itallie and Yang, 1977). Since severely obese people invariably lose FFM during therapeutic starvation, it would seem that starving, nonobese soldiers must inevitably experience FFM loss. It should be borne in mind, however, that severe obesity increases the lean tissue compartment (Drenick, 1975; Henry et al., 1986), and at least some of the lean tissue loss during therapeutic starvation occurs simply because a smaller body requires less muscle to be lifted and moved. From this perspective, normal-weight or only mildly obese people may actually conserve body protein more effectively than severely obese ones during starvation.
It is certainly true that very obese people tolerate starvation with a greater sense of well-being and survive it for longer time. Adults of normal weight die after fasting for approximately 2 months, whereas severely obese people have survived fasts lasting many months (Barnard et al., 1969; Thomson et al., 1966). The longest monitored fast on record was by a 27-year-old man who initially weighed 207 kg and survived 382 days of uninterrupted fasting (Stewart and Fleming, 1973). Friedl and colleages (1994) cite a description of the starvation that occurred among the British forces during the Turkish siege of Kut from December 1915 to April 1916. Soldiers who began with superabundant fat stores survived, whereas those who began with a smaller fat reserve soon died in the cold winter climate (Hehir, 1922).
A study by Friedl and colleagues (1994) provides important insight into this phenomenon. The body composition of 55 men was determined before and after their successful completion of an 8-week US Army Ranger course in which energy intake was unusually low (1,300 kcal per day during the restriction periods). Body fat was an average of 14.3 percent at the start and decreased to an average of 5.8 percent at the end of the course. The remarkable finding was that, during progressive starvation, body fat stabilized at a minimum of 5 percent. The men who reached this limit continued to lose weight, but the source of the weight loss was now FFM. This observation suggests that 5 percent body fat represents a biological limit that is essential for successful adaptation to starvation. US Army data reviewed by Friedl (1995) suggest the contribution of lean tissue loss to total weight loss increases gradually as body fat falls below 10 percent. Nevertheless, the 5 percent limit appears to represent a “metabolic floor” that also approximately coincides with the psychological limit of voluntary participation (Friedl, 1995). As long as body fat remains above this critical level, a high-protein intake can spare body protein, but once it falls below it, protein-sparing is no longer possible. Obese people therefore survive prolonged starvation longer than lean ones because it takes longer to deplete their fat store to the critical level below which protein catabolism accelerates and disability becomes marked.
Comparison has been made between the Minnesota study and modern calorie-restriction studies like the Biosphere 2 study which involved normal-weight individuals (Heilbronn and Ravussin, 2003). This comparison is complicated by the fact that the average BMI of the Minnesota volunteers was only 21.4 when
they embarked on their starvation regimen. Thus, even before they began to starve, the Minnesota volunteers had BMIs comparable to modern adults who would be considered calorie restricted. The BMIs of most fit young men of the 21st century typically range from 23 to 25. The crucial determinant of well-being and physical function is more likely to be the absolute lean tissue mass that exists for a given body structure than a percentage reduction. At the end of the starvation phase of the Minnesota study, the average BMI had fallen to 16.3; this was associated with a severity of fat and lean tissue depletion that was incompatible with normal physiologic function. Had the baseline BMI of the Minnesota volunteers been greater, their final BMI would presumably have been closer to the range reported in modern calorie-restriction experiences. Taken together, these considerations suggest that elite soldiers being considered for extended hypocaloric field maneuvers should have normal fat and muscle stores as indicated by a normal physical examination, a BMI not less than approximately 23, and body fat not less than an appropriate minimum, such as 15 percent.
Protein Intake
While it is true that energy deficiency worsens nitrogen balance, it is also true that an increase in protein intake improves nitrogen balance over a wide range of energy intakes from deficient to maintenance (Munro, 1964; Shaw et al., 1983). It was not at first appreciated that a high protein intake protects the lean tissue store during starvation because the early studies were of short duration. Short-duration studies are confounded by a transient loss of body nitrogen, called “labile protein,” that occurs immediately after energy (or protein) intake is reduced, and by the failure to allow sufficient time for physiologic adaptation to the new diet. When nitrogen balance studies are carried out long enough for the transient effect of labile protein loss to end and metabolic adaptation to take effect—a process that requires several days (Hoffer, 1999a, b)—high protein intakes are protein sparing in many energy-deficient states (Hoffer, 2003).
The protein-sparing effect of increased dietary protein is not necessarily limited to obese persons. In one study, nonobese men were calorie restricted for 10 weeks while consuming 93 g of protein per day. The men lost 7.4 kg (BMI fell from 24.9 to 22.6), 83 percent of which was fat (Velthuis-te Wierik et al., 1995). This is the composition of adipose tissue, which is approximately 85 percent fat and 15 percent FFM (Garrow, 1982; Grande and Keys 1980; Waki et al., 1991). In the Biosphere 2 experiment, eight normal men and women starved for 6 months, then remained in energy equilibrium for 18 months during which they consumed 2,200 kcal per day including 82 g of protein (Walford et al., 2002; Weyer et al., 2000). The men’s BMI decreased from 23.7 to 19.3 and the women’s from 21.2 to 18.5 (Walford et al., 2002). In the five persons in whom it was measured, body fat fell to 10 percent (significantly less than the 20 percent body fat in a matched normal comparison group), but their FFM was normal
(Weyer et al., 2000). Several studies in the obesity field have demonstrated zero nitrogen balance in normally active, moderately obese people adhering to very low energy diets supplying 1.5 g of protein per kg of ideal body weight per day (Gelfand and Hendler, 1989; Piatti et al., 1994). The proposition that lean tissues need not be lost during starvation is further supported by data from military trainees who lost body fat (i.e., starved) during basic training without food restriction. In one study, moderately obese female army trainees lost 2.2 kg of fat but simultaneously gained 1.7 kg of FFM (Friedl, 1995). In a study of obese male army recruits, basic training was associated with an average of loss of 12.5 kg within the first 3 months, virtually all of it as fat (Lee et al., 1994). Protein intakes in all these studies were substantially greater than in the Minnesota study and in published US Army Ranger field studies. One may conclude that important protein sparing may well be achievable in starving, normal-weight soldiers by increasing the protein content of their rations.
Another issue to be considered is whether the high level of physical activity required during maneuvers increases the protein requirement. Sports experts advise athletes to consume approximately 1.5 g of protein per kg of body weight every day. This is nearly twice the RDA (IOM, 2002) and nearly three times the average minimum requirement (Fielding and Parkington, 2002; Tipton and Wolfe, 2004). Could the increased protein requirement incurred by exercise add to the high-protein requirement induced by starvation? This issue was addressed by the CMNR (IOM, 1999), who found there was insufficient evidence to conclude that high-level physical activity increases the normal protein requirement. More recent reviews have come to the same conclusion (Fielding and Parkington, 2002; Tipton and Wolfe, 2004). Since the increased food intake necessary to meet a high-energy requirement automatically increases protein intake, the most cogent reason for advising athletes to consume a lot of protein is that this is what they naturally do.
In summary, lean tissues can be conserved during starvation by four mechanisms: (1) the only modest adipose tissue unloading—and hence less absolute weight loss and less disuse muscle atrophy—that occurs when baseline fat stores are normal or only moderately increased; (2) a high, or at least maintained, level of physical activity (Ballor and Poehlman 1994; Prentice et al., 1991); (3) the prevention of a severe depletion of the fat reserve; and (4) substantially greater protein intake than in the Minnesota experiment. Despite the common assumption that protein wasting is unavoidable in the presence of energy deficiency (IOM, 1995), there are good reasons to predict that the lean tissue stores of soldiers in the field can in fact be largely, if not completely, protected by increasing their protein intake. The daily ration currently under consideration provides 2,400 kcal. If, like other US Army rations (Cline and Warber, 1999), 15 percent of the energy is from protein, this ration will provide 90 g of protein per day, or 1.15 g of protein per kilogram of body weight for the average 78 kg US male soldier (IOM, 1999). This may be sufficient to prevent starvation-induced
lean tissue loss. Indeed, US Army Ranger course participants who consumed considerably less than 90 g/day suffered only modest losses of FFM and their physical performance appeared to be maintained (Friedl, 1995, 1997). In the Ranger study described earlier, in which daily energy and protein intakes during restriction periods were 1,800 kcal and 67 g of protein, respectively, FFM decreased by 6 percent or 4 kg (Friedl et al., 2000). Upper arm muscle cross-sectional area decreased from 68 to 60 cm2, but 60 cm2 remains at the upper 85th percentile for normal age-matched men. Will 90 g of protein per day prevent even these losses? Would 120 g of protein per day (1.5 g of protein per kg) be even more effective? This larger amount of protein would be provided by a 2,400 kcal ration with 20 percent of energy from protein and would conform to the current expert recommendation of 1.5 g/kg for athletes (Fielding and Parkington, 2002; Tipton and Wolfe, 2004). Although 120 g of protein per day seems to exceed the military protein RDA (MRDA) of 100 g per day, first established in 1947, it actually does not. In 1947 the average male soldier weighed only 68 kg (IOM, 1999); the original MRDA was therefore equivalent to a protein intake of 1.5 g/kg of body weight.
Potential drawbacks to a high-protein diet include unpalatability, early satiety, and increased obligatory urine volume to eliminate the additional urea osmoles (Friedl, 1999). However, an increase of protein intake from 90 to 120 g per day would not increase obligatory urine volume because urea is an “ineffective osmole” (Kamel et al., 2004). With regard to satiety and palatability, it is worth recalling that protein comprised 30 to 35 percent of the daily energy intake of paleolithic humans. This is equivalent to 2.5 to 3.5 g/kg (Eaton and Cordain, 1997; Eaton et al., 1996). It seems very likely that a palatable diet providing a “mere” 1.5 g/kg of protein could be devised.
CONCLUSIONS
If soldiers are required to starve for only three to seven days before refeeding, it does not matter how much protein they consume, and the design of the ration should focus on features such as palatability. Field studies do show, however, that ad libitum feeding over the few days following seven to ten days of food restriction is insufficient to make up the energy deficit incurred, so soldiers returning too quickly to the field will experience progressive starvation (Friedl et al., 1994). It is also possible that a highly successful ration could be put to use for longer periods than initially planned for. The following conclusions and suggestions are offered with these possibilities in mind.
Contrary to what is commonly assumed, it is very likely that the lean tissue store of normal-weight persons can be largely conserved during starvation through a combination of a high-protein intake, physical activity, and avoidance of severe body fat loss. This hypothesis has not been tested in a field situation. The prediction is that a hypocaloric ration providing 2,400 kcal and 90 g of
protein per day will spare the lean tissue store of soldiers on extended stressful maneuvers. It would be interesting and useful to determine whether a ration providing 2,400 kcal and 120 g of protein is even more effective, as well as practical and palatable. The protein-sparing effect of high-protein intakes will not be demonstrable unless balance studies are long enough to allow for metabolic adaptation.
The energy deficit should be minimized. Protein sparing is only possible when body fat is above a minimum of approximately 5 percent of body weight. Energy-deficient states are psychologically very unpleasant and appear to be increasingly so as the lower fat limit is approached (Friedl, 1997). The psychological unpleasantness of hypocaloric rations may well be their most debilitating short-term feature (Keys et al., 1950). As has already been pointed out (Friedl, 1995; Friedl et al., 1994), it is important, when selecting elite soldiers for stressful missions, to consider their starting body composition. Under starvation conditions, very lean and muscular does not translate into better.
REFERENCES
Ballor DL, Poehlman ET. 1994. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: A meta-analytical finding. Int J Obes Relat Metab Disord 18(1):35–40.
Barnard DL, Ford J, Garnett ES, Mardell RJ, Whyman AE. 1969. Changes in body composition produced by prolonged total starvation and refeeding. Metabolism 18(7):564–569.
Cline AD, Warber JP. 1999. Overview of garrison, field, and supplemental protein intake by US military personnel. In: The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington, DC: National Academy Press. Pp. 93–108.
Drenick EJ. 1975. Weight reduction by prolonged fasting. In: Bray GA, ed. Obesity in Perspective: A Conference. John E. Fogarty International Center for Advanced Study in the Health Sciences, October 1–3, 1973. DHEW Publication No. NIH 75-708. Washington, DC: US Government Printing Office. Pp. 341–360.
Eaton SB, Cordain L. 1997. Evolutionary aspects of diet: Old genes, new fuels. Nutritional changes since agriculture. World Rev Nutr Diet 81:26–37.
Eaton SB, Eaton SB 3rd, Konner MJ, Shostak M. 1996. An evolutionary perspective enhances understanding of human nutritional requirements. J Nutr 126(6):1732–1740.
Elia M, Stubbs RJ, Henry CJ. 1999. Differences in fat, carbohydrate, and protein metabolism between lean and obese subjects undergoing total starvation. Obes Res 7(6):597–604.
Elwyn DH, Gump FE, Munro HN, Iles M, Kinney JM. 1979. Changes in nitrogen balance of depleted patients with increasing infusions of glucose. Am J Clin Nutr 32(8):1597–1611.
Fielding RA, Parkington J. 2002. What are the dietary protein requirements of physically active individuals? New evidence on the effects of exercise on protein utilization during post-exercise recovery. Nutr Clin Care 5(4):191–196.
Friedl KE. 1995. When does energy deficit affect soldier physical performance? In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 253–283.
Friedl KE. 1997. Variability of fat and lean tissue loss during physical exertion with energy deficit. In: Kinney JM, Tucker HN, eds. Physiology, Stress, and Malnutrition: Functional Correlates, Nutritional Intervention. Philadelphia: Lippincott-Raven. Pp. 431–450.
Friedl KE. 1999. Protein and amino acids: Physiological optimization for current and future military operational scenarios. In: The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington, DC: National Academy Press. Pp. 85–91.
Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW. 2000. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol 88(5):1820–1830.
Friedl KE, Moore RJ, Martinez-Lopez LE, Vogel JA, Askew EW, Marchitelli LJ, Hoyt RW, Gordon CC. 1994. Lower limit of body fat in healthy active men. J Appl Physiol 77(2):933–940.
Garrow JS. 1982. New approaches to body composition. Am J Clin Nutr (5):1152–1158.
Gelfand RA, Hendler R. 1989. Effect of nutrient composition on the metabolic response to very low calorie diets: Learning more and more about less and less. Diabetes Metab Rev 5(1):17–30.
Grande F, Keys A. 1980. Body weight, body composition and calorie status. In: Goodhart RS, Shils ME, eds. Modern Nutrition in Health and Disease. 6th ed. Philadelphia: Lea & Febiger. Pp. 3–34.
Hehir P. 1922. Effects of chronic starvation during the siege of Kut. Br Med J 1:865–869.
Heilbronn LK, Ravussin E. 2003. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am J Clin Nutr 78(3):361–369.
Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. 1986. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 35(2):155–164.
Hoffer LJ. 1999a. Evaluation of the adaptation to protein restriction in humans. In: El-Khoury AE, ed. Methods for the Investigation of Amino Acid and Protein Metabolism. Boca Raton, FL: CRC Press. Pp. 83–102.
Hoffer LJ. 1999b. Metabolic consequences of starvation. In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease. Baltimore, MD: Williams & Wilkins. Pp. 645–665.
Hoffer LJ. 2003. Protein and energy provision in critical illness. Am J Clin Nutr 78(5):906–911.
IOM (Institute of Medicine). 1995. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 41–54.
IOM. 1999. The Role of Protein and Amino Acids in Sustaining and Enhanding Performance. Washington, DC: National Academy Press.
IOM. 2002. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press.
Kamel KS, Cheema-Dhadli S, Shafiee MA, Halperin ML. 2004. Dogmas and controversies in the handling of nitrogenous wastes: Excretion of nitrogenous wastes in human subjects. J Exp Biol 207(Pt 12):1985–1991.
Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. 1950. The Biology of Human Starvation. Minneapolis, MN: The University of Minnesota Press.
Khanum S, Alam AN, Anwar I, Akbar Ali M, Mujibur Rahaman M. 1988. Effect of zinc supplementation on the dietary intake and weight gain of Bangladeshi children recovering from protein-energy malnutrition. Eur J Clin Nutr 42(8):709–714.
Lee L, Kumar S, Leong LC. 1994. The impact of five-month basic military training on the body weight and body fat of 197 moderately to severely obese Singaporean males aged 17 to 19 years. Int J Obes Relat Metab Disord 18(2):105–109.
Millward DJ. 2004. Macronutrient intakes as determinants of dietary protein and amino acid adequacy. J Nutr 134(6 Suppl):1588S–1596S.
Munro HN. 1964. General aspects of the regulation of protein metabolism by diet and hormones. In: Munro HN, Allison JB, eds. Mammalian Protein Metabolism. Vol. 1. New York: Academic Press. Pp. 381–481.
Pellett PL, Young VR. 1992. The effects of different levels of energy intake on protein metabolism and of different levels of protein intake on energy metabolism: A statistical evaluation from the published literature. In: Scrimshaw NS, Schurch B, eds. Protein-Energy Interactions. Proceedings of an I/D/E/C/G Workshop, Held in Waterville Valley, NH, USA, October 21 to 25, 1991. Lausanne, Switzerland: Nestlé Foundation. Pp. 81–121.
Piatti PM, Monti F, Fermo I, Baruffaldi L, Nasser R, Santambrogio G, Librenti MC, Galli-Kienle M, Pontiroli AE, Pozza G. 1994. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: Comparison to hypocaloric high-carbohydrate diet. Metabolism 43(12):1481–1487.
Prentice AM, Goldberg GR, Jebb SA, Black AE, Murgatroyd PR, Diaz EO. 1991. Physiological responses to slimming. Proc Nutr Soc 50(2):441–458.
Rudman D, Millikan WJ, Richardson TJ, Bixler TJ 2nd, Stackhouse J, McGarrity WC. 1975. Elemental balances during intravenous hyperalimentation of underweight adult subjects. J Clin Invest 55(1):94–104.
Shaw SN, Elwyn DH, Askanazi J, Iles M, Schwarz Y, Kinney JM. 1983. Effects of increasing nitrogen intake on nitrogen balance and energy expenditure in nutritionally depleted adult patients receiving parenteral nutrition. Am J Clin Nutr 37(6):930–940.
Stewart WK, Fleming LW. 1973. Features of a successful therapeutic fast of 382 days’ duration. Postgrad Med J 49(569):203–209.
Thomson TJ, Glasg MB, Runcie J, Miller V. 1966. Treatment of obesity by total fasting for up to 249 days. Lancet 2(7471):992–996.
Tipton KD, Wolfe RR. 2004. Protein and amino acids for athletes. J Sports Sci 22(1):65–79.
Van Itallie TB, Yang MU. 1977. Diet and weight loss. N Engl J Med 297(21):1158–1161.
Velthuis-te Wierik EJ, Westerterp KR, van den Berg H. 1995. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. Int J Obes Relat Metab Disord 19(5):318–324.
Waki M, Kral JG, Mazariegos M, Wang J, Pierson RN Jr, Heymsfield SB. 1991. Relative expansion of extracellular fluid in obese vs. nonobese women. Am J Physiol 261(2 Pt 1):E199–E203.
Walford RL, Mock D, Verdery R, MacCallum T. 2002. Calorie restriction in biosphere 2: Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol 57A(6):B211–B224.
Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA, Ravussin E. 2000. Energy metabolism after 2 y of energy restriction: The biosphere 2 experiment. Am J Clin Nutr 72(4):946–953.
Wolman SL, Anderson GH, Marliss EB, Jeejeebhoy KN. 1979. Zinc in total parenteral nutrition: Requirements and metabolic effects. Gastroenterology 76(3):458–467.
Vitamins C and E in the Prevention of Oxidative Stress, Inflammation, and Fatigue from Exhaustive Exercise
Maret G. Traber, Oregon State University
Angela Mastaloudis, Pharmanex
INTRODUCTION
This paper attempts to address the effects of exercise on oxidative stress and immune function and whether increasing the intake of antioxidants would reduce this stress, first in energy balance conditions and then in energy deficit conditions. Findings from our studies in ultramarathon runners may help answer this and the specific following questions:
-
What are the types and levels of direct antioxidants (e.g., vitamins C and E, carotenoids) that could be added to rations for short-term use by soldiers during high-tempo, stressful, repetitive combat missions to enhance performance and/or improve recovery during combat missions?
-
Would certain nonvitamin antioxidants help performance?
-
What is the impact of exercise on oxidative stress and immune function? Will increasing the intake of antioxidants reduce this stress in energy balance conditions and then in energy deficit conditions?
-
Is there any concern with megadoses of antioxidants—should soldiers be already taking supplements? For example, could megadoses of antioxidants negatively affect performance or adaptation?
OXIDATIVE STRESS GENERATED DURING EXERCISE
The human body continuously produces reactive oxygen species (ROS) as a result of normal metabolism in the mitochondria (Halliwell and Gutteridge, 1999). ROS are an unavoidable but necessary byproduct of cellular respiration. ROS also have a beneficial role in that leukocytes use radicals to help kill bacteria. This action produces a large increase in oxygen use, called a “respiratory burst,” to catalyze hydrogen peroxide with chloride ions to create a strongly antiseptic hypochlorite ion. Unfortunately, hypochlorite is also a strong oxidizing agent and can produce free radicals.
In response to endurance exercise, the body’s oxygen consumption can increase 10 to 20 times (Åstrand and Rodahl, 1986), while skeletal muscle oxygen consumption can increase 100 to 200 times (Halliwell and Gutteridge, 1999). This increased oxygen consumption may produce ROS in amounts that exceed the body’s antioxidant supplies. Clearly, exercise can cause oxidative stress resulting in lipid peroxidation (Alessio, 2000; Child et al., 1998; Duthie et al., 1990; Hessel et al., 2000; Marzatico et al., 1997; Mastaloudis et al., 2001; Rokitzki et al., 1994), DNA damage (Hartmann and Niess, 2000), and, possibly, protein oxidation (Alessio, 2000; Tirosh and Reznick, 2000).
Electrons “leaking” from the mitochondria during exercise are considered a main source of oxidative stress (Halliwell and Gutteridge, 1999). Other potential sources of ROS during exercise include enhanced purine oxidation, damage to iron-containing proteins, disruption of Ca2+ homeostasis (Jackson, 2000), and NADPH oxidase (Hessel et al., 2000). These exercise-induced ROS are also thought to modulate acute phase inflammatory responses (Cannon and Blumberg, 2000).
We found that during an ultramarathon race (32-mile forest trail through hilly terrain), as compared with a sedentary trial, deuterium-labeled vitamin E disappeared from the plasma faster (disappearance rates of 2.8 × 10–4 versus 2.3 × 10–4, p < 0.03) and lipid peroxidation increased (75 ± 7 pg/ml at prerace to 131 ± 17 at postrace (p < 0.02) (Mastaloudis et al., 2001). Lipid peroxidation
was assessed by measuring plasma F2-isoprostanes (F2-IsoPs), prostaglandin-like compounds produced by free-radical catalyzed lipid peroxidation of arachidonic acid (20:4 n-6, a long-chain polyunsaturated fatty acid) (Morrow et al., 1990). F2-IsoPs are widely accepted as sensitive and reliable measures of in vivo lipid peroxidation (Roberts, 1997). Importantly, F2-IsoPs have proatherogenic biological activity, including vasoconstriction and activation of platelet aggregation (Nieman et al., 2002; Roberts and Morrow, 2000). Moreover, they have been shown to recruit proatherogenic monocytes and induce monocyte adhesion (Leitinger et al., 2001). Thus, endurance exercise not only increases oxidative stress, but also increases a proatherogenic response.
MODULATION OF OXIDATIVE STRESS DURING AN ULTRAMARATHON BY VITAMINS C AND E
Based on our findings that an ultramarathon race increases oxidative stress (Mastaloudis et al., 2001), we hypothesized that prior supplementation with antioxidants (vitamins C and E) would decrease oxidative stress during distance running (Mastaloudis, 2004; Mastaloudis et al., 2004a, b). If the antioxidant supplements decreased oxidative stress, they should decrease lipid peroxidation and inflammation, slow α-tocopherol use, decrease DNA damage, decrease muscle damage, and improve recovery. To test these hypotheses, we carried out a randomized, double-blind study in ultramarathon runners (n = 11 women, 11 men) who consumed either (1) antioxidants (AO) [1,000 mg vitamin C (500 mg twice daily) and 300 mg vitamin E (400 IU RRR-α-tocopheryl acetate)] or (2) matching placebos (PL) for seven weeks (six weeks before through one week after the race). The race was a 50 km (32 mile) ultramarathon that took place in the hills of Corvallis, Oregon. The study design is shown in Figure B-18.
Subject Characteristics and Plasma Antioxidant Concentrations
Subjects were approximately 40 years of age and were recreationally trained endurance runners. Complete descriptions of the subjects have been published (Mastaloudis, 2004; Mastaloudis et al., 2004b). Plasma α-tocopherol and ascorbic acid concentrations were similar in the two groups before supplementation (Mastaloudis, 2004). Following six weeks of supplementation, the PL-group plasma concentrations were unchanged. In contrast, the AO-group α-tocopherol plasma concentrations increased from 28 ± 2 to 45 ± 3 µM (p < 0.0001) and were higher than in the PL group (p < 0.0007). Similarly, the AO group had a higher ascorbic acid plasma concentration, 121 ± 9 µM, than did the PL group, 78 ± 9 µM (p < 0.0007) following supplementation. Note that in both the PL and AO groups the ascorbic acid concentrations are well into the range for repleted subjects (Levine et al., 1996, 2001).
All subjects completed the race. Run times and intensity were similar among
FIGURE B-18 Study Design. Subjects (n = 22) were randomly assigned in a double-blind fashion to one of two groups: (1) PL [(1,000 mg of citric acid (500 mg twice daily) and 300 mg of soybean oil] or (2) AO [1,000 mg ascorbic acid (500 mg twice daily) and 300 mg RRR-α-tocopheryl acetate]. Blood samples were obtained before supplementation (baseline); after three weeks of supplementation (compliance); 24, 12, and 1 hours before the race. Samples were also taken at mid-race at kilometer 27 (~ 5 h), immediately postrace, 2 h postrace (approximately 10 h), and daily for six days postrace (1–6 days, 24–144 h). All samples were fasting morning blood draws except 12 h, mid-, post-, and two h post-race.
NOTE: AO = antioxidant; PL = placebo.
SOURCE: Summarized from Mastaloudis (2004); Mastaloudis et al. (2004a), used with permission from Elsevier.
treatment groups and genders: 7.1 ± 0.2 h at a pace of 13.7 ± 0.4 minutes per mile and a heart rate of 146 ± 2 beats per minute. Energy expenditure was calculated based on the average heart rate during the run and the corresponding oxygen consumption (VO2), multiplied by the time it took each subject to finish the race (Mastaloudis et al., 2004b). Energy expenditure was approximately 7,000 kcal for men and 5,000 kcal for women; both consumed about 2,000 kcal on race day and were in caloric deficit (Table B-6).
TABLE B-6 Race Results
|
|
Women |
Men |
||
|
AO (n = 6) |
PL (n = 5) |
AO (n = 6) |
PL (n = 5) |
|
|
Run Time (hr) |
7.1 ± 0.4 |
7.3 ± 0.6 |
6.8 ± 0.4 |
6.8 ± 0.2 |
|
Energy Expenditure (kcal) |
4,997 ± 207 |
4,958 ± 187 |
6,928 ± 558 |
7,006 ± 297 |
|
Energy Intakea (kcal) |
1,844 ± 137 |
2,040 ± 221 |
2,530 ± 325 |
2,468 ± 279 |
|
Vitamin E Intake (mg) |
3 ± 1 |
2 ± 1 |
3 ± 1 |
4 ± 1 |
|
Vitamin C Intake (mg) |
39 ± 14 |
32 ± 9 |
28 ± 5 |
17 ± 5 |
|
NOTE: AO = antioxidant; PL = placebo. aWomen versus men p < 0.05. SOURCE: Mastaloudis et al. (2004b), used with permission from Elsevier. |
||||
Vitamins C and E intakes from foods consumed during race day were compiled. During the run vitamin C intake was < 50 mg for most subjects, and vitamin E intake was < 5 mg.
Vitamin C, Vitamin E, and Lipid Peroxidation
Plasma F2-isoprostanes (F2-IsoP) concentrations increased in the PL group in response to the race, but prior supplementation with vitamins C and E completely suppressed the increase in the AO group (Figure B-19) (Mastaloudis et al., 2004a). At postrace, when oxidative stress was maximal, F2-IsoP concentrations were inversely correlated with both the ratio for α-tocopherol:lipids (R = −0.61, p < 0.003) and with ascorbic acid (R = −0.41, p = 0.05) (Mastaloudis et al., 2004a), providing further documentation that antioxidants were responsible for preventing lipid peroxidation.
Both men and women in the PL group responded to the run with similar F2-IsoP concentration increases (Mastaloudis et al., 2004a). However, men’s and

FIGURE B-19 Antioxidant supplementation prevents the increase in lipid peroxidation as observed in ultramarathon runners. Plasma F2-isoprostanes concentrations increased from pre- to postrace only in the placebo (PL) group (28 ± 2 to 41 ± 3 pg/ml; p < 0.01). Additionally, postrace F2-isoprostanes concentrations were significantly higher in the PL group than in the antioxidant group (p < 0.001).
SOURCE: Mastaloudis et al. (2004a), used with permission from Elsevier.
women’s responses were markedly different during recovery. In PL women, F2-IsoP concentrations returned to baseline within two hours postrace, while in PL men, higher F2-IsoP concentrations persisted for the duration of the study (six days) (Mastaloudis et al., 2004a). This difference in oxidative stress in men and women has been observed previously. Healthy young men compared with age-matched women had greater concentrations of markers of oxidative stress (plasma TBARS [thiobarbituric acid reactive substances] and urinary 8-Iso-PGF2). Following antioxidant supplementation (600 mg vitamin C and 300 mg vitamin E daily for 4 weeks), levels of lipid peroxidation markers were normalized to concentrations similar to those observed in the women (Ide et al., 2002). Thus, men in comparison with women are subjected to continued higher oxidative stress in the absence of antioxidant supplementation.
Cytokine Levels after the Race
Very few studies have examined the effects of antioxidants on both exercise-induced oxidative stress and inflammation (Childs et al., 2001; Nieman et al., 2002). We found that AO supplementation had no effect on exercise-induced increases in tumor necrosis factor (TNF)-a, interleukin (IL)-6, C-reactive protein (CRP), or IL-1, despite the finding that increases in lipid peroxidation were prevented (Mastaloudis et al., 2004a). Similarly, ascorbic acid (1,500 mg/day) consumption for one week before an ultramarathon did not prevent exercise-induced increases in plasma F2-IsoPs, lipid hydroperoxides, or IL-6 (proinflammatory cytokine) (Nieman et al., 2002). Postexercise supplementation with vitamin C (approximately 1,000 mg/day) and n-acetyl-cysteine for one week also had no effect on exercise-induced IL-6 increases (Childs et al., 2001). Army recruits that participated in an intensive 48-hour final military endurance exercise were also shown to have increased CRP, but the effect of antioxidants were not tested (Brull et al., 2004). In general, antioxidants do not modulate increases in cytokine concentrations in response to exercise.
Assessing DNA Damage
The ultramarathon was a sufficiently strenuous exercise that, by mid-race, the runners exhibited DNA damage as assessed with the comet assay (a whole-cell electrophoresis assay that estimates DNA damage) (Mastaloudis et al., 2004b). However, the proportion of cells with DNA damage returned to baseline by the end of the race, declined below baseline values two days after the race, and remained low for six days after the race (Mastaloudis et al., 2004b). Both men and women within each treatment group had similar circulating AO levels, but the women had a higher proportion of DNA damage after the race. In addition, the women in the AO group were more protected, showing a decrease in the
proportion of DNA damage on the day following the ultramarathon race, while men experienced little benefit.
Overall, this exercise appeared to induce a temporary increase in DNA damage. This increase, however, has no apparent adverse effects and may be beneficial because it appears to induce the replacement of damaged cells.
Muscle Damage Following Running
Runners experienced muscle damage after the ultramarathon: deficits in maximal force production by the knee extensors and flexors were documented (Mastaloudis et al., in press). Prior supplementation with vitamins C and E did not prevent muscle damage or fatigue or improve recovery. The ultramarathon run may have been so damaging that it overwhelmed the protective effects of the antioxidants. For example, vitamin C was found to be protective in a more moderate exercise protocol (60 minutes of box-stepping exercise), in which supplementation enhanced the rate of recovery from maximal force deficit (Jakeman and Maxwell, 1993).
Plasma markers of muscle damage were increased by the endurance exercise in our study (Mastaloudis et al., in press) and were unaffected by AO supplementation. Both lactate dehydrogenase (LDH) and creatine kinase (CK) increased in response to the race; LDH peaked at postrace and CK reached maximal values 2 h and 1 day postrace; neither was affected by AO treatment. These observations are in agreement with others. There was no influence on increased CK concentrations in response to a 90 minute treadmill run by two weeks prior supplementation with 500 mg vitamin C and 400 mg vitamin E (Petersen et al., 2001). Our findings are in contrast, however, with other studies (Rokitzki et al., 1994) that reported prior supplementation with 200 mg vitamin C and 400 IU vitamin E for 4.5 weeks diminished increases in CK following a 90 km ultramarathon.
Our study also showed that vitamins C and E did not prevent LDH increases after distance running. However, daily supplementation with 1,200 IU α-tocopherol for four weeks prior to running diminished LDH increases following six successive days of running (Itoh et al., 2000), suggesting that a larger dose of vitamin E is required to reduce muscle damage resulting from endurance exercise. The results seem influenced not only by the level of exercise but also by the amount, duration, and type of supplemental AO.
Endogenous Mechanisms to Increase Antioxidant Defenses
Plasma ascorbic acid concentrations increased in both the AO and PL groups during the 50 km ultramarathon run, with significant increases at mid-race, and at postrace compared with levels measured prerace; levels returned to prerace values two hours after the race (Mastaloudis et al., 2004a, b). Increases in plasma
ascorbic acid in response to vigorous exercise have been reported in some (Duthie et al., 1990; Kaikkonen et al., 1998; Mastaloudis et al., 2001; Petersen et al., 2001; Rokitzki et al., 1994; Viguie et al., 1993), but not all (Meydani et al., 1993; Peters et al., 2001a, b) studies. Exercise-related increases in circulating cortisol has been suggested to promote efflux of ascorbic acid from the adrenal gland and the mobilization of ascorbic acid from other tissue sites such as leukocytes or erythrocytes (Gleeson et al., 1987). Similarly, some (Nieman et al., 2000; Peters et al., 2001a, b), but not all (Nieman et al., 2002), studies demonstrated that exercise-related increases in circulating cortisol diminish with vitamin C supplementation. Taken together, these findings suggest that oxidative stress may regulate cortisol secretion.
Plasma uric acid concentrations also increased in response to the run, consistent with the findings of others (Hellsten et al., 1997; Liu et al., 1999; Rokitzki et al., 1994), and the increase may be explained by enhanced purine oxidation resulting from exercise (Hellsten et al., 1997; Liu et al., 1999; Rokitzki et al., 1994).
Concurrent increases in plasma ascorbic and uric acids may reflect the body’s response to extreme exercise by enhancing AO defenses, including increased AO enzymes (Marzatico et al., 1997) and AO nutrients (Child et al., 1998; Rokitzki et al., 1994; Viguie et al., 1993).
Some studies investigating the effects of vitamin E supplementation on endurance runners have reported increases in plasma α-tocopherol concentrations in both the AO and the PL groups following exercise (Buchman et al., 1999; Rokitzki et al., 1994; Vasankari et al., 1997). We (Mastaloudis et al., 2004a) observed increased plasma α-tocopherol concentrations during exercise in the AO group only. Plasma tocopherols are transported entirely within lipoproteins and fluctuate with lipoprotein concentrations (Traber and Jialal, 2000) and after correcting α-tocopherol for lipids, no significant changes in α-tocopherol/lipid concentrations were observed in either group (Mastaloudis et al., 2004a). In the only other study to report both α-tocopherol and α-tocopherol/lipid concentrations (Buchman et al., 1999), fluctuations in lipoproteins explained the differential responses in the AO and PL groups. Thus, the increase in plasma α-tocopherol concentrations during the exercise may be a result of fluctuations in lipoprotein concentrations rather than an actual increase due to exercise.
Increases and Depletions of Plasma Lipids
Plasma lipids (total cholesterol plus triglycerides) increased during the race, but decreased to below prerace levels two hours after the race and remained low for three days (Figure B-20). These data suggest that extreme endurance exercise depletes lipid stores that subsequently remain depleted for several days. Rations should provide adequate fat content and total kcals to ensure that tissue stores of lipids are maintained for endurance and multi-day physical work in the field.
FIGURE B-20 Plasma lipids increase during the race but are depleted for three days postrace. No differences in plasma total lipids (total cholesterol plus triglycerides) were observed between genders or among treatment groups within each gender.
NOTE: *Compared with prerace levels, plasma total lipids were higher at mid-race (p < 0.004), but were lower two hours (p < 0.01), one day (p < 0.0001), two days (p < 0.0001), and three days (p < 0.01) postrace.
SOURCE: Adapted from Mastaloudis (2004), used with permission.
Conclusions
ROS generated in response to exercise cause oxidative damage (Mastaloudis et al., 2001, 2004a), stimulate an inflammatory response (Vassilakopoulos et al., 2003), and damage skeletal muscle (Cannon and Blumberg, 2000; Sjodin et al., 1990). Hypothetically, AO supplementation could prevent exercise-induced oxidative damage, inflammation, and muscle damage. We found, however, that while supplementation with vitamins C and E prevented increases in lipid peroxidation in response to endurance exercise (Mastaloudis et al., 2004a), they had no apparent effect on DNA damage (Mastaloudis et al., 2004b), inflammation (Mastaloudis et al., 2004a), or muscle damage (Mastaloudis et al., in press). These results suggest that the mechanism of oxidative damage is operating independently of the inflammatory and muscle damage responses (Nieman et al., 2002).
Preventing production and enhancing clearance of F2-IsoPs may be more beneficial than preventing inflammation. F2-IsoPs have demonstrated proatherogenic biological activity, including vasoconstriction and activation of platelet aggregation (Nieman et al., 2002; Roberts and Morrow, 2000). In addition, they are known to recruit proatherogenic monocytes and induce monocyte
adhesion (Leitinger et al., 2001). In contrast, the muscle damage-induced inflammatory response stimulates recovery from exercise by inducing regeneration of damaged tissue and recruitment of satellite cell proliferation (Malm, 2001). In our studies, AO supplementation proved to prevent the damaging increase in lipid peroxidation without influencing inflammation. This is especially important because preventing exercise-induced inflammation could inhibit muscular adaptation to physical activity, the so-called “training effect” of exercise.
Supplementation of both vitamins C and E appears beneficial in exercise. Certainly, the ultramarathon runners were adequately nourished with respect to vitamin C, but studies in cigarette smokers suggest that vitamin C is necessary to maintain vitamin E concentrations (Bruno et al., 2005). Prior supplementation with vitamin E alone may be possible, but the assault ration should contain vitamin C not only for its own benefits but also for maintaining vitamin E concentrations.
In healthy adults, there are no obvious adverse effects of either vitamin C or E supplements alone or in combination (Hathcock et al., 2005), and the Tolerable Upper Intake Levels are 2,000 and 1,000 mg, respectively (IOM, 2000). A recent meta-analysis assessed the combined results of 19 clinical trials of vitamin E supplementation for various diseases reported that patients who took supplements of 400 IU/day or more were 6 percent more likely to die from any cause than those who did not take vitamin E supplements (Miller et al., 2005). However, three other meta-analyses that combined the results of randomized controlled trials designed to evaluate the efficacy of vitamin E supplementation in cardiovascular disease found no evidence that vitamin E supplementation up to 800 IU/day significantly increased or decreased cardiovascular disease mortality or all-cause mortality (Eidelman et al., 2004; Shekelle et al., 2004; Vivekananthan et al., 2003). At present, there is no convincing evidence that vitamin E itself increases the risk of death from cardiovascular disease or other causes. Therefore, AO vitamin supplements could be added to assault rations to prevent the adverse increase in lipid peroxidation as described in the ultramarathon runners in our study (Mastaloudis et al., 2004a).
ACKNOWLEDGMENTS
We thank the runners and the support from National Institute of Environmental Health Sciences Grant ES11536 and National Institutes of Health Grant DK59576. The following individuals provided supplements and ergogenic aids: Jim Clark, Cognis Health and Nutrition; Klaus Krämer, BASF; Tim Corliss, Clif Bar; Jeff Zachwieja, Gatorade.
The following individuals played key roles in our study: Dawn W. Hopkins, The Department of Exercise and Sport Science, Linus Pauling Institute, Oregon State University, Corvallis; Scott Leonard, Linus Pauling Institute, Oregon State University, Corvallis; David Yu, Linus Pauling Institute, Oregon State Univer-
sity, Corvallis; Robert P. O’Donnell, Statistics, Oregon State University, Corvallis; Roderick H. Dashwood, Linus Pauling Institute, Oregon State University, Corvallis; Balz Frei, Linus Pauling Institute, Oregon State University, Corvallis; Jason D. Morrow, Departments of Medicine and Pharmacology, Vanderbilt University School of Medicine, Nashville, Tennessee; and Sridevi Devaraj, Department of Pathology, the University of California Medical Center, Sacramento; Jeffrey J. Widrick, The Department of Exercise and Sport Science, Oregon State University, Corvallis.
REFERENCES
Alessio HM. 2000. Lipid peroxidation in healthy and diseased models: Influence of different types of exercise. In: Sen CK, Packer L, Hanninen, O, eds. 1st ed. Handbook of Oxidants and Antioxidants in Exercise. New York: Elselvier. Pp. 115–128.
Åstrand P-O, Rodahl K. 1986. Textbook of Work Physiology: Physiological Basis of Exercise. 3rd ed. New York: McGraw Hill.
Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, Sharma P, Lowe GDO, World MJ, Humphries SE, Hingorani AD. 2004. Human CRP gene polymorphism influences CRP levels: Implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol 23(11):2063–2069.
Bruno RS, Ramakrishnan R, Montine TJ, Bray TM, Traber MG. 2005. α-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am J Clin Nutr 81(1):95–103.
Buchman AL, Killip D, Ou C, Rognerud CL, Pownall H, Dennis K, Dunn JK. 1999. Short-term vitamin E supplementation before marathon running: A placebo-controlled trial. Nutr 15(4):278–283.
Cannon JG, Blumberg JB. 2000. Acute phase immune responses in exercise. In: Sen CK, Packer L, Hanninen O, eds. Handbook of Oxidants and Antioxidants in Exercise. 1st ed. New York: Elsevier. Pp. 177–194.
Child RB, Wilkinson DM, Fallowfield JL, Donnelly AE. 1998. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med Sci Sports Exerc 30(11):1603–1607.
Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. 2001. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med 31(6):745–753.
Duthie GG, Robertson JD, Maughan RJ, Morrice PC. 1990. Blood antioxidant status and erythrocyte lipid peroxidation following distance running. Arch Biochem Biophys 282(1):78–83.
Eidelman RS, Hollar D, Herbert PR, Lamas GA, Hennekens CH. 2004. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med 164(14):1552–1556.
Gleeson M, Robertson JD, Maughan RJ. 1987. Influence of exercise on ascorbic acid status in man. Clin Sci 73(5):501–505.
Halliwell B, Gutteridge JMC. 1999. Free Radicals in Biology and Medicine. 3rd ed. New York: Oxford University Press Inc.
Hartmann A, Niess AM. 2000. Oxidative DNA damage in exercise. In: Sen CK, Packer L, Hanninen O, eds. Handbook of Oxidants and Antioxidants in Exercise. 1st ed. New York: Elsevier. Pp. 195–217.
Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Johnston CS, Kelly FJ, Krämer K, Packer L, Parthasarathy S, Sies H, Traber MG. 2005. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr 81(4):736–745.
Hellsten Y, Tullson PC, Richter EA, Bangsbo J. 1997. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med 22(1/2):169–174.
Hessel E, Haberland A, Muller M, Lerche D, Schimke I. 2000. Oxygen radical generation of neutrophils: A reason for oxidative stress during marathon running? Clin Chim Acta 298(1-2): 145–156.
Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suimatsu N, Tsuchihashi M, Tamai H, Takeshita A. 2002. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 22(3):438–442.
IOM (Institute of Medicine). 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. Washington, DC: National Academy Press.
Itoh H, Ohkuwa T, Yamazaki Y, Shimoda T, Wakayama A, Tamura S, Yamamoto T, Sato Y, Miyamura M. 2000. Vitamin E supplementation attenuates leakage of enzymes following 6 successive days of running training. Int J Sports Med 21(5):369–374.
Jackson M. 2000. Exercise and oxygen radical production by muscle. In: Sen C, Packer L, Hanninen O, eds. 1st ed. Handbook of Oxidants and Antioxidants in Exercise. 1st ed. New York: Elsevier. Pp. 57–68.
Jakeman P, Maxwell S. 1993. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur J Appl Physiol Occup Physiol 67(5):426–430.
Kaikkonen J, Kosonen L, Nyyssonen K, Porkkala-Sarataho E, Salonen R, Dorpela H, Salonen JT. 1998. Effect of combined coenzyme Q10 and d-a-tocopheryl acetate supplementation on exercise-induced lipid peroxidation and muscular damage: A placebo-controlled double-blind study in marathon runners. Free Radic Res 29(1):85–92.
Leitinger N, Huber J, Rizza C, Mechtcheriakova D, Bochkov V, Koshelnick Y, Berliner JA, Binder BR. 2001. The isoprostane 8-iso-PGF(2α) stimulates endothelial cells to bind monocytes: Difference to thromboxane-mediated endothelial activation. FASEB J 15(7):1254–1256.
Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. 1996. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93(8):3704–3709.
Levine M, Wang Y, Padayatty SJ, Morrow J. 2001. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA 98(17):9842–9846.
Liu ML, Bergholm R, Makimattila S, Lahdenpera S, Valkonen M, Hilden H, Yki-Jarvinen H, Taskinen MR. 1999. A marathon run increases the susceptibility of LDL to oxidation in vitro and modifies plasma antioxidants. Am J Physiol 276(6 Pt 1):E1083–E1091.
Malm C. 2001. Exercise-induced muscle damage and inflammation: Fact or fiction? Acta Physiol Scand 171(3):233–239.
Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della Valle G. 1997. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint trained athletes. J Sports Med Phys Fitness 37(4):235–239.
Mastaloudis A. 2004. Inhibition of Exercise-Induced Oxidative Stress, Inflammation and Muscle Damage by Prior Supplementation with the Antioxidant Vitamins E and C. Ph.D. dissertation. Oregon State University, Corvallis.
Mastaloudis A, Leonard SW, Traber MG. 2001. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med 31(7):911–922.
Mastaloudis A, Morrow JD, Hopkins DW, Deveraj S, Traber MG. 2004a. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med 36(10):1329–1341.
Mastaloudis A, Traber MG, Carstensen K, Widrick J. In press. Antioxidants do not prevent muscle damage in response to an ultramarathon run. Med Sci Sports Exerc Jan. 2006.
Mastaloudis A, Yu TW, Frei B, Dashwood RH, Traber MG. 2004b. Endurance exercise results in DNA damage as detected by the comet assay. Free Radic Biol Med 36(8):966–975.
Meydani M, Evans WJ, Handelman G, Biddle L, Fielding RA, Meydani SN, Burrill J, Fiatarone MA, Blumberg JB, Cannon JG. 1993. Protective effect of vitamin E on exercise-induced oxidative damage in young and older adults. Am J Physiol 33(5 Pt 2):R992–R998.
Miller ER 3rd, Paston-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. 2005. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142(1):37–46.
Morrow J, Hill K, Burk R, Nammour T, Badr K, Roberts J. 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical catalyzed mechanism. Proc Natl Acad Sci USA 87(23):9383–9387.
Nieman DC, Henson DA, McAnulty SR, McAnulty L, Swick NS, Utter AC, Vinci DM, Opiela SJ, Morrow JD. 2002. Influence of vitamin C supplementation on oxidative and immune changes after an ultramarathon. J Appl Physiol 92(5):1970–1977.
Nieman DC, Peters EM, Henson DA, Nevines EI, Thompson MM. 2000. Influence of vitamin C supplementation on cytokine changes following an ultramarathon. J Interferon Cytokine Res 20(11):1029–1030.
Peters EM, Anderson R, Nieman DC, Fickl H, Iogessa V. 2001a. Vitamin C supplementation attenuates the increases in circulating cortisol, adrenaline, and anti-inflammatory polypeptides following ultramarathon running. Int J Sports Med 22(7):537–543.
Peters EM, Anderson R, Theron AJ. 2001b. Attenuation of increase in circulating cortisol and enhancement of the acute phase protein response in vitamin C-supplemented ultramarathoners. Int J Sports Med 22(2):120–126.
Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer-Kristensen J, Pedersen BK. 2001. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol 280(6):C1570–C1575.
Roberts J. 1997. The generation and actions of isoprostanes. Biochim Biophys Acta 1345(2): 121–135.
Roberts JL II., Morrow JD. 2000. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28(4):505–513.
Rokitzki L, Logemann E, Sagredos AN, Murphy M, Wetzel-Roth W, Keul J. 1994. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiol Scand 151(2):149–158.
Shekelle PG, Morton SC, Jungvig LK, Udani J, Spar M, Tu W, M JS, Coulter I, Newberry SJ, Hardy M. 2004. Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med 19(4):380–389.
Sjodin B, Hellsten Westing Y, Apple F. 1990. Biochemical mechanisms for oxygen free radical formation during exercise. Sports Med 10(4):236–254.
Tirosh O, Reznick O. 2000. Chemical bases and biological relevance of protein oxidation. In: Sen CK, Packer L, Hanninen O, eds. Handbook of Oxidants and Antioxidants in Exercise. 1st ed. New York: Elsevier. Pp. 89–114.
Traber M, Jialal I. 2000. Measurement of lipid-soluble vitamins-further adjustment needed? Lancet 355(9220):2013–2014.
Vasankari TJ, Kujala UM, Vasankari TM, Vuorimaa T, Ahotupa M. 1997. Increased serum and low-density-lipoprotein antioxidant potential after antioxidant supplementation in endurance athletes. Am J Clin Nutr 65(4):1052–1056.
Vassilakopoulos T, Karatza MH, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C. 2003. Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol 94(3):1025–1032.
Viguie C, Frei B, Shigenaga M, Ames B, Packer L, Brooks G. 1993. Antioxidant status and indexes of oxidative stress during consecutive days of exercise. J Appl Physiol 75(2):566–572.
Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. 2003. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 361(9374):2017–2023.
Zinc, Magnesium, Copper, Iron, Selenium, and Calcium in Assault Rations: Roles in Promotion of Physical and Mental Performance
Henry C. Lukaski and James G. Penland, USDA-ARS Grand Forks Human Nutrition Research Center
INTRODUCTION
Military personnel are exposed to environmental, physical, and mental stressors during combat and require adequate dietary intakes of energy, water, and micronutrients for optimal performance. During deployment, soldiers decrease food consumption up to 50 percent, resulting in a suboptimal intake of energy and micronutrients (Baker-Fulco, 1995). Thus, reduced food intake and increased losses of minerals during assault operations suggest the need to evaluate mineral nutrition of military personnel (Shippee, 1993).
Only male soldiers participate in first-strike assault operations. These missions occur for repetitive brief periods (three to seven days), followed by short recovery periods (one to three days) that can last for months. Under these circumstances, it is unlikely that such brief durations of reduced micronutrient intake will elicit severe mineral deficiencies. However, repeated bouts of first-strike assaults without adequate replenishment of minerals may predispose soldiers to a reduced mineral nutritional status and result in impaired physiological and psychological function and performance.
This review addresses the mineral needs of male soldiers. It does so by comparing the prevalence of inadequate mineral intakes of civilian and military men and summarizing the effects of restricted and supplemental mineral intakes on their performance. Additionally, it provides a strategy for increasing food and nutrient intake to promote optimal performance of the male combat soldier. Findings from studies of women with documented mineral depletion are described to highlight impairments in performance that would be applicable to men with similarly reduced mineral nutritional status.
The following are specific questions that this review attempts to answer:
-
Which of the minerals might reach deficit levels, given the high-stress, high-intensity scenario? Given existing information that suggests that soldiers probably consume insufficient amounts of zinc during military
-
operations, and that they could become zinc deficient, are there potential effects on health or performance?
-
What are the types and levels of cofactors in antioxidant and other biochemical reactions with high metabolic flux (e.g., zinc, manganese, copper, selenium, or others) that could be added to assault rations to enhance performance during combat missions? Does the presence of preexisting malnutrition make a difference?
-
Is there any concern with taking megadoses of minerals; should soldiers be already taking supplements?
LIMITING MINERALS IN THE DIET
Estimating a group’s adequate intake of a nutrient is achieved through comparison with the Estimated Average Requirement (EAR). If an EAR is not available, then the Adequate Intake (AI) is used. The Recommended Dietary Allowance (RDA) is not used to assess the adequacy of nutrient intakes by groups (IOM, 2000a).
Inadequate Dietary Mineral Intakes in Adults
Epidemiological surveys using dietary recall reveal (NHANES III, Continuing Survey of Food Intake of Individuals, and US Food and Drug Administration Total Diet Study) that intakes of some minerals by the US population do not meet the Dietary Recommended Intake (DRI) recommendations. Among men ages 19 to 50 years, 50 percent did not meet the AI for calcium or the EAR for magnesium (Table B-7). Inadequate zinc intakes were found among 10 percent of men, but iron intakes generally met the EAR (6 mg) (IOM, 1997, 2000b, 2001).
Nutritional surveys reveal wide-ranging estimates of possible copper deficiency in the US population (IOM, 2001). Although findings of the NHANES III and Continuing Survey of Food Intake of Individuals showed no men with copper intakes less than the EAR, the US Food and Drug Administration Total Diet Study revealed that 25 percent of men consume less than the EAR for copper. Analyses of diets from ten multinational studies indicated that copper intakes were less than the EAR in 11 percent of the population (Klevay et al., 1993). Another study analyzed duplicate diets of randomly selected adults in Baltimore, Maryland, and found that 36 percent of the adults had dietary copper intakes less than the EAR (Pang et al., 2001).
Inadequate Dietary Mineral Intakes in Military Personnel
Based on observation of the foods soldiers consume (Rose et al., 1987), soldiers frequently have inadequate intakes of certain minerals. Zinc and magne-
TABLE B-7 Dietary Reference Intakes of Minerals in Diets of American Men Ages 19–50 Years and Actual Intakes
|
|
Dietary Reference Intake |
Not Attaining (%) |
||
|
Mineral |
RDA |
EAR or AI |
RDA |
EAR or AI |
|
Calcium |
|
1,000 mga |
|
50a |
|
Copper |
900 mg |
700 mg |
5 |
0 |
|
Iron |
8 mg |
6 mg |
10 |
0 |
|
Magnesium |
400–420 mg |
350 mg |
50 |
50 |
|
Selenium |
55 mg |
45 mg |
0 |
0 |
|
Zinc |
11 mg |
9.4 mg |
20 |
10 |
|
NOTE: The percentage of men not attaining the RDA/EAR/AI has been assessed using the US national survey intake data found in the appendices of the source below. An RDA is set from an EAR; calcium does not have an EAR therefore also no RDA. AI = Adequate Intake; EAR = Estimated Average Requirement; RDA = Recommended Dietary Allowance. aIndicates the value is an AI rather than an EAR. SOURCE: IOM (1997, 2000b, 2001). |
||||
sium intakes of male marine engineers fed standard rations were less than the recommended amounts (Tharion et al., 2000). The addition of hot meals to standard rations, compared with provision of only standard rations, promoted adequate mineral intakes for the soldiers (Thomas et al., 1995). However, feeding a customized, high-carbohydrate diet, compared with standard rations, during training in a hot, humid environment decreased the proportion of elite male soldiers with inadequate intakes of calcium (50 versus 95 percent) but not magnesium (75 versus 75 percent) or zinc (20 versus 25 percent) (personal communication, S. Montain, USARIEM, August 9, 2004; Savannah Ranger Study, 1996). Male hospital personnel were studied during operational training to compare the effects of meal-based versus standard rations on the resulting adequacy of mineral intakes (Baker-Fulco et al., 2002). Compared with the standard ration, the high-carbohydrate, meal-based ration was associated with more soldiers consuming adequate calcium, similar percentages consuming adequate magnesium, but fewer consuming adequate zinc (Table B-8). Thus, magnesium, zinc, and calcium intake levels are limited in civilian and military men, and data on copper and selenium in soldiers are lacking.
FUNCTIONAL RESPONSES TO DIFFERENCES IN MINERAL INTAKES
Mineral intakes affect human biological functions. Controlled studies of restriction and supplementation of mineral intakes reveal deficits and enhance-
TABLE B-8 Male Hospital Workers During Training Consumptiona of Limiting Minerals
ments, respectively, in measures of physiological and psychological function and performance.
Zinc
Zinc is required for the structure and activity of more than 300 enzymes (Vallee and Falchuk, 1993). Because zinc functions in all physiological systems, adequate zinc status is needed for optimal physiological and psychological performance.
Low zinc status impairs muscle and cardiorespiratory functions (Table B-9). Adolescent gymnasts with decreased serum zinc concentrations, compared with age-matched, nonathletic controls, experienced reduced muscle strength (Brun et al., 1995). Male athletes with low, in contrast with normal, serum zinc concentrations had decreased power output (physical work capacity, watts) and increased blood lactate concentrations during peak exercise tests (Khaled et al., 1997).
Controlled feeding studies with low zinc intakes show adverse physiological function. Men fed severely zinc deficient diet (< 1 versus 12 mg/day of zinc) had decreased muscle strength and work capacity (Van Loan et al., 1999). Physically active men fed a moderately low zinc diet (5 versus 18 mg/day) had impaired cardiorespiratory function during peak and prolonged submaximal exercise (Lukaski, 2005).
Low zinc intakes and status have been related to deficits in memory, perception, attention, and motor skills, while zinc supplementation has improved memory (Table B-10). Low serum zinc in otherwise well-nourished men fed 5, compared with 15, milligrams per day of zinc was associated with faster, but less accurate, performance on memory for digits and several perceptual tasks (Tucker and Sandstead, 1984). Contrasted to a control period when men were fed adequate zinc (10 mg/day), low zinc intakes (1, 2, 3, or 4 mg/day) resulted in
TABLE B-9 Effects of Selected Minerals on Measures of Physical Function and Performance
|
Mineral |
Subjects |
Study Design |
Outcome |
Reference |
|
Zinc |
Adolescents |
Observation |
↓Strength |
Brun et al., 1995 |
|
Men |
Observation |
↓Power |
Khaled et al., 1997 |
|
|
Men |
5 vs. 18 mg/day |
a↓Aerobic capacity, ↑Ventilation, ↑HR |
Lukaski, 2005 |
|
|
Men |
<1 vs. 12 mg/day as zinc sulfate |
a↓Strength |
Van Loan et al., 1999 |
|
|
Magnesium |
Men |
+250 mg/day as magnesium oxide |
b↑Endurance, ↓Oxygen use |
Brilla and Gunther, 1995 |
|
Men |
+250 mg/day as magnesium oxide |
b↑Strength |
Brilla and Haley, 1992 |
|
|
Men |
+370 mg/day as magnesium pidolinate |
b↓Oxygen use, ↓Lactate |
Golf et al., 1994 |
|
|
Copper |
Men |
0.9 vs. 2 mg/day as copper amino acid chelate |
b↑Oxygen use, ↑HR, ↑Lactate, ↓Muscle cytochrome c oxidase activity |
Lukaski and Johnson, 2005 |
|
Iron |
Women |
+100 mg/day as ferrous sulfate |
↑Training |
Brownlie et al., 2002 |
|
Women |
+100 mg/day as ferrous sulfate |
↑Strength |
Brutsaert et al., 2003 |
|
|
Women |
+100 mg/day as ferrous sulfate |
↓Time trial |
Hinton et al., 2000 |
|
|
NOTE: See text for details. aEffect of lower, compared with higher, intake. bEffect of supplement. |
||||
decreased performance on psychomotor (tracking and connect-the-dots), attention (orienting and misdirection), memory (letter, shape, and cube recognition), perceptual (search-count), and spatial (maze) tasks. However, there was no evidence of a “dose–response” effect of dietary zinc on cognitive performance (Penland, 1991). Reaction times during word recall were significantly slower when men were fed low amounts of zinc (5 versus 14 mg/day) (Kretsch et al., 2000).
Magnesium
Magnesium, a cofactor in more than 300 enzyme reactions in which food is metabolized and new products are formed, regulates many biological functions (Shils, 1997). Thus, it is a potentially limiting nutrient for human performance.
TABLE B-10 Effects of Selected Minerals on Measures of Psychological Function and Performance
|
Mineral |
Subjects |
Study Design |
Outcome |
Reference |
|
Zinc |
Men |
5 vs. 15 mg/day |
a↓Memory, ↓Perception |
Tucker and Sandstead, 1984 |
|
Men |
5 vs. 14 mg/day |
↓Memory |
Kretsch et al., 2000 |
|
|
Men |
1, 2, 3, 4 vs. 10 mg/daya |
a↓Psychomotor, ↓Attention, ↓Perception, ↓Memory |
Penland, 1991 |
|
|
Magnesium |
Men |
Observation |
↓EEG alpha |
Delorme et al., activity 1992 |
|
Copper |
Women |
1 vs. 3 mg/day |
a↑Sleep time, ↑Confusion, ↑Latency to sleep, ↑Depression, ↓Feeling rested |
Penland, 1988 |
|
Women |
1 vs. 3 mg/day |
a↓Short-term memory, ↑Distraction |
Penland et al., 2000 |
|
|
Iron |
Women |
+90 mg/day of ferrous fumarate |
b↑Attention, ↑Memory |
Groner et al., 1986 |
|
Women |
Not reported |
↑Learning, ↑Memory |
Murray-Kolb et al., 2004 |
|
|
Women |
5 vs. 15 mg |
a↑Sleep duration, ↑Awakenings |
Penland, 1988 |
|
|
Men |
Not reported |
↓Alertness, ↓Visual detection |
Tucker et al., 1981, 1982, 1984 |
|
|
Selenium |
Women |
+100 mg/day |
b↓Anxiety, ↓Depression |
Benton and Cook, 1991 |
|
Men |
30 vs. 230 µg/day |
b↓Confusion, ↓Depression |
Penland and Finley, 1995 |
|
|
↑Positive mood |
Finley and Penland, 1998 |
|||
|
NOTE: See text for details. aEffect of lower, compared with higher, intake. bEffect of supplement. |
||||
The dietary restriction of magnesium reduced the magnesium status and impaired physiological function and performance in untrained adults. As shown in Table B-9, physically active men supplemented with magnesium (250 mg/day for four weeks) experienced increased endurance and decreased oxygen use during submaximal exercise (Brilla and Gunter, 1995).
Supplementation with magnesium salts improved cellular function. Men receiving 370 mg of magnesium daily for four weeks had reduced serum lactate concentration and oxygen uptake during a progressive rowing test (Golf et al., 1994). Magnesium supplementation (250 mg/day for seven weeks) increased muscle strength and power in men participating in a strength training regimen (Brilla and Haley, 1992). Although modest strength gains occurred with magnesium intakes of 540 mg/day (290 mg from diet and 250 mg from supplements), increases were achieved at intakes greater than the RDA of 420 mg/day (IOM, 1997).
Whereas severe magnesium deficiency has been associated with numerous neurological and psychological disturbances (Dubray and Rayssiguier, 1997), few reports have described neuropsychological effects from marginal magnesium restriction (Delorme et al., 1992; Table B-10). Male athletes with low, compared with normal, erythrocyte magnesium had significantly less alpha activity in the right occipital region as recorded by an electroencephalogram (EEG) (Delorme et al., 1992), which suggests that magnesium is involved in regulating cortical activity related to motor function.
Copper
Copper is a cofactor of many metalloenzymes and, thus, copper status may affect diverse biological functions. It regulates iron absorption, neurotransmitter metabolism, antioxidant defense, and oxygen use. Longitudinal studies of diet and physical training showed an adaptation in antioxidant protection (Table B-9). Collegiate swimmers training for competition increased dietary copper from 1 to 1.4 mg/day, resulting in an increased erythrocyte superoxide dismutase activity and no change in plasma copper, whereas nontraining control subjects did not change enzyme activity at the same intakes of copper (Lukaski et al., 1989, 1990). Adequate copper intake is needed for adaptation in antioxidant protection during physical training.
Marginal copper intake reduces energy metabolism. Men fed less (0.9 versus 1.6 mg/day) dietary copper showed increased oxygen use, heart rate, and postexercise lactate concentrations during submaximal exercise (Lukaski and Johnson, 2005). Muscle cyctochrome c oxidase activity decreased with the marginal dietary copper (Table B-9). Thus, restricted copper intake appears to increase energy use during low-level work.
Many of the studies to measure cognition and behavioral effects of copper deficiencies have been conducted with women. Restricted dietary copper has been associated with impaired verbal memory, and disrupted sleep and mood states in women (Table B-10). Increased sleep times, longer latency to sleep, and feeling less rested upon awakening as well as increased confusion, depression, and total mood disturbances were reported when dietary copper was low (0.8 versus 2 mg/day) (Penland, 1988). Short-term memory and immediate recall of verbally presented words (list recall) worsened with low dietary copper (1 versus
3 mg) (Penland et al., 2000). Low copper intakes were also associated with increased difficulty in discriminating between relevant and irrelevant responses. Sufficient plasma copper and ceruloplasmin are associated with improved verbal and long-term memory, increased clustering of verbal material (strategy), and fewer distractions (Penland et al., 2000).
Iron
Iron is needed to deliver oxygen to tissues and to use oxygen at the cellular level. It serves as a functional component of iron-containing proteins needed for efficient energy use as well as for catecholamine metabolism.
Whereas the adverse effects of iron deficiency anemia on work capacity and endurance are well established (Tobin and Beard, 1997), there is growing interest in the functional effects of tissue iron depletion without anemia. Iron deficiency anemia in men is rare, except with excessive blood loss. Estimates of tissue iron depletion in men range from 5 to 15 percent despite only a 5 percent prevalence of low iron intake (IOM, 2001). Accumulating evidence shows that a low-iron status without anemia (e.g., low serum ferritin or transferrin saturation) elicits impaired physical performance, including time used to complete standard running distance (Hinton et al., 2000), endurance training adaptation (Brownlie et al., 2002), and muscle function (Brutsaert et al., 2003) that are all ameliorated with increased iron intake.
Male soldiers participating in Ranger training maintained normal hematology with increased ferritin and decreased serum iron (Shippee, 1993). Various measures of muscle strength and endurance decreased with the training. Because food intake as well as body and fat-free mass concomitantly decreased, one may conclude that reduced iron status contributed to the impaired physical performance.
Iron status has been related to attention, memory, and learning. Iron supplementation in young women (180 mg/day for 30 days) improved attention and short-term memory (Groner et al., 1986), while verbal learning and memory improved significantly in nonanemic, iron-deficient women supplemented similarly with iron (Bruner et al., 1996). Accuracy and reaction times on tasks measuring attention, memory, and learning were improved in women with the highest, compared with the lowest, ferritin and transferrin saturation in the absence of anemia (Murray-Kolb et al., 2004).
Sleep and mood disturbances have been related to dietary iron restriction. Nonanemic women and menstruating women fed low dietary iron (5 versus 15 mg/day) reported more frequent night-time awakenings and more total sleep duration (Penland, 1988). This low iron intake also increased reports of depression, fatigue, and total mood disturbances (Penland, 1989).
Select patterns of cortical activity (measured by EEG) have been predicted by iron status in nonanemic men. Consistent with depressed alertness, lower serum iron and ferritin were associated with more low-frequency EEG activity
and lower amplitude-evoked potentials in response to visual stimuli (Tucker and Sandstead, 1981; Tucker et al., 1982, 1984).
Selenium
Selenium acts through its association with proteins as an antioxidant and a regulator of thyroid hormone metabolism. The independent role of selenium in exercise performance and metabolism is not well understood because of its interdependence with vitamin E and other nutrients in antioxidant defense.
Endurance-trained men supplemented with selenium (180 µg/day as selenomethionine versus a placebo) significantly increased plasma selenium and glutathione peroxidase activity with no effect on performance (Tessier et al., 1995b). Improvements in peak oxygen uptake were significantly correlated with glutathione peroxidase activity only in the men supplemented with selenium. Young men supplemented with 240 µg/day of selenium (selenomethionine) and trained for endurance had no performance benefit but a significant increase in muscle glutathione peroxidase activity (Tessier et al., 1995a). Thus, supplemental selenium upregulates biochemical markers of selenium status without enhancing performance.
Several studies have shown an effect of selenium intakes and status on mood states in both men and women (Table B-10). Women and men supplemented with selenium (100 µg/day) reported less anxiety and depression and more energy (Benton and Cook, 1991). Men fed supplemental selenium (230 versus 30 µg/day) reported less confusion and depression (Penland and Finley, 1995). Activity of glutathione peroxidase, a selenium enzyme, in platelets was positively correlated with all mood states (Finley and Penland, 1998).
Calcium
Although its role in bone metabolism is emphasized, calcium acts in mediating vascular, muscular, and nerve functions. Adverse effects of inadequate calcium intake on physical performance are not well studied. However, calcium is required to regulate glycolysis and glycogenolysis and to control protein breakdown. Studies of the effects of calcium restriction or supplementation on human metabolism are lacking despite evidence that calcium intake for 50 percent of adult men is less than the AI (IOM, 1997).
DIETARY INTAKE, NUTRITIONAL STATUS, AND PERFORMANCE IN FIELD STUDIES
Military personnel participating in training and operational exercises consistently decrease food intake, thus consuming inadequate amounts of minerals. Blood biochemical markers of nutritional status, however, do not reveal overt
mineral deficiencies although assessments of hormonal and immune functions show decreases (Booth et al., 2003; Shippee, 1993). Failure to detect nutritional deficiencies is explained by a lack of sensitive biochemical markers of mineral status, brief durations of restricted intakes, and mineral mobilization from stores into the blood with increased metabolic demands and loss of body weight.
Measurable impairments in aerobic capacity or muscle power occur when body weight decreases about 10 percent with a 5 percent loss in muscle mass (Friedl, 1995). Cognitive function declines when significant weight loss (> 6 percent) occurs (Mays, 1995). When energy restriction (50 percent decrease) occurs with strenuous activity, sleep deprivation and mental stress, cognitive performance may fall by more than 33 percent within a few days (Mays, 1993). Decreased food intake plus physical and mental stressors for 12 days increase reports of fatigue and sleep impairment, even at modest levels of weight loss (3 percent) (Booth et al., 2003). Thus, brief and prolonged periods of exposure to nutritional, environmental, physical, and mental stressors impair cognitive performance, including attention, perception, memory, and reasoning (Mays, 1993).
Some reports describe decreased nutritional status during military operations. Shippee (1993) found decreased iron and altered copper and zinc status in Ranger II. Also, Booth and colleagues (2003) reported a decline in iron status in both 12- and 23-day training activities. It is unclear if these findings reflect a response to stress and inflammation or to inadequate intakes of these minerals.
Repeated assault operations may result in mineral depletion because of inadequate intake, and increased turnover and losses of minerals may promote marginal deficiencies under stressful conditions. Also, entry into operations with marginal mineral status may predispose individuals to deficiency status and functional deficits, particularly with repetitive assaults without replenishment of depleted mineral reserves.
FOOD QUALITY AND NUTRIENT DENSITY
Replacing standard combat rations with fresh food and hot meals may alleviate the adverse effects on body weight, physical performance, and cognitive function (Booth et al., 2003; Tharion et al., 2004). This approach, however, is not feasible for soldiers in assault operations. Therefore, new strategies are needed to provide soldiers with proper nutrition.
One approach is to increase the nutrient density of assault rations. Mineral nutrient densities should meet the military standards for reduced-energy rations for men (Baker-Fulco et al., 2001; US Departments of the Army, Navy, and Air Force, 2001). Thus, with reduced energy intake, soldiers would still have adequate mineral intakes.
Steps to improve food intake should be further explored (Hirsch and Kramer, 1993). These include adding new, fortified foods and using stages of change
models to foster healthful nutrition habits (Veverka et al., 2003). Increasing emphasis on maintaining adequate hydration and sleep, particularly during recovery periods, will support these efforts.
Active efforts to prevent marginal mineral depletion should be employed. Some suggestions include:
-
Develop and support an active nutrition education program.
-
Provide palatable, mineral-rich food items in the rations, including on-the-go foods.
-
Increase the availability of mineral-rich foods during recovery periods.
-
Encourage unit leaders and peers to be models of healthful eating behaviors.
SUMMARY AND CONCLUSIONS
Evidence shows that military personnel fail to consume adequate amounts of magnesium, zinc, and calcium. These minerals, as well as copper, selenium, and iron, play key roles in promoting optimal physiological and psychological function and performance. Limited data on mineral intakes and the resulting status of soldiers in various types of training do not provide evidence of overt nutritional deficiencies. A lack of sensitive biochemical markers of nutritional status hinders interpretation of available data. It is difficult to discriminate the independent effect of severely restricted energy intake on potential micronutrient impairments. Nevertheless, physiological and psychological impairments found in civilians with marginal mineral deficits are consistent with perturbations reported in soldiers during operations and suggest similarities, particularly when the low mineral intakes have been noted in the soldiers.
Based on limited evidence of inadequate intakes of zinc and magnesium, and the presumption of increased zinc losses associated with increased physical activity and elevated rates of sweat, zinc and magnesium status may be compromised. With repeated bouts of these conditions and inadequate replenishment of zinc and magnesium stores, soldiers may manifest marginal zinc and magnesium depletion and experience limitations in work capacity, recovery after deployment, perception, and attention. Furthermore, zinc depletion may attenuate immune function and increase the potential for acute bouts of gastrointestinal distress. Evidence of reduced antioxidant defense is generally lacking in military personnel during short-term assault missions. However, recurrent intermittent periods of inadequate intakes of copper, selenium, zinc, and manganese without adequate intakes during recovery may lead to decreased activity of protective antioxidant enzymes.
Generalized use of multiple vitamin and mineral supplements at intakes not exceeding recommended levels should not be hazardous to soldiers participating in assault missions. The use of single nutrient supplements in amounts exceeding
the recommended intake should be avoided to eliminate potential adverse interactions with other nutrients, particularly mineral elements.
Initiating a proactive approach to decrease the potential of adverse effects of limited mineral intakes is recommended. Increasing the mineral densities of assault rations should be advantageous. Adopting an active nutrition education program with palatable, mineral-rich foods and encouraging leaders to model healthy eating behaviors also should be useful to reverse the high rates of low-mineral intakes of soldiers.
RESEARCH NEEDS
There is a need to determine the nutrient intakes, markers of nutritional status, and physiological and psychological performance of military personnel during training and operations. This information is needed to critically evaluate the adequacy of rations provided to and consumed by soldiers exposed to multiple stressors. Findings of this research will enhance the development of rations that promote optimal nutrition and, accordingly, the performance of soldiers engaged in assault operations.
ACKNOWLEDGMENTS
Mention of a trademark or proprietary product does not constitute a guarantee of the product by the US Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable. US Department of Agriculture, Agricultural Research, Northern Plains Area, is an equal opportunity/affirmative action employer and all agency services are available without discrimination.
REFERENCES
Baker-Fulco CJ. 1995. Overview of dietary intakes during military exercises. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 121–149.
Baker-Fulco CJ, Bathalon GP, Bovill ME, Lieberman HR. 2001. Military Dietary Reference Intakes: Rationale for Tables Values. Technical Note TN-00/10. Natick, MA: US Army Research Insitute of Environmental Medicine.
Baker-Fulco CJ, Kramer FM, Lesher LL, Merrill E, Johnson J, DeLany J. 2002. Dietary Intakes of Female and Male Combat Support Hosptial Personnel Subsisting on Meal-Focused or Standard Versions of the Meal, Ready-to-Eat. Technical Report T-01/23. Natick, MA: US Army Research Institute of Environmental Medicine.
Benton D, Cook R. 1991. The impact of selenium supplementation on mood. Biol Psychiatry 29(11):1092–1098.
Booth CK, Coad RA, Forbes-Ewan CH, Thomson GF, Niro PJ. 2003. The physiological and psychological effects of combat ration feeding during a 12-day training exercise in the tropics. Mil Med 168(1):63–70.
Brilla LR, Gunter KB. 1995. Effect of magnesium supplementation on exercise time to exhaustion. Med Exerc Nutr Health 4:230–233.
Brilla LR, Haley TF. 1992. Effect of magnesium supplementation on strength training in humans. J Am Coll Nutr 11(3):326–329.
Brownlie T 4th, Utermohlen V, Hinton PS, Giordano C, Haas JD. 2002. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr 75(4):734–742.
Brun JF, Dieu-Cambrezy C, Charpiat A, Fons C, Fedou C, Micallef JP, Fussellier M, Bardet L, Orsetti A. 1995. Serum zinc in highly trained adolescent gymnasts. Biol Trace Elem Res 47(1–3):273–278.
Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. 1996. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet 348(9033):992–996.
Brutsaert TD, Hernandez-Cordero S, Rivera J, Viola T, Hughes G, Haas JD. 2003. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr 77(2):441–448.
Delorme O, Bourdin H, Viel JF, Rigaud ML, Kantelip JP. 1992. Spectral analysis of electro-encephalography data in athletes with low erythrocyte magnesium. Magnes Res 5(4):261–264.
Dubray C, Rayssiguier Y. 1997. Magnesium, inflammation and pain. In: Theophanides TM, Anastassopoulou J, eds. Magnesium: Current Status and New Developments:Theoretical, Biological, and Medical Aspects. Dordrecht, Netherlands: Kluwer Academic. Pp. 303–311.
Finley JW, Penland JG. 1998. Adequacy or deprivation of dietary selenium in healthy men: Clinical and psychological findings. J Trace Elem Exp Med 11:11–27.
Friedl KE. 1995. When does energy deficit affect soldier physical performance? In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 253–283.
Golf SW, Bohmer D, Nowacki PE. 1994. Is magnesium a limiting factor in competitive exercise? A summary of relevant scientific data. In: Golf S, Dralle D, Vecchiet L, eds. Magnesium 1993. London: John Libbey & Co. Pp. 209–219.
Groner JA, Holtzman NA, Charney E, Mellits ED. 1986. A randomized trial of oral iron on tests of short-term memory and attention span in young pregnant women. J Adolesc Health Care 7(1):44–48.
Hinton PS, Giordano C, Brownlie T, Haas JD. 2000. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol 88(3):1103–1111.
Hirsch ES, Kramer FM. 1993. Situational influences on food intake. In: Marriott BM, ed. Nutritional Needs in Hot Environments. Washington, DC: National Academy Press. Pp. 215–243.
IOM (Institute of Medicine). 1997. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluorine. Washington, DC: National Academy Press.
IOM. 2000a. Dietary Reference Intakes: Applications in Dietary Assessment. Washington, DC: National Academy Press.
IOM. 2000b. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press.
IOM. 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press.
Khaled S, Brun JF, Micallel JP, Bardet L, Cassanas G, Monnier JF, Orsetti A. 1997. Serum zinc and blood rheology in sportsmen (football players). Clin Hemorheol Microcirc 17(1):47–58.
Klevay LM, Buchet JP, Bunker VW, Clayton BE, Gibson RS, Medeiros DM, Moser-Veillon PB, Patterson KY, Taper LJ, Wolf WR. 1993. Copper in the western diet (Belgium, Canada, UK, and USA). In: Anke M, Meissner D, Mills CF, eds. Trace Elements in Man and Animals. TEMA 8. Gersdorf, Germany: Verlag Media Touristik. Pp. 207–210.
Kretsch MJ, Fong, AKH, Penland JG, Sutherland B, King JC. 2000. Cognitive effects of adaptation to a low zinc diet in healthy men. In: Roussel AM, Favier AE, Anderson RA, eds. Trace Elements in Man and Animals: TEMA 10: Proceedings of the Tenth International Symposium on Trace Elements in Man and Animals. New York: Kluwer Academic/Plenum Publishers. Pp. 999–1001.
Lukaski HC. 2005. Low dietary zinc decreases erythrocyte carbonic anhydrase activities and impairs cardiorespiratory function during exercise in men. Am J Clin Nutr 81(5):1045–1051.
Lukaski HC, Johnson PE. 2005. Dietary copper at the recommended intake decreases muscle cytochrome c oxidase activity and alters metabolic responses during exercise in men. FASEB J 19:A982.
Lukaski HC, Hoverson BS, Milne DB, Bolonchuk WW. 1989. Copper, zinc, and iron status of female swimmers. Nutr Res 9(5):493–502.
Lukaski HC, Hoverson BS, Gallagher SK, Bolonchuk WW. 1990. Physical training and copper, iron, and zinc status of swimmers. Am J Clin Nutr 51(6):1093–1099.
Mays MZ. 1993. Cognitive function in a sustained multi-stressor environment. In: Marriott BM, ed. Review of the Results of Nutritional Intervention, Ranger Training Class 11/92 (Ranger II). Washington, DC: National Academy Press. Pp. 199–214.
Mays MZ. 1995. Impact of underconsumption on cognitive performance. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 285–302.
Murray-Kolb LE, Whitfield KE, Beard JL. 2004. Iron status alters cognitive functioning in women during reproductive years. 2004 Experimental Biology Meeting Abstracts. FASEB J 18:Abstract #500.8. [Online]. Available at http://select.biosis.org/faseb [accessed April 13, 2005].
Pang Y, MacIntosh DL, Ryan PB. 2001. A longitudinal investigation of aggregate oral intake of copper. J Nutr 131(8):2171–2176.
Penland JG. 1988. Effects of trace element nutrition on sleep patterns in adult women. FASEB J 2(4):A434.
Penland JG. 1989. Relationship between essential trace element nutrition and self-reported mood states. N Dak Acad Sci Proc 43:68.
Penland JG. 1991. Cognitive performance effects of low zinc (Zn) intakes in healthy adult men. FASEB J 5(5):A938.
Penland JG, Finley JW. 1995. Dietary selenium and mood states in healthy young men. N Dak Acad Sci Proc 49:26.
Penland JG, Milne DB, Davis CD. 2000. Moderately high zinc intake impairs verbal memory of healthy postmenopausal women on a low copper diet. In: Roussel AM, Favier AE, Anderson RA, eds. Trace Elements in Man and Animals: TEMA 10: Proceedings of the Tenth International Symposium on Trace Elements in Man and Animals. New York: Kluwer Academic/Plenum Publishers. Pp. 1025–1030.
Rose MS, Buchbinder JC, Dugan TB, Szeto EG, Allegretto JD, Rose DW, Carlson DE, Sammonds KW, Schnakenberg DD. 1987. Determination of Nutrient Intakes by a Modified Visual Estimation Method and Computerized Nutritional Analysis for Dietary Assessments. Technical Report T6-88. Natick, MA: US Army Research Institute of Environmental Medicine.
Shils ME. 1997. Magnesium. In: O’Dell BL, Sunde RA, eds. Handbook of Nutritionally Essential Mineral Elements. New York: Marcel Dekker. Pp. 117–152.
Shippee RL. 1993. Nutritional status and immune function of Ranger trainees given increased caloric intake. Briefing for the National Academy of Sciences. In: Marriott BM, ed. Review of the Results of Nutritional Intervention, Ranger Training Class 11/92 (Ranger II). Washington, DC: National Academy Press. Pp. 86–104.
Tessier F, Hida H, Favier A, Marconnet P. 1995a. Muscle GSH-Px activity after prolonged exercise, training, and selenium supplementation. Biol Trace Elem Res 47(1–3):279–285.
Tessier F, Margaritis I, Richard MJ, Moynot C, Marconnet P. 1995b. Selenium and training effects on the glutathione system and aerobic performance. Med Sci Sports Exerc 27(3):390–396.
Tharion WJ, Baker-Fulco CJ, Bovill ME, Montain SM, DeLany JP, Champagne CM, Hoyt RW, Lieberman HR. 2004. Adequacy of Garrison feeding for Special Forces soldiers during training. Mil Med 169(6):483–490.
Tharion WJ, Baker-Fulco CJ, McGraw S, Johnson Wk, Niron P, Warber JP, Kramer FM, Allen R, Champagne CM, Falco C, Hoyt RW, DeLany JP, Lesher LL. 2000. The Effects of 60 Days of Tray Ration Consumption in Marine Combat Engineers While Deployed on Great Inagua Island, Bahamas. Technical Report T-00-16. Natick, MA: US Army Research Institute of Environmental Medicine.
Thomas CD, Friedl KE, Mays MZ, Mutter SH, Moore RJ, Jezior DA, Baker-Fulco CJ, Marchitelli LJ, Tulley RT, Askew EW. 1995. Nutrient Intakes and Nutritional Status of Soldiers Consuming the Meal, Ready-to-Eat (MREXII) During a 30-Day Field Training Exercise. Technical Report T95-6. Natick, MA: US Army Research Institute of Environmental Medicine.
Tobin BW, Beard J. 1997. Iron. In: Wolinsky I, Driskell JA, eds. Sports Nutrition: Vitamins and Trace Elements. Boca Raton, FL: CRC Press. Pp. 137–156.
Tucker DM, Sandstead HH. 1981. Spectral electroencephalographic correlates of iron status: Tired blood revisited. Physiol Behav 26(3):439–449.
Tucker DM, Sandstead HH. 1984. Neuropsychological function in experimental zinc deficiency in humans. In: Frederickson CJ, Howell GA, Kasarskis EJ, eds. The Neurobiology of Zinc. Part B: Deficiency, Toxicity, and Pathology. Vol. 11B. New York: Alan R. Liss. Pp. 139–152.
Tucker DM, Sandstead HH, Penland JG, Dawson SL, Milne DB. 1984. Iron status and brain function: Serum ferritin levels associated with asymmetries of cortical electrophysiology and cognitive performance. Am J Clin Nutr 39(1):105–113.
Tucker DM, Sandstead HH, Swenson RA, Sawler BG, Penland JG. 1982. Longitudinal study of brain function and depletion of iron stores in individual subjects. Physiol Behav 29(4):737–740.
US Departments of Army, Navy, and Air Force. 2001. Nutrition Standards and Education. AR 40-25/BUMEDINST 10110.6/AFI 44-141. Washington, DC: US Department of Defense Headquarters.
Vallee BL, Falchuk KH. 1993. The biochemical basis of zinc physiology. Physiol Rev 73(1):79–118.
Van Loan MD, Sutherland B, Lowe NM, Turnlund JR, King JC. 1999. The effects of zinc depletion on peak force and total work of knee and shoulder extensor and flexor muscles. Int J Sport Nutr 9(2):125–135.
Veverka DV, Anderson J, Auld GW, Coulter GR, Kennedy C, Chapman PL. 2003. Use of the stages of change model in improving nutrition and exercise habits in enlisted Air Force men. Mil Med 168(5):373–379.
Effect of Inadequate B Vitamin Intake and Extreme Physical Stress
Lynn B. Bailey and Kristina von Castel-Dunwoody, University of Florida
INTRODUCTION
The primary objective of this paper is to evaluate the potential effect of inadequate B vitamin intake in relation to the consumption of assault rations under extreme physical stress during intense military combat operations. The goal is to characterize the optimal B vitamin content for future rations that will be designed to maximize the physical and mental performance of soldiers
engaged in intensely stressful combat operations. The specific questions that were posed by the Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations are the following:
-
What are the types and levels of B vitamins and choline that could be added to such rations to enhance performance and/or improve recovery during combat missions?
-
Does exercise increase B vitamin use? Does decreased energy intake impact this? Will supplementation regardless of energy level meet B vitamin requirement during exercise?
-
Do B vitamins and choline affect the bioavailability of other nutrients?
The current ration is hypocaloric in that it provides approximately 2,400 kcal for an expected energy expenditures of 4,000 to 4,500 kcal/day. The B vitamin content of the assault ration has not been analyzed chemically; however, the ration is designed to meet the nutrition standards of a restricted energy ration in which the vitamin content is proportionally reduced with the energy content. The estimated B vitamin content of the current assault ration is as follows: (1) thiamin, 0.6 mg; (2) riboflavin, 0.7 mg; (3) niacin, 8 mg NE; (4) vitamin B6, 0.7 mg; (5) folic acid, 200 µg dietary folate equivalents (DFE); and (6) vitamin B12, 1.2 µg [personal communication, S. Montain, USARIEM, July 16, 2004)]. These estimated quantities are approximately 50 percent of the Recommended Dietary Allowance (RDA) or Adequate Intake (AI) for young adult males (IOM, 1998); therefore, the current ration is both hypocaloric and deficient in B vitamin content (Table B-11). This paper presents examples of data supporting the conclusions
TABLE B-11 Dietary B Vitamins in a Ration Compared with the RDA or AI for Men Ages 19 to 30 Years
|
Vitamin |
Estimated B Vitamin Content in Current Ration |
RDA or AI |
|
Thiamin |
0.6 mg |
1.2 mg |
|
Riboflavin |
0.7 mg |
1.3 mg |
|
Niacin |
8 mg NE |
16 mg NE |
|
Vitamin B6 |
0.7 mg |
1.3 mg |
|
Folic Acid |
200 µg |
400 µg DFE |
|
Vitamin B12 |
1.2 µg |
2.4 µg |
|
Biotin |
— |
30 µga |
|
Pantothenic Acid |
— |
5 mga |
|
NOTE: AI = Adequate Intake; DFE = dietary folate equivalents; NE = niacin equivalents; RDA = Recommended Dietary Allowance. aIndicates the value is an AI rather than a RDA. SOURCE: IOM (1998); personal communication, S. Montain, USARIEM, July 16, 2004. |
||
that (1) inadequate B vitamin intake and caloric restriction impair physical and cognitive performance, and (2) extreme physical exertion coupled with caloric restriction significantly increases the requirement for B vitamins.
THE EFFECT OF INCREASED ENERGY DEMAND ON B VITAMIN USE
The B vitamins are required coenzymes for energy production in hundreds of metabolic reactions, including those required for glycolysis, the citric acid cycle, β-oxidation, amino acid metabolism, glycogenolysis, and gluconeogenesis (Bowman and Russel, 2001). Evidence suggests that when energy needs are increased by physical exertion, the B vitamin requirement increases to sustain energy production and maintain normal vitamin status.
Van der Beek and colleagues (1988) investigated the effect of low B vitamin intake on vitamin status and physical performance. These investigators conducted an eight-week, double-blind, controlled metabolic study in healthy young adult males (n = 24) described as moderately active. The study compared the effect of consumption of a diet low in thiamin, riboflavin, and vitamin B6 with that of a deficient diet plus a supplement providing twice the Dutch RDA for these B vitamins. (2.5 mg, 4 mg, and 4 mg, respectively). The quantities of dietary B vitamins in the low vitamin group were similar to the quantities in the current military assault rations (i.e., 0.42 mg/day of thiamin; 0.53 mg/day of riboflavin; and 0.32 mg/day of vitamin B6). The subjects consuming the low B vitamin diet were supplemented with twice the Dutch RDA for all vitamins except thiamin, riboflavin, and vitamin B6 during the eight-week period of low vitamin intake. Unlike the combat ration, both experimental diets contained adequate calories (3,070 kcal/day).
Thiamin status was determined by measuring thiamin diphosphate concentration (TDP) and erythrocyte transketolase (ETK) activity as well as its in vitro stimulation by TDP (α-ETK or activation coefficient). Riboflavin status was assessed by means of flavin adenine dinucleotide (FAD) and erythrocyte glutathione reductase (EGR) activity as well as its in vitro stimulation by FAD (α-EGR). Vitamin B6 status assessment was based on pyridoxal-5′-phosphate (PLP, the active form of the vitamin) concentration and erythrocyte glutamate oxaloacetate (EGOT) activity as well as its in vitro stimulation by PLP (α-EGOT). Within three to six weeks, deterioration of the vitamin status was indicated by decreased B vitamin coenzyme concentrations in blood, decreased erythrocyte enzyme activities, and elevation of stimulation tests of these enzymes, indicating an insufficient supply of coenzyme to maintain normal enzyme activity.
Physical performance was quantified by means of submaximal and maximal oxygen consumption (VO2max). The onset of blood lactate accumulation (OBLA) was measured as well. To determine VO2max and OBLA, the subjects performed incremental bicycle exercise tests. Both the aerobic power and maxi-
FIGURE B-21 Effect of combined low B vitamin intake on aerobic power (A) and work capacity (B). This effect is illustrated by the change of maximal performance from the baseline to 10 weeks during the study.
*Significantly different compared to control at p < 0.01.
SOURCE: van der Beek et al. (1988).
mal workload were significantly lower in the low vitamin group than in the control group (Figure B-21). The blood lactate concentration increase occurred at a lower intensity of physical exertion in the low vitamin group than in the control group. In summary, consumption of a diet adequate in calories but low in thiamin, riboflavin, and vitamin B6 resulted in significantly impaired biochemical
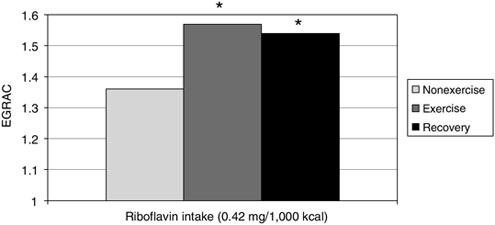
FIGURE B-22 Effect of exercise on riboflavin requirement in men.
NOTE: EGRAC = erythrocyte glutathione reductase activation coefficient.
*Significantly different compared to control (nonexercise) at p < 0.05.
SOURCE: Soares et al. (1993).
status, impaired aerobic power, decreased work capacity, and increased blood lactate at a lower intensity of work.
Soares and colleagues (1993) evaluated the effect of exercise on the riboflavin status of adult men whose baseline riboflavin status was inadequate. Energy balance was maintained throughout the study. Riboflavin status, based on increases in the erythrocyte glutathione reductase activation coefficient (EGRAC), deteriorated significantly and the impaired status persisted during the subsequent recovery period when the study participants exercised (Figure B-22). These data indicate that riboflavin status further deteriorates during a short period of increased physical activity in individuals whose riboflavin status is marginal.
As reviewed by Manore (2000), the results of a number of controlled metabolic studies in women indicate that exercise, dieting for weight loss, or a combination of both all increase riboflavin requirements. In one study by Belko and colleagues (1983), young women consumed various amounts of riboflavin over a 10-week period, and their EGRAC values were determined. The EGRAC values were above the cutoff of 1.25, indicating poor riboflavin status during the first two weeks, when they consumed 0.14 mg of riboflavin/MJ (239 kcal). In response to the consumption of 0.24 mg/MJ during the second two-week period, the EGRAC values returned to normal. For the next three weeks, the riboflavin intake was 0.24 mg/MJ, and the women started to exercise (20 to 50 minutes, six days a week). The initiation of exercise increased the mean EGRAC values above the cutoff. During the last three weeks, the women continued to exercise, and their riboflavin intake increased to 0.33 mg/MJ. At this higher riboflavin
intake, the mean EGRAC values were normal, indicating that exercise led to an increase in the riboflavin requirement.
In two other metabolic studies, Belko and colleagues (1984, 1985) examined the effect of energy restriction and energy restriction plus physical exertion on riboflavin status. Overweight women consumed a metabolic diet that provided 1,195 to 1,266 kcal/day and various quantities of riboflavin (0.14 to 0.19 mg/MJ). The amount of riboflavin required to maintain good status was increased by energy restriction and increased even more by energy restriction plus exercise. It was concluded that a 0.38 mg/MJ level of riboflavin is required to keep EGRAC values in the normal range when female subjects dieted and exercised three to four hours week at 75 to 85 percent of the maximal heart rate.
The effect of exercise and energy restriction on riboflavin status was also evaluated by Winters and colleagues (1992), who fed older female subjects (50 to 67 years old) a metabolic diet that contained adequate calories to maintain weight and either 0.15 or 0.22 mg/MJ of riboflavin for five weeks. During the period when no exercise was performed, EGRAC values increased significantly. When subjects were exercising, 0.22 mg/MJ was required to maintain mean EGRAC values within the normal range. The conclusion from this investigation was that while energy restriction alone or exercise alone may increase riboflavin requirements above the RDA, calorie restriction plus exercise increases the requirement even more.
Manore and colleagues (1987) evaluated the effect of exercise on vitamin B6 status. Plasma PLP concentrations increased significantly in response to exercise and returned to baseline within 60 minutes after exercise stopped (Figure B-23). The marked increase in plasma PLP in response to exercise increases the probability that the active coenzyme form of the vitamin (PLP) may be metabolized to the major excretory form (4-pyridoxic acid) and lost in the urine (Crozier et al., 1994). It has been proposed, therefore, that exercise may increase the turnover and loss of vitamin B6 in active individuals.
In addition, Fogelholm and colleagues (1993) evaluated the effect of energy restriction coupled with exercise on vitamin B6 status. A diet (1,673 kcal/day) consumed by elite male wrestlers over a three-week period resulted in a significant increase in vitamin B6-dependent enzyme stimulation. Since no dietary intake data were obtained, there is a possibility that poor vitamin B6 dietary intake may have also contributed to the impairment of vitamin B6 status. In a different study, van Dale and colleagues (1990) compared the vitamin B6 status in two groups of obese adult males who consumed low-calorie diets (716–931 kcal/day) for 14 weeks. In the group that consumed the weight-reduction diet coupled with exercise, plasma PLP concentration decreased from 54 to 40 mmol/L, but no such change occurred in the group who consumed the same diet but did not exercise. In addition, riboflavin status was also decreased in the diet-plus-exercise group.
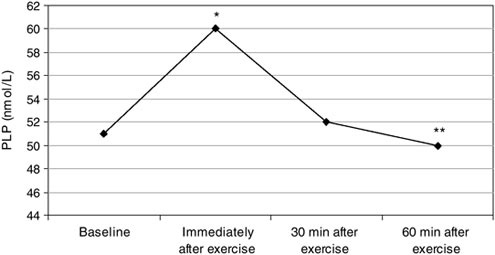
FIGURE B-23 Changes in a blood coenzyme form of vitamin B6 (PLP) in response to very moderate exercise.
NOTE: PLP = pyridoxal 5′-phosphate.
*Sgnificant increase from baseline to immediately after exercise at α = 0.05 level.
**Significant decrease from immediately after exercise to 60 minutes after exercise at α = 0.05 level.
SOURCE: Manore et al. (1987).
EFFECT OF LOW B VITAMIN INTAKE ON MENTAL PERFORMANCE
The B vitamins are essential for normal neurological function. Deficiencies of vitamin B6 and thiamin result in rapid neurological abnormalities (McCormick, 2001; Wood and Currie, 1995). The synthesis of key neurotransmittors, including serotonin, dopamine, norepinephrine, and γ-amniobutyric acid, is dependent on an adequate supply of vitamin B6. Thiamin is required for normal neural cell function with different mechanisms of action proposed for normal nerve cell function (Bates, 2001). Folate is also required for the synthesis of a number of different neuroactive substances through its role as a methyl group donor (Bottiglieri, 1996). In addition, folate is a required coenzyme for homocysteine remethylation; low folate intake results in elevated homocysteine concentrations that have been associated with excitotic properties (Fava et al., 1997).
The effect of a low intake of thiamin (0.42 mg/day), riboflavin (0.53 mg/day), and vitamin B6 (0.32 mg/day) on mental performance was evaluated in an eight-week double-blind controlled study in healthy young adult males (van der Beek et al., 1988). The mental performance test results indicated that subjects who
consumed the low B vitamin diet made more errors (described as displaying a more risky behavior) and needed more time to complete tasks than subjects consuming the control diet, which contained twice the Dutch RDA (2.5 mg/d thiamin, 4 mg/day riboflavin and vitamin B6) for these substances.
Low folate status has been associated with depression, cognitive dysfunction, psychosocial disorders, insomnia, irritability, impaired memory, and fatigue (Alpert et al., 2000; Bottiglieri et al., 1995). A meta-analysis of randomized controlled trials suggests that folate may have a potential role as a supplement to other treatments for depression (Taylor et al., 2004). Table B-12 includes a summary of studies that evaluated the association between folate status and mental performance.
TABLE B-12 Studies on Folate Levels and Mental Status
RESPONSE TO SPECIFIC QUESTIONS RELATED TO REVISING THE B VITAMIN CONTENT OF THE PROPOSED MILITARY COMBAT RATION
A key question related to formulation of the proposed military ration is whether increasing the B vitamin content will enhance the performance of combat soldiers. The current combat ration is hypocaloric and estimated to have approximately 50 percent of the RDA for the B vitamins (personal communication, S. Montain, USARIEM, July 16, 2004). Data from the controlled studies reviewed above support the conclusion that increasing the B vitamin content in the current combat ration will likely enhance the physical and mental performance of soldiers. The studies consistently indicate that either exercise or energy restriction will increase the requirement of select B vitamins, and combining physical exertion with energy restriction will decrease B vitamin status and performance.
A second key question is whether the energy content of the combat ration should be maximized to reduce the energy debt. Based on findings from the controlled metabolic studies discussed above, energy restriction alone will result in impaired B vitamin status. There are no data that support the conclusion that supplemental B vitamins will compensate for all of the metabolic changes that occur due to energy restriction, but to maintain the B vitamin status, it is recommended that the B vitamins be added to the diet in excess of the RDA.
SUMMARY AND CONCLUSIONS
Energy restriction or moderate exercise increases the body’s use of B vitamins to maintain metabolic pathways involved in energy production. A combination of energy restriction and moderate exercise increases B vitamin use to an even greater extent. In contrast to the short, intermittent periods of moderate exercise evaluated in the controlled studies, the intensity and duration of physical exertion during military combat operations are much greater and sustained for much longer periods. It is logical to assume that the effect of energy restriction and extreme physical exertion on B vitamin metabolism would be exacerbated during military combat. The recommended B vitamin content of the proposed new combat ration is twice the current RDA (Table B-13). For the B vitamins and choline not considered in this review, it is also recommended that the ration contain twice the RDA or AI (Table B-13).
REFERENCES
Alpert JE, Mischoulon D, Nierenberg AA, Fava M. 2000. Nutrition and depression: Focus on folate. Nutrition 16(7–8):544–546.
Bates CJ. 2001. Thiamin. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. Washington, DC: ILSI Press. Pp. 184–190.
TABLE B-13 Suggested B Vitamin Content for the Ration
|
Vitamin |
RDA or AI Doubled for Daily Intake |
|
Thiamin |
2.4 mg |
|
Riboflavin |
2.6 mg |
|
Niacin |
32 mg NE |
|
Vitamin B6 |
2.6 mg |
|
Folate |
800 µg DFE (475 mg folic acid × 1.7) |
|
Vitamin B12 |
4.8 µg |
|
Biotin |
60 µga |
|
Pantothenic Acid |
10 µga |
|
Choline |
1,100 mga |
|
NOTE: AI = Adequate Intake; DFE = dietary folate equivalents; NE = niacin equivalents; RDA = Recommended Dietary Allowance. aIndicates the value is an AI rather than a RDA. SOURCE: IOM (1998). |
|
Belko AZ, Meredith MP, Kalkwarf HJ, Obarzanek E, Weinberg S, Roach R, McKeon G, Roe DA. 1985. Effects of exercise on riboflavin requirements: Biological validation in weight reducing women. Am J Clin Nutr 41(2):270–277.
Belko AZ, Obarzanek E, Kalkwarf HJ, Rotter MA, Bogusz S, Miller D, Haas JD, Roe DA. 1983. Effects of exercise on riboflavin requirements of young women. Am J Clin Nutr 37(4):509–517.
Belko AZ, Obarzanek E, Roach R, Rotter M, Urban G, Weinberg S, Roe DA. 1984. Effects of aerobic exercise and weight loss on riboflavin requirements of moderately obese, marginally deficient young women. Am J Clin Nutr 40(3):553–561.
Bottiglieri T. 1996. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev 54(12):382–390.
Bottiglieri T, Crellin R, Renolds EH. 1995. Folates and neuropsychiatry. In: Bailey LB, ed. Folate in Health and Disease. New York: Marcel Dekker. Pp. 435–462.
Bowman BA, Russel RM. 2001. Present Knowledge in Nutrition. 8th ed. Washington, DC: ILSI Press.
Crozier PG, Cordain L, Sampson DA. 1994. Exercise-induced changes in plasma vitamin B-6 concentrations do not vary with exercise intensity. Am J Clin Nutr 60(4):552–558.
Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. 1997. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 154(3):426–428.
Fogelholm M, Ruokonen I, Laakso JT, Vuorimaa T, Himberg JJ. 1993. Lack of association between indices of vitamin B1, B2, and B6 status and exercise-induced blood lactate in young adults. Int J Sport Nutr 3(2):165–176.
Goodwin JS, Goodwin JM, Garry PJ. 1983. Association between nutritional status and cognitive functioning in a healthy elderly population. J Am Med Assoc 249(21):2917–2921.
IOM (Institute of Medicine). 1998. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitmain B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press.
Joosten E, Lesaffre E, Riezler R, Ghekiere V, Dereymaeker L, Pelemans W, Dejaeger E. 1997. Is metabolic evidence for vitamin B-12 and folate deficiency more frequent in elderly patients with Alzheimer’s disease? J Gerontol A Biol Sci Med Sci 52(2):M76–M79.
Kristensen MO, Gulmann NC, Christensen JE, Ostergaard K, Rasmussen K. 1993. Serum cobalamin and methylmalonic acid in Alzheimer dementia. Acta Neurol Scand 87(6):475–481.
Levitt AJ, Karlinsky H. 1992. Folate, vitamin B12 and cognitive impairment in patients with Alzheimer’s disease. Acta Psychiatr Scand 86(4):301–305.
Manore MM. 2000. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr 72(2 Suppl):598S–606S.
Manore MN, Leklem JE, Walter MC. 1987. Vitamin B-6 metabolism as affected by exercise in trained and untrained women fed diets differing in carbohydrate and vitamin B6 content. Am J Clin Nutr 46(6):995–1004.
McCormick DB. 2001. Vitamin B-6. In: Bowman BA, Russel RM, eds. Present Knowledge in Nutrition. Washington, DC: ILSI Press. Pp. 207–213.
Nilsson K, Gustafson L, Faldt R, Andersson A, Brattstrom L, Lindgren A, Israelsson B, Hultberg B. 1996. Hyperhomocysteinaemia—A common finding in a psychogeriatric population. Eur J Clin Invest 26(10):853–859.
Renvall MJ, Spindler AA, Ramsdell JW, Paskvan M. 1989. Nutritional status of free-living Alzheimer’s patients. Am J Med Sci 298(1):20–27.
Riggs KM, Spiro A 3rd, Tucker K, Rush D. 1996. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr 63(3):306–314.
Soares MJ, Satyanarayana K, Bamji MS, Jacob CM, Ramana YV, Rao SS. 1993. The effect of exercise on the riboflavin status of adult men. Br J Nutr 69(2):541–551.
Taylor MJ, Carney SM, Goodwin GM, Geddes JR. 2004. Folate for depressive disorders: Systematic review and meta-analysis of randomized controlled trials. J Psychopharmacol 18(2):251–256.
van Dale D, Schrijver J, Saris WH. 1990. Changes in vitamin status in plasma during dieting and exercise. Int J Vitam Nutr Res 60(1):67–74.
van der Beek EJ, van Dokkum W, Schrijver J, Wedel M, Gaillard AW, Wesstra A, van de Weerd H, Hermus RJ. 1988. Thiamin, riboflavin, and vitamins B-6 and C: Impact of combined restricted intake on functional performance in man. Am J Clin Nutr 48(6):1451–1462.
Winters LR, Yoon JS, Kalkwarf HJ, Davies JC, Berkowitz MG, Haas J, Roe DA. 1992. Riboflavin requirements and exercise adaptation in older women. Am J Clin Nutr 56(3):526–532.
Wood B, Currie J. 1995. Presentation of acute Wernicke’s encephalopathy and treatment with thiamine. Metab Brain Dis 10(1):57–72.
Optimization of the Nutrient Composition in Military Rations for Short-Term, High-Stress Situations: Sodium, Potassium, and Other Electrolytes
Susan Shirreffs, Loughborough University, UK
INTRODUCTION
The specific questions addressed in this manuscript are as follows:
-
What are the types and levels or ratios of electrolytes that could be added to such rations to enhance performance during combat missions? Specifically address the effects of environmental stress.
-
What are the interactions between electrolytes (e.g., sodium and potassium)?
Certain illnesses and medications influence electrolyte balance. In this manuscript, however, dietary electrolyte requirements have not been considered for individuals with illnesses that may cause repeated vomiting, prolonged diarrhea, heat illness, or collapse or for individuals with ongoing medical treatment.
One recent publication from the Institute of Medicine (IOM, 2004) provides an up-to-date review of sodium and potassium dietary requirements. The recommendations given within this publication have been used in this manuscript as a starting point for determining a healthy diet with specific requirements for short-term, high stress situations. Adequate Intakes (AIs) were set for both sodium and potassium because an Estimated Average Requirement, and thus a Recommended Dietary Allowance could not be established.
POTASSIUM
Potassium is the major cation in the intracellular fluid, and it is maintained at a concentration of approximately 145 mmol/L; the extracellular concentration is approximately 4 to 5 mmol/L. Potassium is required for normal cellular function, and relatively small changes in the extracellular potassium can greatly affect the extra- to intracellular ratio, thereby affecting neural transmission, muscle contraction, and vascular tone.
Severe potassium deficiency, characterized by hypokalemia and indicated by a serum potassium concentration of less than 3.5 mmol/L, has consequences that include cardiac arrhythmia, muscle weakness, and glucose intolerance. Moderate potassium deficiency is characterized by increased blood pressure, salt sensitivity, and risk of kidney stones and shows evidence of increased bone turnover.
In normal circumstances, 77 to 90 percent of dietary potassium is excreted in urine with the remaining excreted in feces and sweat (Holbrook et al., 1984).
The AI for potassium set by the Panel on Dietary Reference Intakes for Electrolytes and Water (IOM, 2004) is 4.7 g (120 mmol) per day. This recommendation, however, is based on potassium intake from food, excluding supplementation, and is therefore based on forms of potassium with bicarbonate precursors. The rationale for this AI is that it should help maintain lower blood pressure levels, reduce the effects of sodium chloride intake on blood pressure, reduce the risk of kidney stones, and, possibly, decrease bone loss. The Panel assigned no Tolerable Upper Intake Level (UL) because for healthy individuals with normal kidney function, any excess dietary potassium intake from foods is excreted in the urine, feces, and sweat. However, the National Health and Nutrition Examination Survey (NHANES III, 1988–1994) in the United States indicated that the median potassium intake by male adults was approximately 2.8 to 3.3 g (72 to 84 mmol) per day. Only 10 percent of men consumed potassium in a quantity equal to or greater than the AI.
SODIUM
Sodium is the major cation in extracellular fluid, and it is maintained at a concentration of approximately 140 mmol/L; the intracellular concentration is approximately 4 mmol/L. Along with the anion chloride, sodium maintains extracellular volume, and therefore, plasma volume and serum osmolality. It is also an important determinant of cell membrane potential and an active transporter of molecules across membranes.
In the absence of substantial sweating, individuals in sodium and water balance typically excrete 90 to 95 percent of their dietary sodium intake in their urine (Table B-14). The total obligatory sodium losses are very small, amounting to approximately 0.18 g (8 mmol) per day (Dahl, 1958).
On the basis of available data, the AI for sodium set by the Panel on Dietary Reference Intakes for Electrolytes and Water (IOM, 2004) is 1.5 g (65 mmol) per day. Based on the effects of higher sodium intake levels on blood pressure, the UL for sodium is 2.3 g (100 mmol) per day. However, while both the AI and UL were said to be appropriate for unacclimatized individuals exposed to high environmental temperatures or for people who are physically active, they were deemed inappropriate for highly active individuals or individuals who are exposed to prolonged heat and lose large amounts of sweat daily. Although the AI and UL parameters were not quantified, it is clear that the AI allows for an intake of approximately 1.3 g (57 mmol) above the maximum obligatory losses (Table B-14), meaning that this amount may be taken to accommodate increased sweat sodium loss.
Hyponatremia, which is defined as a serum sodium concentration of less than 135 mmol/L, does not generally occur with low sodium intakes (Kirkendall et al., 1976; Overlack et al., 1995), but rather is related to excessive sodium loss from the body or to excessive consumption of fluids low in sodium.
The National Health and Nutrition Examination Survey (NHANES III, 1988–1994) in the United States indicated that the median sodium intake by male adults ranged from 3.1 to 4.7 g (135 to 204 mmol) per day (IOM, 2004). This estimate, however, excludes any salt added at the table, so the intake levels
TABLE B-14 Obligatory Losses of Sodium
|
Source |
g/day |
mmol/day |
|
Urine |
0.005–0.035 |
0.2–1.5 |
|
Skin (nonsweating) |
0.025 |
1.1 |
|
Feces |
0.010–0.125 |
0.4–5.4 |
|
Total |
0.040–0.185 |
1.7–8.0 |
|
SOURCE: Dahl (1958). |
||
may be an underestimate for many individuals. Total dietary sodium intake was recently estimated to be 4.2 g (183 mmol) per day as shown in a study that quantified intake based on 24-hour urine collections (Zhou et al., 2003).
CHLORIDE
Sodium and chloride are commonly found together in foods as sodium chloride. For this reason, and because both are commonly in association in the body, the AI for chloride set by the Panel on Dietary Reference Intakes for Electrolytes and Water (IOM, 2004) is at a level equivalent, on a molar basis, to that of sodium and thus amounts to 2.3 g (65 mmol) per day.
SODIUM-WATER INTERACTION
A brief mention of water intake is warranted because of its links with body sodium balance and sodium’s role in maintaining extracellular fluid volume. Water is the largest single constituent in the body in nonobese individuals. It acts as a solvent for biochemical reactions and as a medium of transport of other compounds, and it has a high specific heat to absorb metabolic heat, maintains vascular volume, supports the supply of nutrients, and removes waste. However, an individual’s total water intake includes drinking water, water in other drinks, and water in foods. The AI for water was established at a sufficient level to prevent the acute deleterious effects of dehydration, including functional and metabolic abnormalities. For example, the AI for total water intake for young men is 3.7 liters per day (IOM, 2004); however, higher intakes will be required for individuals who are physically active or are exposed to hot environments. In very unusual circumstances, an excess consumption of fluids combined with a low sodium intake may lead to excess body water, resulting in hyponatremia.
THE EFFECTS OF ENVIRONMENTAL STRESS ON ELECTROLYTE REQUIREMENTS
An increased environmental temperature is more physiologically stressful to free living humans than is a cold environment. Exposure to a cold environment, provided that appropriate clothing is available and, perhaps, some form of exercise to generate heat is possible, presents no special consideration with regard to electrolyte requirements. They should be no different from requirements for a “normal” environment for that individual. However, the same is not true for a warm environment because of the sweating mechanism that is activated to regulate body temperature.
When sweating it stimulated, it can have a significant influence on electrolyte losses. Sodium is the main electrolyte present in sweat, but significant amounts of other electrolytes are present (Table B-15).
TABLE B-15 The Normal Concentration Range of Some of the Main Electrolytes in Sweat
|
Electrolyte |
Concentration (mmol/L) |
|
Sodium |
10–80 |
|
Chloride |
10–60 |
|
Potassium |
4–14 |
|
Calcium |
0.3–2 |
|
Magnesium |
0.2–1.5 |
|
Sulfate |
0.1–0.2 |
|
SOURCE: Costill (1977); Lentner (1981); Maughan (1991); Pitts (1959); Schmidt and Thews (1989). |
|
Clearly, the extent of any electrolyte loss incurred will also depend on the sweat rate. As summarized by IOM (2004) when an individual is at rest and not visibly sweating, the rate of water loss through the skin is approximately 0.020 L per hour. At the other extreme, an individual that experiences shorter periods of intense activity in a warm environment could have a maximum sweat rate of 3 L per hour (Sawka and Pandolf, 1990). Other data from the literature suggest sweat rates of 0.3 to 1.2 L per hour (Adolph, 1947) for residents of desert climates performing occupational activities, while persons performing low-intensity exercise but wearing protective clothing commonly have sweat rates of 1 to 2 L per hour (Levine et al., 1990). Finally, when calculated on a daily basis from water turnover studies, sweat water losses of 0.2 to 2 L per day have been reported for sedentary individuals in a temperate climate while volumes of 1 to 9 L per day have been reported for individuals undertaking manual work or exercise in varying climates (Leiper et al., 1996, 2001; Singh et al., 1989). These very different sweat rates clearly have the potential to induce electrolyte losses, thus affecting total body electrolyte loss. Taking 0.20 L per hour (4.8 L per day) as the lowest sweat rate and 9 L per day as the highest, the losses of the main electrolytes could range as shown in Table B-16.
Many other factors, however, influence sweat composition. These include the dietary electrolyte intake (Allsopp et al., 1998; Armstrong et al., 1985b), the heat acclimation status of an individual, and the individual’s sweat rate at any time. However, as shown in Box B-2, while sodium levels are influenced by all three factors noted, divergent findings for potassium have been reported in the literature.
Many studies have attempted to quantify sodium and potassium loss through sweat and urine in a variety of settings, and some of these have been summarized in Table B-17. However, a key factor when considering dietary electrolyte intakes for groups of individuals is the vast differences individuals have in both sweat rate and sweat electrolyte losses when doing the same task, at the same time, in the same environment, and wearing the same clothing.
TABLE B-16 The Range of Losses for the Main Electrolytes Attributed to Sweat Rates Ranging from 4.8 L per Day to 9 L per Day
|
Electrolyte |
Loss (mmol/day)a |
|
Sodium |
5–720 |
|
Chloride |
5–540 |
|
Potassium |
4–126 |
|
aSee Table B-15 for the range of normal sweat electrolyte concentrations. |
|
|
BOX B-2 SODIUM
POTASSIUM
|
SODIUM AND POSTEXERCISE REHYDRATION
In situations when large sweat losses occur over a relatively short time, effective recovery from dehydration will not occur when replacing water loss until the sodium loss is replaced as well. Solid food consumed along with the water will replace the sodium, but if food is not consumed, sodium salts should be added to the water (Maughan et al., 1996; Ray et al., 1998; Shirreffs and Maughan, 1998).
SODIUM–POTASSIUM INTERACTIONS
Sodium and potassium are complementary to each other in a number of ways and their consumption has particular effects on urinary excretion of the alternate cation, salt sensitivity, and blood pressure. However, in the studies referred to in the final sections below, the subjects were not stressed to a level that would add to significant sweat electrolyte losses in addition to the urinary losses described.
TABLE B-17 Sweat (S) and Urinary (U) Loss of Potassium and Sodium
Dietary Intake and Sodium Excretion
With dietary sodium intakes of up to 3.2 g (140 mmol) per day, urinary potassium excretion generally remains lower than dietary potassium intake for at least 28 days (Sacks et al., 2001). However, with dietary sodium intakes greater that 6.9 g (300 mmol) per day, the urinary potassium excretion was greater than the dietary potassium intake over three days (Luft et al., 1982).
When potassium in the form of potassium bicarbonate or potassium chloride was supplemented in quantities greater than 4.7 g (120 mmol) per day, an increase in urinary sodium excretion was reported (van Buren et al., 1992). However, a potassium bicarbonate intake of either 2.7 or 4.7 g (70 to 120 mmol) per day was sufficient to reverse this increased loss when dietary sodium chloride intake was increased from 1.8 to 14.6 g (30 to 250 mmol) per day (Morris et al., 1999).
Salt Sensitivity
Salt sensitivity refers to the degree of blood pressure response to a change in dietary sodium intake. It is frequently classified by a dietary sodium chloride-induced increase in mean arterial blood pressure of 3 mm Hg or more. Studies have demonstrated that the expression of salt sensitivity is modulated by dietary
potassium intake, and this is one of the main rationales given for encouraging a high potassium intake. Morris and colleagues (1999) reported a 50 to 80 percent decrease of the identified salt-sensitive individuals who had a sodium chloride intake of 14.6 g (250 mmol) per day while they increased their potassium bicarbonate intake from 1.2 g (30 mmol) to 2.7 g (70 mmol) per day. It has been postulated that these effects on salt sensitivity occur with the natriuretic (high level of sodium loss in urine) effects of the potassium, increasing renal tubule excretion of sodium chloride, which seems to be unaffected by the anion accompanying potassium (IOM, 2004).
Blood Pressure
The modulating effects of dietary potassium intake on blood pressure appear to be related to the sodium/potassium ratio, and the effects increase with higher sodium chloride intake (Morris and Sebastian, 1995; Whelton et al., 1997). Reductions in systolic blood pressure of 2.5 mm Hg and in diastolic blood pressure of 1.5 mm Hg have been reported when 2 g (50 mmol) of urinary potassium was excreted, indicative of higher potassium intakes (Intersalt Cooperative Research Group, 1988).
CONCLUSIONS
For the most part, appropriate electrolyte intake for combat rations would be similar to that for daily intake in a healthy, balanced diet. Sodium requirements may be the main exception because of environmental influences. Because adaptations to significant changes in dietary intake of sodium take a number of days to fully develop, there are compelling reasons to have sodium in a short-term ration be present in a least the same quantities as those in the preceding daily diet.
The types of stresses experienced and their effects on sweat production may influence dietary electrolyte requirements. After a period of heavy sweating, sodium should be consumed, if possible, with any large volume of water to minimize diuresis. It is possible that sodium requirements may well differ with prolonged low sweat rates in comparison with the high sweat rates seen during periods of intense activity.
In keeping with the conclusions of the Panel on Dietary Reference Intakes for Electrolytes and Water, “data are presently insufficient to set different potassium intake recommendations according to the level of sodium intake, and vice versa. Likewise, data are insufficient to set requirements based on the sodium/potassium ratio” (IOM, 2004, p. 230).
REFERENCES
Adolph EF. 1947. Physiology of Man in the Desert. New York: Intersciences.
Allsopp AJ, Sutherland R, Wood P, Wootton SA. 1998. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. Eur J Appl Physiol 78(6):516–521.
Armstrong LW, Costill DL, Fink WJ, Bassett D, Hargreaves M, Nishibata I, King DS. 1985a. Effects of dietary sodium on body and muscle potassium content during heat acclimation. Eur J Appl Physiol 54(4):391–397.
Armstrong LE, Hubbard RW, Szlyk PC, Matthew WT, Sils IV. 1985b. Voluntary dehydration and electrolyte losses during prolonged exercise in the heat. Aviat Space Environ Med 56(8): 765–770.
Costill D. 1977. Sweating: Its composition and effects on body fluids. Ann N Y Acad Sci 301: 160–174.
Costill DL, Cote R, Fink WJ. 1982. Dietary potassium and heavy exercise: Effects on muscle water and electrolytes. Am J Clin Nutr 36(2):266–275.
Conn JW. 1949. The mechanism of acclimatization to heat. Adv Int Med 3:373–393.
Dahl LK. 1958. Salt intake and salt need. N Engl J Med 258(23):1152–1157.
Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. 1984. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr 40(4):786–793.
Intersalt Cooperative Research Group. 1988. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Br Med J 297(6644):319–328.
IOM (Institute of Medicine). 2004. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride and Sulfate. Washington, DC: The National Academies Press.
Kirkendall AM, Connor WE, Abboud F, Rastogi SP, Anderson TA, Fry M. 1976. The effect of dietary sodium chloride on blood pressure, body fluids, electrolytes, renal function, and serum lipids of normotensive man. J Lab Clin Med 87(3):411–434.
Leiper JB, Carnie A, Maughan RJ. 1996. Water turnover rates in sedentary and exercising middle aged men. Br J Sports Med 30(1):24–26.
Leiper JB, Pitsiladis Y, Maughan RJ. 2001. Comparison of water turnover rates in men undertaking prolonged cycling exercise and sedentary men. Int J Sports Med 22(3):181–185.
Lentner C, ed. 1981. Geigy Scientific Tables. 8th ed. Basel: Ciba-Geigy Ltd.
Levine L, Quigley MD, Cadarette BS, Sawka MN, Pandolf KB. 1990. Physiologic strain associated with wearing toxic-environment protective systems during exercise in the heat. In: Das B, ed. Advances in Industrial Ergonomics and Safety II. London: Taylor & Francis. Pp. 897–904.
Luft FC, Weinberger MH, Grim CE. 1982. Sodium sensitivity and resistance in normotensive humans. Am J Med 72(5):726–736.
Malhotra MS, Sridharan K, Venkataswamy Y. 1976. Potassium losses in sweat under heat stress. Aviat Space Environ Med 47(5):503–504.
Maughan RJ. 1991. Fluid and electrolyte loss and replacement in exercise. J Sports Sci 9 (Special):117–142.
Maughan RJ, Leiper JB, Shirreffs SM. 1996. Resortation of fluid balance after exercise-induced dehydration: Effects of food and fluid intake. Eur J Appl Physiol 73:317–325.
Morris RC, Sebastian A. 1995. Potassium-responsive hypertension. In: Laragh JH, Brenner BM, eds. Hypertension: Pathophysiology, Diagnosis, and Management. 2nd ed. New York: Raven Press. Pp. 2715–2726.
Morris RC Jr, Schmidlin O, Tanaka M, Forman A, Frassetto L, Sebastian A. 1999. Differing effects of supplemental KCl and KHCO3: Pathophysiological and clinical implications. Semin Nephrol 19(5):487–493.
Overlack A, Ruppert M, Kolloch R, Kraft K, Stumpe KO. 1995. Age is a major determinant of the divergent blood pressure responses to varying salt intake in essential hypertension. Am J Hypertens 8(8):829–836.
Pitts RF. 1959. The Physiological Basis of Diuretic Therapy. Springfield, IL: C.C. Thomas.
Ray ML, Bryan MW, Ruden TM, Baier SM, Sharp RL, King DS. 1998. Effect of sodium in a rehydration beverage when consumed as a fluid or meal. J Appl Physiol 85(4):1329–1336.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH. 2001. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344(1):3–10.
Sawka M, Pandolf K. 1990. Effects of body water loss on physiological function and exercise performance. In: Gisolfi C, Lamb D, eds. Perspectives In Exercise Science and Sports Medicine. Vol. 3. Fluid homeostasis during exercise. Carmel, IN: Benchmark Press Inc. Pp. 1–38.
Schmidt RF, Thews G, eds. 1989. Human Physiology. 2nd ed. Berlin: Springer-Verlag.
Shirreffs SM, Maughan RJ. 1998. Volume repletion after exercise-induced volume depletion in humans: Replacement of water and sodium losses. Am J Physiol 274(5 Pt 2):F868–F875.
Singh J, Prentice AM, Diaz E, Coward WA, Ashford J, Sawyer M, Whitehead RG. 1989. Energy expenditure of Gambian women during peak agricultural activity measured by the doubly-labelled water method. Br J Nutr 62(2):315–329.
van Buren M, Rabelink TJ, van Rijn HJ, Koomans HA. 1992. Effects of acute NaCl, KCl and KHCO3 loads on renal electrolyte excretion in humans. Clin Sci (Lond) 83(5):567–574.
Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. 1997. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. J Am Med Assoc 277(20):1624–1632.
Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P. 2003. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP study. J Hum Hypertens 17(9):623–630.
Other Bioactive Food Components and Dietary Supplements
Rebecca B. Costello and George P. Chrousos, National Institutes of Health
INTRODUCTION
Evaluation of the potential beneficial effects of nutrition supplements or bioactive food components should consider an individual’s baseline status and the many different stress states in response to discrete types of stressors. Also, we need to distinguish between acute, subacute, and chronic stressors and the type, amount, and duration of demands that each imposes on the individual. Another issue is the constitution of the individual, dictated by genetics, developmental intrauterine and early life history, and late postnatal and current environment.
Psychosocial or physical stressors or a combination of the two produce different kinds of adaptive responses and have different types of material requirements depending on both the size and duration of the stressor. During stress, be it psychosocial or physical, all four major neurotransmitter systems—noradrenergic, serotonergic, cholinergic, and γ-aminobutyric acid (GABA)ergic—are activated. This poses increased needs for precursor amino acid molecules as well as for the
energy necessary for their synthesis, secretion, and electrochemical effects. Also, there is increased demand for the necessary coenzymes, some of which are essential vitamins that must be taken from the environment. When physical stress is present, one has to consider both the increased needs for the stress neurotransmitter synthesis, secretion, and effects, and the peripheral energy demands, the latter becoming of major importance. In this instance, the availability of nutrients and oxygen and the presence of healthy energy-producing machinery, including adequate numbers of healthy mitochondria and the ability to switch from aerobic to anaerobic metabolism, are crucial components of a successful adaptation.
This chapter reviews a number of bioactive food components and dietary supplements that have been tested or suggested for their performance-enhancing effects. Among these are the well-characterized and extensively studied amino acids tyrosine and tryptophan for their role in modulating neurotransmitter concentrations. Also reviewed are L-carnitine and coenzyme Q10 for their role in supporting mitochondrial energy metabolism, as well as several botanical ingredients, Eleutherococcus senticosus (Siberian ginseng), Cordyceps sinensis, and Rhodiola, for their adaptogenic qualities, and Ginkgo biloba for cognitive function and mitigation of acute mountain sickness.
NEUROTRANSMITTER PRECURSORS
Tyrosine and tryptophan, two of the large neutral amino acids (LNAA), when given in single, nonphysiologic doses or within special diets, have been shown to alter brain activity. Tyrosine is the precursor for the neurotransmitters dopamine, norepinephrine, and epinephrine. The working hypothesis is that tyrosine can mitigate the adverse effects of acute stress by modulating levels of norepinephrine. Tryptophan is the precursor for the neurotransmitter serotonin. Its role in regulating mood (particularly depression) and alertness but also pain sensitivity, aggression and food consumption may, under certain circumstances, support various aspects of performance. This topic has been well described in previous IOM reports (IOM, 1994, 1999) and in reviews by Lieberman (1994, 1999, 2003) and thus this chapter will concentrate on more recent research findings dealing with the roles of tyrosine and tryptophan in modulating performance.
TYROSINE
Animal studies have demonstrated that performance decline observed in highly stressed animals can be restored by supplementation with tyrosine (IOM, 1994). This has recently been confirmed (Yeghiayan et al., 2001); rats pretreated with L-tyrosine (200 to 400 mg/kg body weight) in a dose-dependent fashion improved performance in a forced-swim test following acute cold stress. Tyrosine also improved mood and performance concomitant with increases in hippocampal norepinephrine concentrations following cold exposure.
Human studies involved in supplementing with L-tyrosine have shown that the adverse effects of hypoxia and cold, lower body negative-pressure stress, and psychological stress can be reduced by tyrosine supplementation in dose ranges of 85 to 100 mg/kg body weight (IOM, 1994). Work by Neri and colleagues (1995) in a clinical study of a small number of US Marine research subjects, demonstrated that 75 mg/kg of L-tyrosine, compared to the results of a cornstarch control given in banana-flavored yogurt on two occasions to the soldiers after one night’s sleep loss, ameliorated the usual performance decline on a set of psychomotor tasks over a 13-hour test session. A significant reduction in lapse probability on a high-event rate vigilance task was also demonstrated with improvements lasting approximately three hours. When two grams of L-tyrosine was administered in the form of a protein-rich drink for a period of five days, military cadets performed better on enhanced memory and tracking tests compared with those who ingested an isocaloric placebo drink (Deijen et al., 1999). Most recently in a study designed to test whether repeated ingestions of tyrosine during prolonged exercise would improve physical performance in competitive cyclists, Chinevere and colleagues (2002) administered L-tyrosine alone (25 mg/kg), tyrosine with a carbohydrate supplement, or a placebo in a double-blind controlled study and found a lack of effect on physical endurance parameters of oxygen uptake, heart rate, rate of perceived exertion (RPE) at any time during the 90-minute cycling session. Although an increase in the plasma to free-tyrosine:tryptophan ratio (a more sensitive marker of amino acid transport across the blood-brain barrier than plasma levels alone) was noted for those who consumed the tyrosine-containing supplements, it did not translate into an increase in performance. Thus, it appears that the benefits of tyrosine supplementation continue to be accrued for enhancements in cognitive function but not for physical performance.
L-Tyrosine is generally recognized as safe (GRAS) status in the United States. It is safe in doses up to 150 mg/kg/day for two weeks (IOM, 2002), although a number of issues remain to be addressed concerning routine supplementation with tyrosine. Yet to be demonstrated is tyrosine’s effects across a wider range of stressors. There is a need to establish a dose-response function for tyrosine in humans and to estimate an upper level of intake. Documentation in human studies is still lacking regarding its efficacy in situations of chronic stress. The most effective method of supplementation for the soldiers (foods versus supplements) has yet to be determined.
TRYPTOPHAN
Tryptophan has been shown to have sedative-like effects on humans when administered in pure form and in sufficient quantity (IOM, 1994; Lieberman et al., 1986). It may also be useful as a mild sleep aid in military operations as it does not appear to impair some aspects of performance (IOM, 1994; Lieberman
et al., 1986). Research on supplementation with L-tryptophan was halted in 1990 when products were removed from the market due to an outbreak of a rare condition called eosinophilia-myalgia syndrome (EMS). The exact etiology of this outbreak remains undefined. L-Tryptophan however is available in the United States in special dietary products for limited use under medical supervision, such as infant formulas. Consumption of increased levels of L-tryptophan when “balanced” with other amino acids in foods or as a fortificant has not been associated with EMS.
Dietary carbohydrates produce major, insulin-mediated decreases in the availability of LNAA, with lesser reductions in plasma tryptophan, which in effect raises the plasma tryptophan:LNAA ratio, facilitating tryptophan’s entry into the brain. In contrast, intake of dietary protein has been shown to lower this ratio as they contribute less tryptophan to the circulation than do other LNAAs. Strüder and Weicker (2001) reviewed more than 20 human studies and concluded that nutritional manipulation of serotonin in the brain has variable effects on reducing fatigue and enhancing performance outcomes. This physiologic response has been recently reexamined by Wurtman et al. (2003) evaluating a high-carbohydrate versus a high-protein breakfast meal, as typically eaten by Americans, on the plasma tryptophan:LNAA ratio. It was found that the carbohydrate-rich and protein-rich breakfasts had significantly different effects on the plasma tryptophan:LNAA ratio (54 percent median difference) and tyrosine:LNAA ratio (28 percent median difference). In evaluating carbohydrate-rich and protein-poor diets in stress-prone individuals, Markus et al. (1998, 1999), have demonstrated that a carbohydrate-rich/protein-poor diet compared with a protein-rich/carbohydrate-poor diet increased the ratio of tryptophan:LNAA in the plasma and improved stress coping during high, uncontrollable laboratory stress in the high stress subjects only; thus suggesting a higher requirement for tryptophan and enhanced serotonergic functioning in the high-stress compared with low-stress subjects.
Following on these findings and further manipulating dietary constituents, Markus and colleagues (2000; 2002) designed clinical studies to evaluate mood and cognitive performance in high-stress-vulnerable subjects by using an enriched whey protein supplement high in tryptophan content (6 percent from α-lactalbumin) in a double-blind, placebo crossover study. Tryptophan 12.32 g/kg (in the form of α-lactabumin), when given in a chocolate beverage drink versus a sodium caseinate control beverage containing 9.51 g/kg of tryptophan twice daily, increased the plasma ratio of tryptophan:LNAA, enhanced plasma prolactin levels (a measure of enhanced brain serotonin function), and decreased cortisol levels (a measure of the reactivity of the stress response system) with improvements in cognitive performance in high-stress-vulnerable subjects only, as compared with low-stress subjects. Additional work in this area has been performed by Beulens and colleagues (2004) in healthy male subjects randomized to either an α-lactalbumin supplement plus carbohydrate or carbohydrate supplement only consumed after
a breakfast consisting of 19 percent protein. Measurements were obtained at baseline, 30 minutes, and 90 minutes postingestion. The team demonstrated moderately increased levels (16 percent) of the plasma tryptophan:LNAA ratio with the α-lactalbumin supplement with no effect on appetite, food intake, macronutrient preference, or mood as compared with the carbohydrate-only supplemented group.
What is yet to be determined from these studies is the threshold level of tryptophan necessary in a protein-rich/carbohydrate-poor diet that will elicit the maximum response on the plasma tryptophan:LNAA ratio and subsequent levels of serotonin in the brain needed to effect changes in cognitive performance. A study by Teff and colleagues (1989) suggests that only an increase in tryptophan:LNAA ratio of 50 percent or more causes meaningful increases in brain serotonin; however, animal studies have shown effects at lower levels. What can be ascertained at present from this work is the relative safety of use from naturally occurring, enriched levels of tryptophan in food-based products. Improvements in cognitive function were evident together with immediate and short-term effects on plasma ratios of amino acids. Table B-18 summarizes the details of these studies.
METABOLIC COFACTORS
Carnitine
Carnitine is a conditionally essential nutrient because, under certain conditions, its requirements may exceed the body’s capacity to synthesize it. Orally, L-carnitine is used for treating primary carnitine deficiency, secondary carnitine deficiency due to inborn errors of metabolism, and carnitine deficiency in people requiring hemodialysis. Aside from primary or secondary deficiency states, typical intake of carnitine from foods provides 50 to 100 mg/day in the United States. No evidence suggests a greater amount is needed to support normal metabolic functions. The role of carnitine is to chaperone the transport of medium- to long-chain fatty acids across mitochondrial membranes to facilitate their oxidation with subsequent energy production. By formation of acylcarnitines from acyl-CoA metabolic intermediates, carnitine may optimize the intracellular milieu for complete oxidation of glucose and fatty acids. Carnitine also has an important metabolic role in the exercising muscle. The hypothesis is that supplementation might enhance the oxidation of fatty acids during exercise, sparing the use of muscle glycogen, thereby delaying the onset of fatigue and culminating in enhanced physical performance. The data in this area are mixed and are not robust. For a number of reasons, carnitine supplementation would not be expected to enhance physical performance: whole-body carnitine homeostasis is highly regulated and compartmentalized; it exists in a large endogenous pool refractive to rapid change; exhibits low bioavailability (16 to 18 percent), with a rapid renal excretion; and it is regulated by a number of saturable transport
TABLE B-18 Results of Human Studies Utilizing Whole Foods on Tryptophan:LNAA Ratio
|
Reference |
Subjects |
Study Design |
Preparation |
|
Beulens et al., 2004 |
N = 18 healthy males, mean age 22 ± 4 y |
DBRPC-CO |
CHO only (10 g CHO, 0 protein) versus α-lactalbumin plus CHO (CHO 20 g, protein 12 g as α-lactalbumin enriched whey protein) |
|
Markus et al., 1998 |
N = 48 healthy men and women; mean age 21.2 ± 0.4 years 24 high stress prone; 24 low stress prone |
Open |
CR/PP (62% CHO, 3.6% protein) versus PR/CP (26.3% CHO, 40.4% protein) |
|
Markus et al., 1999 |
N= 43 healthy men and women; mean age 22.5 years 22 high stress prone and 21 low stress prone |
Open |
Same as above |
|
Markus et al., 2000 |
N = 58 |
DBPC healthy mean and women; mean age 20.5 years. 29 high stress prone and 21 low stress prone |
α-lactalbumin enriched whey protein (12.32 g/kg tryptophan; ratio tryptophan:LNAA 8.7) versus Sodium caseinate control (9.51 g/kg tryptophan; ratio tryptophan:LNAA 4.7) |
|
Dosing Schedule |
Test Exposure |
Effects |
|
Drink supplement—one hour after breakfast containing CHO 50%, protein 15% and fat 35%. |
2 test sessions, each separated by 2 weeks. Assessment of plasma amino acids, serum prolactin, insulin concentrations and mood (POMS) at baseline, 60 and 90 minutes. |
Mean tryptophan: LNAA ratio increased (16%) from 0.084 ± 0.003 on α-lactalbumin diet to 0.097 ± 0.003 and decreased 17% from 0.087 ± 0.003 to 0.073 ± 0.002 after CHO only diet. Decrease in serum prolactin slightly smaller after α-lactalbumin than CHO alone. No significant differences in appetite, food intake, macronutrient preference, insulin levels or mood between diets. After α-lactalbumin, changes in tryptophan:LNAA ratio correlated significantly with changes in anger score (r=-0.65), and tension score (r=0.59). |
|
Consumed as breakfast, snack and lunch; diet test periods were 4 weeks apart. |
Mental arithmetic and memory scanning test during uncontrollable stress (POMS). |
Mean tryptophan:LNAA ratio increased from 0.074 ± 0.01 on PR/CP to 0.105 ± 0.015 on CR/PP (42% increase). High stress subjects on CR/PP did not show stress-induced rise in depression (POMS). Mean reaction time was not significantly different between high stress and low stress subjects and increased for both groups on CR/PP diet. |
|
Same as above |
Mental arithmetic and memory scanning test and mood assessment (POMS) during controllable and uncontrollable stress. |
Memory scanning after controllable stress improved in high stress subjects on CR/PP diet. Mean reaction time for high stress on PR/CP increased significantly after controllable stress from 700 to 900 minutes. |
|
Active ingredients given in matching chocolate drink given before breakfast and before lunch. Diets were isoenergetic with equal amounts of CHO, fats, and protein. |
Stress-inducing timed mental arithmetic task with industrial noise stress, and POMS. Salivary and plasma cortisol levels. Skin conductance. Heart rate. |
Mean tryptophan:LNAA ratio increased from 0.071 ± 0.012 on casein diet to 0.104 ± 0.013 on α-lactalbumin diet (48%). High stress subjects had higher (40%) prolactin concentrations, decreased salivary and plasma cortisol levels and reduced depressive (POMS) feelings under stress on α-lactalbumin. Rise in the cortisol stress response was prevented in high stress but not in low stress on α-lactalbumin diet. |
mechanisms. Studies suggest that muscle function is sensitive to carnitine supplementation when muscle carnitine content is < 25 to 50 percent of normal but insensitive to changes in muscle carnitine content around normal levels (Brass, 2004). Exercise studies in healthy subjects have failed to consistently define an effect of supplementation on more than one metabolic parameter of interest. Many of the studies suffer from small sample sizes, lack of appropriate control groups, and short duration. In general, L-carnitine appears to be well tolerated, and no serious toxicity has been demonstrated when given in oral doses up to several grams (Goa and Brogden, 1987). The D-isomer is not biologically active and D-isomer formulations can compete with the L-isomer. At this time, it appears that L-carnitine does not have a significant role in enhancing physical performance in the short term, but additional research is needed to ascertain the benefits of L-carnitine in certain states that alter the requirements for L-carnitine, such as physical or psychological stress. These studies would benefit from well-designed, adequately powered clinical trials with robust clinical performance endpoints.
Also of interest is L-carnitine’s emerging role in immunomodulation, as
|
Dosing Schedule |
Test Exposure |
Effects |
|
Same as above |
Computerized stress-inducing timed mental task |
Mean tryptophan: LNAA ratio increased from 0.073 ± 0.012 on casein diet to 0.104 ± 0.013 on α-lactalbumin diet (43%). Mean reaction time for high stress on α-lactalbumin was significantly lower (758 ± 137 ms) compared to casein control (800 ± 173 minutes). |
|
2 breakfasts, 3–7 days apart |
Timed collection of plasma for assessment of tryptophan:LNAA ratio at baseline, 40, 80, 120, and 240 min |
Tryptophan:LNAA and tyrosine/LNAA ratios on CR diet at 40, 80, 120, 240 were not different from baseline. Tryptophan:LNAA and tyrosine/LNAA ratios on PR diet at 40, 80, 120, 240 minutes were statistically significant from baseline. Median difference for tryptophan:LNAA was 54% (range 36–88%) and for tyrosine:LNAA was 28 % (range 10–64%). |
demonstrated in both animal and human studies. In rodent studies, carnitine (50 to 100 mg/kg body weight) was shown to reduce lipopolysaccharide (LPS)-induced cytokine production with improved survival during cachexia and septic shock (Ruggiero et al., 1993; Winter et al., 1995). Carnitine was also shown to reduce the ex vivo release of tumor necrosis factor alpha (TNF-α) by S. aureus-stimulated human polymorphonuclear white blood cells (Fattorossi et al., 1993). Carnitine administration in patients undergoing surgery (8 g intravenously) or in patients with HIV+ (6 g/day for two weeks) can significantly decrease serum TNF-α levels (De Simone et al., 1993; Delogu et al., 1993). Recently, Alesci and colleagues (2004) have suggested that the immunomodulatory properties of L-carnitine may be mediated by its interaction with the glucocorticoid receptor. Similar to dexamethasone, which is a synthetic glucocorticoid, carnitine at millimolar concentrations triggered nuclear translocation of human glucocorticoid-receptor alpha (GRα), stimulated GRα-mediated transactivation of known glucocorticoid-responsive promoters, suppressed in a GRα-dependent fashion; and stimulated release of proinflammatory cytokines from human monocytes. These proposed modulatory actions of L-carnitine on the glucocorticoid receptor
may be of interest to the military as related to the stressful training undertaken by soldiers in the Army Ranger program. As noted previously by Kramer et al. (1997) and Martinez-Lopez et al. (1993), infection rates in Rangers were notably elevated in association with derangements in indices of immune function. High levels of glucocorticoids during chronic stress suppress most aspects of the immune response (Dhabhar and McEwen, 1997) and the ability to fight infection and mount an antibody response (Dhabhar, 2002). The net effect is a suppression of proinflammatory cytokines by glucocorticoids, with an increase in antiinflammatory cytokines leading to overall immunosuppression (IOM, 2004). New mechanistic data on L-carnitine may warrant research studies in situations of high stress and compromised nutritional status.
Coenzyme Q10
Coenzyme Q10 (CoQ10) is a member of the family of compounds known as ubiquinones. All animals, including humans, can synthesize ubiquinones, and no CoQ10 deficiency syndromes have been reported in humans; therefore, a varied diet provides sufficient CoQ10 for healthy individuals. Oral supplementation with CoQ10 increases the plasma and lipoprotein concentrations of CoQ10 in humans (Crane, 2001; Mohr et al., 1992). What is not clear is whether supplementation increases CoQ10 concentrations in other tissues of individuals with normal endogenous CoQ10 synthesis. Several placebo-controlled clinical trials in either trained or untrained men have demonstrated a lack of effect of CoQ10 on parameters of physical performance. These parameters include VO2max and exercise time to exhaustion (Braun et al., 1991; Malm et al., 1997; Porter et al., 1995; Weston et al., 1997), blood lactate levels, and rate of perceived exertion (Porter et al., 1995) in response to cycle ergometer testing. Doses ranging from 100 to 150 mg of CoQ10, with intervention periods lasting from three to eight weeks in duration, have been reported. Two studies in trained men found significantly greater improvements in measures of anaerobic (Malm et al., 1997) and aerobic exercise performance (Laaksonen et al., 1995) with a placebo as compared with CoQ10. While supplementation with CoQ10 appears safe in doses as high as 1,200 mg/day up to 16 months (Shults et al., 2002) its role for enhancing performance cannot be supported with existing data.
PERFORMANCE-ENHANCING BOTANICALS
Panax ginseng
Among the botanicals considered as adaptogens, agents taken as a general tonic to increase resistance to environmental stress, Panax ginseng (Asian ginseng) is the most studied in terms of enhancing performance. Its ergogenic effects have been characterized in terms of physical performance (exercise out-
comes, physiological changes, metabolic measures, and assessment of hormone levels) as well as in terms of cognitive performance (psychomotor measures, reaction time, mood, cognition, memory, and accuracy of repetitive tests) [see review by Bucci et al. (2004)]. Conclusions drawn from a recent systematic review of the literature note that the evidence is contradictory for benefits to physical performance but may be suggestive of benefit for psychomotor performance and cognitive behavior (Vogler et al., 1999). The majority of the studies found at least one significant change in mental functions with Asian ginseng, but responses were not uniform across studies. While the use of ginseng is associated with a feeling of general well-being, in addition, reaction times to auditory or visual cues improved and fatigue and errors in cognitive tasks reduced. Short-term use (four weeks or less) of ginseng does not result in benefits to mental or physical performance, but long-term use (over 12 weeks) in situations of compromised performance suggests some benefit in mood, reaction times, neuromuscular control, mental functions, and work capacity (Bucci et al., 2004). As with other clinical studies evaluating dietary supplements, ginseng studies also suffer from small sample sizes, short duration of use, and poorly characterized products. Also, it appears that ginseng with its delayed onset of action would not be an appropriate supplement for military use in acute situations.
Ginkgo biloba
Ginkgo biloba extract is one of the most prescribed phytomedicines in Germany and France. Physicians prescribe it for dementia, vertigo, anxiety, headaches, tinnitus, peripheral vascular occlusive disease, and other problems. There are also indications that it is an effective antioxidant with free-radical scavenging activity (Christen, 2004). In recent years, ginkgo has been a top selling dietary supplement in the United States. Ginkgo preparations are derived from the dried, green leaf of Ginkgo biloba. L. Ginkgo biloba extracts (GBEs), contain several constituents including flavonoids, terpenoids, and organic acids. GBE is standardized to contain 24 percent flavonoids and 6 percent terpenes. Although many of ginkgo’s individual constituents have intrinsic pharmacological effects, there is some evidence that the constituents work synergistically to produce more potent pharmacological effects than any individual constituent.
Evidence suggests that chronic administration of standardized extracts of Ginkgo biloba can ameliorate cognitive decline associated with aging (Ernst and Pittler, 1999; Oken et al., 1998). However, relatively few studies of ginkgo’s effect on cognitive measures in healthy adults are available. A systematic review (Canter and Ernst, 2002) identified nine placebo-controlled, double-blind studies utilizing standardized extracts of Ginkgo biloba, the longest trial lasting 30 days. The authors concluded that there were no consistent positive effects of ginkgo on objective measures of cognitive function in healthy populations. Two studies published since the 2002 review provide additional data suggesting beneficial
effects for ginkgo on measures of cognitive function. Kennedy and colleagues (2002), building on a series of previous investigations (Kennedy et al., 2000, 2001), evaluated the acute cognitive effects of herbal extracts. They conducted a randomized placebo-controlled double-blind crossover study to compare the effects of single doses of GBE, ginseng, and a combination product on aspects of mood (Bond-Lader visual analogue scale) and cognitive performance (Cognitive Drug Research computerized assessment battery, CDR) and several subtraction mental arithmetic tasks (Kennedy et al., 2002). Fifteen female and five male healthy young subjects (mean age 21.2) received 360 mg of GBE (GK501), 400 mg of ginseng (G115), 960 mg of a product combining the two extracts (Ginkoba M/E), and a matching placebo on four separate occasions. Each test session was separated by a seven-day washout period. Measurements were obtained at baseline 1, 2.5, 4, and 6 h after dosing. All three treatments were associated with improved secondary memory performance defined as accuracy of immediate and delayed word recall, picture, and word recognition tasks on the CDR battery. Ginseng showed some improvement in the speed of performing memory tasks (speed of performance of spatial and numeric working memory and picture and word recognition tasks) at four hours postdose. Additionally, ginseng showed improvements in the accuracy of attentional tasks (accuracy of performing choice reaction time and digit vigilance tasks) at 2.5 h postdose. GBE and the GBE/ginseng combination improved performance on both the serial threes and serial sevens subtraction tests at six hours postdose. In another study conducted in 60 healthy adults aged 50 to 65 years, Cieza and colleagues (2003) demonstrated positive effects of 240 mg of GBE (EGb761) three times daily over a four-week period. Primary outcome measures included the subject’s judgment of their own mental health, their general health and their quality of life determined on the basis of three difference visual analog scales. Secondary outcomes (15 tests and procedures) were chosen to represent neurobiologically based functions. Results of finger tapping test-maximal tempo indicated favorable effects of GBE on mental function action and reaction times.
Perhaps of greater interest in the military theater, GBE has also been studied for the prevention and mitigation of acute mountain sickness or high-altitude illness. The Lake Louise Consensus Group defined acute mountain sickness (AMS) as the presence of headache in an unacclimatized person who has recently arrived at an altitude above 2,500 meters plus the presence of other symptoms such as: gastrointestinal symptoms (anorexia, nausea, or vomiting), insomnia, dizziness, and lassitude or fatigue (Roach et al., 1993). If left untreated the condition may progress to life threatening pulmonary or cerebral edema. Whether or not AMS occurs is determined by the rate of ascent, the altitude reached, the altitude at which an affected person sleeps, and individual physiology. Physical fitness does not appear to be protective against AMS (Hackett and Roach, 2001). Safe and effective drugs, such as acetazolamide, are available to treat AMS.
Four randomized double-blind placebo-controlled studies (Chow et al., 2002;
Gertsch et al., 2002, 2004; Roncin et al., 1996) and one nonrandomized but double-blind placebo-controlled study (Leadbetter et al., 2001) totaling 781 participants have been reported utilizing GBE for AMS with mixed results. The dosage of GBE ranged from 160 to 240 mg (brands not always identified) daily with pretreatment ranging from one to thirty days prior to ascent. In the studies by Chow et al. (2002) and Gertsch et al. (2004) the comparator drug was acetazolamide, given as 250 mg twice daily, which demonstrated superiority in prevention of acute symptoms as coded using the Lake Louise Scoring system and showed that ginkgo offered no additional benefit in reduction of symptoms compared to placebo. While the study by Gertsch et al. (2004) enrolled 614 healthy western trekkers, mean age 36.6 years old, and predominately male, a number of shortcomings of the study were identified. These included short duration of dosing prior to ascent (3 to 4 doses), administration of the intervention to participants at a high baseline altitude (as opposed to starting the intervention at sea level before ascent), coupled with a 20 percent drop-out rate (although analysis was by intention-to-treat). In the study by Chow et al. (2002), both men and women were enrolled (age range 25 to 65, mean 36.5 years) and were dosed five days prior to ascent, and were driven from a level of 4,000 feet to a final altitude of 12,470 feet over a two-hour period. An earlier study by Gertsch and colleagues (2002) demonstrated that pretreatment of 180 mg of GBE (GK501) one day prior to ascent from sea level to 4,205 meters over three hours by air showed that ginkgo lowered the incidence of AMS but not significantly as compared to placebo. The two studies with positive outcomes for GBE (Leadbetter et al., 2001; Roncin et al., 1996) provided either 160 or 240 mg of GBE daily with pretreatment ranging from 5 to 30 days prior to ascent. The study by Roncin and colleagues (1996) tested EGb761 for the prevention of AMS and also studied vasomotor changes (cold gradient) of the extremities using plethysmorgraphy during a 30-day Himalayan expedition. These investigators found that none of the subjects randomized to EGb761 developed acute mountain sickness versus 40.9 percent of subjects in the placebo group as determined by responses to an Environmental Symptoms Questionnaire (ESQ)–cerebral factor (Sampson et al., 1983). A small percentage (13.6 percent) of subjects on EGb761 compared with subjects on placebo (81.8 percent) developed AMS as determined by the ESQ–respiratory factor. Evaluation of a “cold gradient” was measured by plethysmography and a specific questionnaire. Subjects randomized to EGb 761 showed a mean improvement of the cold gradient of 22.8 percent compared to the placebo group that deteriorated by 104 percent across 20 days of study. There was a marked increase in hand blood flow in the subjects on ginkgo compared to placebo. Also of possible importance to field maneuvers, diuresis of the EGb 761 group was decreased to a much lesser extent than in the placebo group suggesting a tendency for poorly adapted subjects to accumulate excess fluid, which could predispose them to an increased risk of developing altitude edema. The mechanism of action for the effects of GBE cannot be attributed to a single
action or to a single molecule in the extract. Research suggests that ginkgo could act in several different ways to prevent AMS. It may block the enzyme inducible nitric oxide synthase, which produces nitric oxide, act as an antioxidant oxygen radical scavenger, or may block platelet-activating factors (Christen, 2004).
In the studies reviewed above, ginkgo was well tolerated and without side effects and is likely safe when used orally and appropriately. Ginkgo may be contraindicated in individuals with epilepsy or in individuals who are prone to seizures, individuals with diabetes or those taking prescription anticoagulants.
New data on GBE for the prevention of AMS from controlled trials coupled with data from basic and mechanistic studies provides justification for additional investigation of this product. Lastly, if GBE were to be incorporated into food-based products and depending on the intended use of the product, potential regulatory issues (unlike vitamin and mineral nutrients) would need to be addressed prior to distribution.
OTHER ADAPTOGENIC HERBS
Preliminary data exist for a number of other adaptogenic herbs to include Cordyceps sinensis, Eleutherococcus senticosus, and Rhodiola. Cordyceps sinensis gained the interest of Americans in 1994 following the unprecedented record-breaking running performances by Chinese athletes (Steinkraus and Whitfield, 1994). In China, Cordyceps sinensis is an herbal pharmaceutical under investigation for treatment of immune deficiencies, cardiovascular diseases, diabetes, cancer, and inflammatory diseases (Zhu et al., 1998a, b). Five small, controlled, human studies measuring parameters of physical performance (such as VO2max, ventilatory threshold, respiratory exchange ratio, anaerobic threshold, and exercise time) using the same Cordyceps sinensis product (3 g/day) for 4 to 12 weeks suggest that Cordyceps sinensis may be associated with some improvements in untrained persons, but these improvements could not be routinely expected for trained persons (Bucci et al., 2004).
Rhodiola species, also known as goldenroot, roseroot, and Arctic ginseng, have been well studied and characterized by Russian and Scandinavian researchers. The Soviet Ministry of Health has approved a Rhodiola extract liquid (in 40 percent ethanol) for use as a stimulant to relieve fatigue, improve memory, improve attention span and work productivity in healthy persons (Brown et al., 2002; Germano et al., 1999; Kelly, 2001). Sweden and Denmark also have approved Rhodiola extracts (SHR-5) as antifatigue stimulants and adaptogens (Brown et al., 2002; Shevtsov et al., 2003). In one randomized double-blind placebo controlled trial in 121 military cadets on night duty, 185 mg of Rhodiola rosea SHR-5 extract acutely improved an antifatigue index for mental work while fatigued, and it decreased errors on ring attention and numbers tests (Shevtsov et al., 2003). In summary, standardized extracts used in clinical trials show potential for improving some aspects of mental (electroencephalogram, memory, reduction of errors performing
tasks, shooting accuracy, and hearing) and physical (increased work, increased run time to exhaustion, heart rate record post-exercise, and less fatigue) performance in athletes and sedentary subjects (Bucci et al., 2004). A combination formula containing 1,000 mg of Cordyceps sinensis and 300 mg of Rhodiola was recently tested with a two week treatment in 17 competitive amateur cyclists in a randomized, placebo-controlled, double-blind study design. The formula failed to elicit positive changes for peak exercise variables (VO2max, time to exhaustion, peak workload, or peak heart rate) as well as for subpeak exercise variables (power output ventilatory threshold, respiratory compensation or VO2) (Earnest et al., 2004). The authors note that a longer period of supplementation may have provided a more efficacious response, but this awaits further study.
SUMMARY
This chapter has focused on a number of bioactive food and dietary supplement components that have been evaluated for their performance enhancing qualities. The data presented suggest that if amino acid precursors are to be further tested, their incorporation into a food matrix is preferable over that of a dietary supplement. While research on L-carnitine for enhancing physical performance has not been promising, the role for L-carnitine and its interactions with the glucocorticoid receptor, and particularly for its potential effects on immunomodulation, warrant further exploration. The use of botanicals most likely would not provide short-term or immediate enhancement to performance, except possibly for standardized extracts of Rhodiola and Ginkgo biloba, which warrant more rigorous study.
In the design of future studies, important considerations are the increased precursor, coenzyme, and energy demands during stress that would be met differently by the human population, possibly distributed in the form of a bell-shaped curve, with individuals ranging from coping poorly to coping extremely well. Individuals to benefit the most from necessary supplements would most likely be in the “coping poorly” category, as was shown in the studies by Markus et al. (1998, 1999, 2000). Of course, persons with discrete defects in stress coping, constituting a separate group of individuals with their own transposed bell-shaped curve, could also benefit from needed supplements if their defects could be corrected in the presence of excess bioactive food components. The possibility that only a subgroup of the population would benefit from bioactive food components, and only when they are challenged by stress, makes the design of clinical studies of these agents difficult, because it presupposes controlling two continuous variables—degree of coping capacity and amount of stress impose—both of which depend on myriad factors. The former is a product of genetics, developmental history, and environment, while the latter depends on the diverse types of stressors applied with different strengths for different times.
REFERENCES
Alesci S, De Martino MU, Kino T, Ilias I. 2004. L-Carnitine is a modulator of the glucocorticoid receptor alpha. Ann N Y Acad Sci 1024:147–152.
Beulens JW, Bindels JG, de Graaf C, Alles MS, Wouters-Wesseling W. 2004. Alpha-lactalbumin combined with a regular diet increases plasma Trp-LNAA ratio. Physiol Behav 81(4):585–593.
Brass EP. 2004. Carnitine and sports medicine: Use or abuse? Ann N Y Acad Sci 1033:67–78.
Braun B, Clarkson PM, Freedson PS, Kohl RL. 1991. Effects of coenzyme Q10 supplementation on exercise performance, VO2max, and lipid peroxidation in trained cyclists. Int J Sport Nutr 1(4):353–365.
Brown RP, Gerbarg PL, Ramazanov Z. 2002. Rhodiola rosea: A phytomedicinal overview. [Online] HerbalGram. American Botanical Council. 56:40–52. Available at: http://www.herbalgram.org/herbalgram/articleview.asp?a=2333 [accessed April 1, 2005].
Bucci LR, Turpin AA, Beer C, Feliciano J. 2004. Ginseng. In: Wolinsky I, Driskell JA, eds. Nutritional Ergogenic Aids. Boca Raton, FL: CRC Press. Pp. 379–410.
Canter PH, Ernst E. 2002. Ginkgo biloba: A smart drug? A systematic review of controlled trials of the cognitive effects of ginkgo biloba extracts in healthy people. Psychopharmacol Bull 36(3):108–123.
Chinevere TD, Sawyer RD, Creer AR, Conlee RK, Parcell AC. 2002. Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance. J Appl Physiol 93(5):1590–1597.
Chow TK, Browne VA, Heileson HL, Wallace DR, Anholm JD. 2002. Comparison of Ginkgo biloba versus acetazolamide in the prevention of acute mountain sickness. Med Sci Sports Exerc 34(Suppl 1):S246.
Christen Y. 2004. Ginkgo biloba: From traditional medicine to molecular biology. In: Packer L, Ong CN, Halliwell B, eds. Herbal and Traditional Medicine. Molecular Aspects of Health. New York: Marcel Dekker. Pp. 145–164.
Cieza A, Maier P, Poppel E. 2003. Effects of Ginkgo biloba on mental functioning in healthy volunteers. Arch Med Res 34(5):373–381.
Crane FL. 2001. Biochemical functions of coenzyme Q10. J Am Coll Nutr 20(6):591–598.
De Simone C, Tzantzoglou S, Famularo G, Moretti S, Paoletti F, Vullo V, Delia S. 1993. High dose L-carnitine improves immunologic and metabolic parameters in AIDS patients. Immunopharmacol Immunotoxicol 15(1):1–12.
Deijen JB, Wientjes CJ, Vullinghs HF, Cloin PA, Langefeld JJ. 1999. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res Bull 48(2):203–209.
Delogu G, De Simone C, Famularo G, Fegiz A, Paoletti F, Jirillo E. 1993. Anaesthetics modulate turnover necrosis factor alpha: Effects of L-carnitine supplementation in surgical patients. Preliminary results. Med Inflamm 2:S33–S36.
Dhabhar FS. 2002. Stress-induced augmentation of immune function—The role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun 16(6):785–798.
Dhabhar FS, McEwen BS. 1997. Acute stress enhances while chronic stress supresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav Immun 11(4):286–306.
Earnest CP, Morss GM, Wyatt F, Jordan AN, Colson S, Church TS, Fitzgerald Y, Autrey L, Jurca R, Lucia A. 2004. Effects of a commercial herbal-based formula on exercise performance in cyclists. Med Sci Sports Exerc 36(3):504–509.
Ernst E, Pittler MH. 1999. Ginkgo biloba for dementia: A systematic review of double-blind placebo controlled trials. Clin Drug Invest 17(4):301–308.
Fattorossi A, Biselli R, Casciaro A, Tzantzoglou S, De Simone C. 1993. Regulation of normal human polymorphonuclear leucocytes by arnitine. Med Inflamm 2:S37–S41.
Germano C, Ramazanov Z, Del Mar Bernal Suarez M. 1999. Rhodiola rosea and human performance. In: Appell B, ed. Arctic Root (Rhodiola rosea) The Powerful New Ginseng Alternative. New York: Kensington Publishing. P. 112.
Gertsch JH, Basnyat B, Johnson EW, Onopa J, Holck PS. 2004. Randomised, double blind, placebo controlled comparison of Ginkgo biloba and acetazolamide for prevention of acute mountain sickness among Himalayan trekkers: The prevention of high altitude illness trial (PHAIT). Br Med J 328(7443):797–801.
Gertsch JH, Seto TB, Mor J, Onopa J. 2002. Ginkgo biloba for the prevention of severe acute mountain sickness (AMS) starting one day before rapid ascent. High Alt Med Biol 3(1):29–37.
Goa KL, Brogden RN. 1987. l-Carnitine. A preliminary review of its pharmacokinetics, and its therapeutic use in ischaemic cardiac disease and primary and secondary carnitine deficiencies in relationship to its role in fatty acid metabolism. Drugs 34(1):1–24.
Hackett PH, Roach RC. 2001. High-altitude illness. N Eng J Med 345(2):107–114.
IOM (Institute of Medicine). 1994. Food Components to Enhance Performance. Washington, DC: National Academy Press.
IOM. 1999. The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington, DC: National Academy Press.
IOM. 2002. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington DC: The National Academies Press.
IOM. 2004. Monitoring Metabolic Status. Predicting Decrements in Physiological and Cognitive Performance. Washington, DC: The National Academies Press.
Kelly GS. 2001. Rhodiola rosea: A possible plant adaptogen. Altern Med Rev 6(3):293–302.
Kennedy DO, Scholey AB, Wesnes KA. 2000. The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacology (Berl) 151(4):416–423.
Kennedy DO, Scholey AB, Wesnes KA. 2001. Differential, dose dependent changes in cognitive performance following acute administration of a Ginkgo biloba/Panax ginseng combination to healthy young volunteers. Nutr Neurosci 4(5):399–412.
Kennedy DO, Scholey AB, Wesnes KA. 2002. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol Behav 75(5):739–751.
Kramer TR, Moore RJ, Shippee RL, Friedl KE, Martinez-Lopez L, Chan MM, Askew EW. 1997. Effects of food restriction in military training on T-lymphocyte responses. Int J Sports Med 18(Suppl 1):S84–S90.
Laaksonen R, Fogelholm M, Himberg JJ, Kaakso J, Salorinne Y. 1995. Ubiquinone supplemetation and exercise capacity in trained young and older men. Eur J Appl Physiol Occup Physiol 72(1-2):95–100.
Leadbetter G, Maakestad K, Olson S, Hackett P. 2001. Ginkgo biloba reduces the incidence and severity of acute mountain sickness. High Altitude Med Biol 2(1):110.
Lieberman HR. 1994. Tyrosine and stress: Human and animal studies. In: Marriott BM, ed. Food Components to Enhance Performance. Washington, DC: National Academy Press. Pp. 277–299.
Lieberman HR. 1999. Amino acid and protein requirements: Cognitive performance, stress, and brain function. In: The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington, DC: National Academy Press. Pp. 289–307.
Lieberman HR. 2003. Nutrition, brain function and cognitive performance. Appetite 40(3):245–254.
Lieberman HR, Spring BJ, Garfield GS. 1986. The behavioral effects of food constituents: Strategies used in studies of amino acids, protein, carbohydrate and caffeine. Nutr Rev 44(Suppl):61–70.
Malm C, Svensson M, Ekblom B, Sjodin B. 1997. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand 161(3):379–384.
Markus CR, Olivier B, de Haan EH. 2002. Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am J Clin Nutr 75(6):1051–1056.
Markus CR, Olivier B, Panhuysen GE, Van Der Gugten J, Alles MS, Tuiten A, Westenberg HG, Fekkes D, Koppeschaar HF, de Haan EE. 2000. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am J Clin Nutr 71(6):1536–1544.
Markus CR, Panhuysen G, Jonkman LM, Bachman M. 1999. Carbohydrate intake improves cognitive performance of stress-prone individuals under controllable laboratory stress. Br J Nutr 82(6):457–467.
Markus CR, Panhuysen G, Tuiten A, Koppeschaar H, Fekkes D, Peters ML. 1998. Does carbohydrate-rich, protein-poor food prevent a deterioration of mood and cognitive performance of stress-prone subjects when subjected to a stressful task? Appetite 31(1):49–65.
Martinez-Lopez LE, Friedl KE, Moore RJ, Kramer TR. 1993. A longitudinal study of infections and injuries of Ranger students. Mil Med 158(7):433–437.
Mohr D, Bowry VW, Stocker R. 1992. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta 1126(3):247–254.
Neri DF, Wiegmann D, Stanny RR, Shappell SA, McCardie A, McKay DL. 1995. The effects of tyrosine on cognitive performance during extended wakefulness. Aviat Space Environ Med 66(4):313–319.
Oken BS, Storzbach DM, Kaye JA. 1998. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol 55(11):1409–1415.
Porter DA, Costill DL, Zachwieja JJ, Krzeminski K, Fink WJ, Wagner E, Folkers K. 1995. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int J Sports Med 16(7):421–427.
Roach RC, Bartsch P, Oelz O, Hackett PH. 1993. Lake Louise AMS Scoring Consensus Committee. The Lake Louise acute mountain sickness scoring system. In: Sutton JR, Houston CS, Coates G, eds. Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Smposium held at Lake, Louise, Canada, February 9–13, 1993. Burlington, VT: Queen City Printers. Pp. 272–274.
Roncin JP, Schwartz F, D’Arbigny P. 1996. EGb 761 in control of acute mountain sickness and vascular reactivity to cold exposure. Aviat Space Environ Med 67(5):445–452.
Ruggiero V, D’Urso CM, Albertoni C, Campo S, Foresta P, Arrigoni-Martelli E. 1993. LPS-induces serum TNF production and lethality in mice: Effect of L-carnitine and some acyl-derivatives. Med Inflamm 2:S43–S50.
Sampson JB, Cymerman A, Burse RL, Mahr JT, Rock PB. 1983. Procedures for the measurement of acute mountain sickness. Aviat Space Environ Med 54(12 Pt 1):1063–1073.
Shevtsov VA, Zholus BI, Shervarly VI, Vol’skij VB, Korovin YP, Khristich MP, Roslyakova NA, Wikman G. 2003. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine 10(2–3):95–105.
Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M. 2002. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch Neurol 59(10):1541–1550.
Steinkraus DC, Whitfield JB. 1994. Chinese caterpillar fungus and world record runners. Am Entomologist 40:235–239.
Strüder HK, Weicker H. 2001. Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance. Part II. Int J Sports Med 22(7):482–497.
Teff KL, Young SN, Marchand L, Botez MI. 1989. Acute effect of protein or carbohydrate breakfasts on human cerebrospinal fluid monoamine precursor and metabolite levels. J Neurochem 52(1):235–241.
Vogler BK, Pittler MH, Ernst E. 1999. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol 55(8):567–575.
Weston SB, Zhou S, Weatherby RP, Robson SJ. 1997. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int J Sport Nutr 7(3):197–206.
Winter BK, Fiskum G, Gallo LL. 1995. Effects of L-carnitine on serum triglyceride and cytokine levels in rat models of cachexia and septic shock. Br J Cancer 72(5):1173–1179.
Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. 2003. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr 77(1):128–132.
Yeghiayan SK, Luo S, Shukitt-Hale B, Lieberman HR. 2001. Tyrosine improves behavioral and neurochemical deficits caused by cold exposure. Physiol Behav 72(3):311–316.
Zhu JS, Halpern GM, Jones K. 1998a. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: Part II. J Altern Complement Med 4(4):429–457.
Zhu JS, Halpern GM, Jones K. 1998b. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part I. J Altern Complement Med 4(3):289–303.
Effect of Physical Activity and Other Stressors on Appetite: Overcoming Underconsumption of Military Operational Rations, Revisited
R. James Stubbs and Stephen Whybrow, Rowett Research Institute
Neil King and John E. Blundell, Leeds University
Marinos Elia, Southhampton General Hospital
INTRODUCTION
The ready availability of a huge variety of energy-dense, palatable food is considered partly responsible for the current trends in obesity and attendant diseases in Western society. Although intuitively, it would seem that widescale under-consumption should not be a problem, underconsumption of military operational rations has been a subject of some concern to the United States. In 1995, the Committee on Military Nutrition Research (CMNR) produced a report entitled Not Eating Enough (IOM, 1995), a comprehensive report covering the causes and consequences of underconsumption in field operations, the strategies, and need for research to increase intake of military rations in combat (IOM, 1995). The majority of data sourced for this report is related to experimental, comprehensive field studies during military exercises and with garrisoned soldiers. These environments clearly differ from each other and from combat itself. The report acknowledged a lack of data from combat or near-combat situations regarding ration intake, acceptability, appetite and feeding behavior. Understandably, most data are anecdotal; however, the report noted that the nagging hunger of energy restriction differs from the appetite-suppressed state that is characteristic of illness, trauma or emotional distur-
bances. They observed that “Based on the discomforts associated with the field situation—the anxiety, fatigue, aches and pains, and assorted other problems—decline of appetite is likely to be a prominent factor in explaining underconsumption of military operational rations. Field training or combat anorexia thus may be the result of a generalized stress response” (IOM, 1995).
The purpose of this paper is to consider appetite control in relation to the high energy expenditure and additional stressors in a combat operation. Apparently, during combat operations, the expected daily energy expenditure is 4,000 to 4,500 kcal, achieved through intermittent periods of high energy expenditure (> 50 percent VO2max) mixed with longer periods of low intensity movement sustained for about 20 h per day. The daily ration must fit within 0.12 cubic feet and weigh 3 pounds or less. The soldiers rely on this ration for three to seven days followed by one to three days of recovery when they will have access to more nutritionally complete meals (i.e., ad libitum food availability served in field kitchen setting). During combat these troops will therefore experience a 1,600 to 2,100 kcal/day energy deficit. This work focuses on these conditions and time window(s). Specifically, the following questions were addressed:
-
Is the energy deficit that soldiers experience in combat due to the relationship between high levels of physical activity, appetite, and energy intake during periods of high energy requirements?
-
How do the additional stresses of combat impinge on appetite and what do we know of the mechanisms?
-
What types of nutritional ingredients or packaging can be added to maintain appetite and hence, presumably, physiological and cognitive performance?
RELATIONSHIP BETWEEN HIGH LEVELS OF PHYSICAL ACTIVITY, APPETITE, AND ENERGY INTAKE
Extremes of Intake and Expenditure
Extremes of energy intake have been recorded during overfeeding studies (Diaz et al., 1992; Norgan and Durnin, 1980; Ravussin et al., 1985), ceremonial overfeeding (Pasquet et al., 1992), prolonged underfeeding studies (Keys et al., 1950), as well as from energy intake records derived from recovering famine, prisoners of war, and concentration camp victims during World War II. These cases represent states of acclimation to extreme environmental conditions, rather than voluntarily energy intake. Compensatory responses subsequent to such induced extremes of energy balance can be spectacular, especially in relation to energy deficits. During rehabilitation from severe malnutrition, famine and concentration camp victims (with body weights below 55 kg) were recorded as spontaneously ingesting 6,900 to 7,900 kcal/day that ceased as body mass and composition were restored.
TABLE B-19 Effect of Extreme Endurance On Energy Balance In Humans
In healthy subjects, such high levels of energy intake have only been recorded under conditions of extreme physical activity such as the Tour de France (Saris, 1989) or military and polar expeditions (Stroud et al., 1993). In the case of the Tour de France, not all the energy intake is voluntary because the participants receive intravenous nutrition during sleep. These examples illustrate the extremes to which energy intake can adapt to intense physiological demands imposed by very high levels of physical activity (Saris, 1989) or severe malnutrition. The primary stressor in these situations is the physical stress of the event. Under these relatively acute conditions in which every attempt is made to meet energy requirements, subjects are nonetheless typically characterized by a marked negative daily energy balance (Table B-19). This is not surprising because this level of expenditure represents the maximal sustainable level of human endurance. In these situations, it is not easy to eat enough to meet elevated expenditures.
The overconsumption characteristic of Western society (with the exception
of binging disorders) is not rampant but episodic. Attempting to persuade individuals to continually overeat, even to 150 percent of their maintenance requirements, is actually quite difficult when imposed as an acute change. This has implications for underconsumption in both endurance and military contexts.
Human populations have been known to subsist for quite prolonged periods on extreme ranges of diet composition despite our general tendency to consume 12 to 20 percent of our energy from protein, 35 to 40 percent from fat, and most of the rest from carbohydrate (Mela, 1995). For example, the Inuit have been reported as subsisting for months on high-fat, high-protein diets almost entirely comprising of animal matter and virtually devoid of all carbohydrate (Mowat, 1975). This produces markedly ill effects during lean months when the energy to protein ratio of the diet falls too low. Similarly, the traditional Japanese diet has been reported as constituting some 8 percent of energy intake from dietary fat, in extreme cases. It appears clear from these accounts that human populations are capable of adapting (through their physiological plasticity) to meet their physiological requirements from a wide range of macronutrient ratios in the diet. As indicated by Phinney and collegues (1983a, b) in the case of virtually carbohydrate free diets (< 20 g/day), the period of adaptation can take some days, therefore abrupt, extreme changes in the composition of rations should perhaps be avoided.
The Impact of Altered Energy Expenditure on Energy Intake
When studying the relationship between energy expenditure and energy intake, it is useful to consider how high and low levels of energy expenditure influence intake, and vice versa (Stubbs et al., 2003). The main focus of the present discussion is the relationship between appetite and energy expenditure during high levels of energy turnover. The general population is remarkably sedentary as indicated by cross-sectional surveys of levels of activity among adults and children and the fact that average levels of physical fitness are remarkably low in the population at large (Activity and Health Research, 1992). Black et al. (1996) have estimated from tracer studies that the established limits of total daily energy expenditure ranges from 1.2 to 4.5 times the basic metabolic rate (BMR) over periods of two weeks or more. Intense, sustained activity tends to be a little lower for most people at approximately 2.3 to 2.9 times the BMR, which is similar to combat levels of energy expenditure. For the general, “rather sedentary” population the average daily energy expenditures are in the range of 1.4 to 1.8 times the BMR (Black et al., 1996).
Acute increases in physical activity is believed to promote weight loss (Garrow and Summerbell, 1995). However, this is unlikely to happen over a prolonged period (e.g., Sum et al., 1994). Over time, energy intake will begin to track energy expenditure. The exact manner in which changes in levels of physical activity influence feeding behavior over periods long enough to affect
energy balance is not clearly understood. There is a large body of literature on the effect of training programs on body weight and composition in athletes (Barr and Costill, 1992; McGowan et al., 1986; van Baak, 1999; van Etten et al., 1997; Westerterp, 1998). Likewise a number of studies have examined the effects of training programs on weight loss in obese subjects (Saris, 1993; Schoeller et al., 1997). Fewer studies have examined the relationship between changes in energy expenditure and feeding behavior in nonobese subjects who do not have a pre-conceived goal associated with weight reduction or a training program. A review of the effects of exercise regimes on appetite and/or energy intake shows that in short-to medium-term intervention studies (often no longer than two to five days), 19 percent reported an increase in energy intake after exercise; 65 percent showed no change, and 16 percent showed a decrease (Blundell and King, 1999; King et al., 1997). Longer-term studies that measure body composition suggest some fat mass is lost, but lean body mass tends to be preserved in response to exercise regimes, depending on the absolute level of energy balance (Ballor and Poehlman, 1994; Sum et al., 1994). These studies suggest that in the short- to medium-term (1 day to 20 days) energy intake does not accurately track changes in energy expenditure. This raises the critical question: What is the time course over which humans begin to compensate for energy imbalances induced by systematic manipulations of energy intake or of energy expenditure? This is a difficult question to answer because the majority of studies do not track energy intake and energy expenditure on a day-by-day basis. The most precise means of objectively measuring energy expenditure in free-living humans is the doubly labeled water method, which tends to give average estimates of daily energy expenditure over periods of 10 to 20 days (Schoeller and van Santen, 1982). Daily energy expenditure can also be estimated using heart rate monitoring (Ceesay et al., 1989; Spurr et al., 1988), but this technique is widely regarded as having relatively low precision and accuracy, especially with sedentary people (Murgatroyd et al., 1993). However, in studies that employ within-subject comparisons and for which most changes in energy expenditure occur through the same exercise activities that were used to individually calibrate the heart rate monitors, this approach is most efficacious. Stubbs and colleagues (2004b) assessed the effect of no exercise (Nex:control) and high-exercise level (Hex; 4 MJ [955 kcal]/day) and two dietary manipulations, (a high-fat diet [HF]; 50 percent of energy, 167 kcal/100 g) and a low- and high-fat diet [LF]; 20 percent of energy, 71 kcal/100g) on compensatory changes in energy intake and energy expenditure over seven-day periods. Eight lean men were each studied four times, in a 2 × 2 randomized design. Energy intake was directly quantified by weight of food consumed. Energy expenditure was assessed by heart rate monitoring. Body weight was measured daily. Mean daily energy expenditures were 4,200 and 2,750 kcal/day (p < 0.001) on the pooled Hex and Nex treatments, respectively. Energy intake was higher on HF diets (3,200 kcal/day) compared to the LF diets (2,150 kcal/day). Regression analysis showed that these energy imbalances
induced significant compensatory changes in energy balances over time of 70 to 96 kcal/day (p < 0.05). These were caused by changes in both energy intake and energy expenditure in the opposite direction to the small changes in energy balance (Figure B-24). These changes were significant and small, but persistent, amounting to approximately 48 and 84 kcal/day for energy intake and energy expenditure, respectively. Under these relatively acute conditions it would take two to four weeks for a person to adjust to the altered level of energy turnover (Stubbs et al., 2004b). However, it is likely that compensation could be accelerated using, for example, sports drinks.
There is evidence that it takes considerable time for energy intake to adjust to elevations of energy expenditure in ad libitum feeding subjects. One direct intervention also supports this view (Figure B-25). Sum et al. (1994) studied the effects of five-month basic military training on body weight, body fat, and lean body mass in 42 Singapore males, classified as being normal weight (BMI 25 to 29.9 kg/m2), obese (BMI 30 to 34.9 kg/m2), and very obese (BMI > 35 kg/m2). Two key features of this study are that training was incremental, allowing subjects to gradually become fitter, and food intake was ad libitum. Over the 5 months of training, fat-free mass (FFM) did not change, but subjects lost substantial amounts of weight and body fat. Subjects who were initially fatter lost more weight and fat. This suggests that responses of intake to exercise-induced changes in energy expenditure may depend on how much fat one has. In other words, it is likely that fat mass is acting as an energy buffer, and intake rises markedly when lean-body mass is threatened by the exercise induced energy deficit. The importance of changes in lean-body mass and appetite control has been discussed by Stubbs and Elia (2001). If responses of intake to exercise-induced changes in energy expenditure depend on body fat level and compensation is due to small changes in both intake and expenditure, then crosstalk between energy expenditure and intake is initially too weak; that is, soldiers would not spontaneously adjust intake to energy requirements during short missions of three to seven days. Most likely, soldiers will need to consciously overcome this lack of spontaneous appetite response by eating more than they feel is sufficient. It appears that substantial fat loss is possible before intake begins to track a sustained elevation of energy expenditure. Friedl (1995) has considered the implications of the baseline nutritional status for physical performance during energy deficit in modern soldiers. Because modern soldiers tend to be larger and better nourished than in earlier studies, they appear to withstand the insults of an energy deficit with less decrement in performance. However, under conditions close to combat (the Ranger I study), “… soldiers who began training with very low body fat (< 10 percent) were less likely to succeed than were slightly fatter soldiers (Moore et al., 1992)” (Friedl, 1995). These data indicate that soldiers who are slightly fatter withstand the rigors of extreme field training better than those who are very lean; they also point to the importance of ensuring soldiers are fed (or overfed) for the coming mission. The Ranger I and II studies provide further
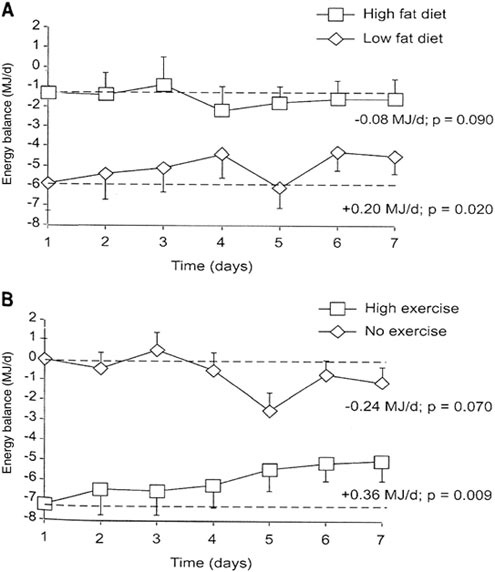
FIGURE B-24 Effect of no-exercise, (Nex) and high exercise level [(Hex); (approximately 4 MJ/day)] and two dietary manipulations [a high-fat diet (HF: 50 percent of energy, 700 kJ/100 g) and low-fat diet (LF: 20 percent of energy, 300 kJ/100 g)] on compensatory changes in energy intake (EI) and energy expenditure (EE) over 7 day periods. Eight lean men were each studied four times, in a 2 × 2 randomised design. EI was directly quantified by weight of food consumed. EE was assessed by heart rate monitoring. Top panel gives plots over time for the HF and LF diet conditions. The bottom panel plots energy balance across days for the Hex and Nex conditions.
SOURCE: Stubbs et al. (2004b). Am J Physiol Regul Integr Comp Physiol used with permission.
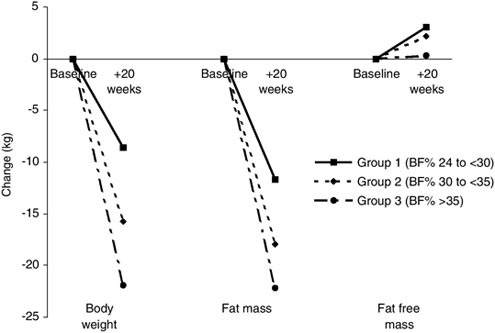
FIGURE B-25 The impact of five-month basic military training on body weight, body fat, and fat-free mass in 42 obese Singapore males.
NOTE: BF = body fat.
SOURCE: Data adapted from Sum et al. (1994).
insights into the time course over which appetite can be suppressed. While energy intake does not accurately track energy expenditure in the short to medium term, over periods of weeks and months, appetite increases again. Friedl (1995) notes that the Ranger students would all have consumed more if food was available and observes that “at least at the extreme level of deprivation of Ranger students (in the studies concerned), there was a strong hunger drive even in the face of multiple stressors” (Friedl, 1995). The same is true of the concentration camp and prisoners of war accounts.
The implications of these data for field training and combat conditions are that (1) when energy expenditure is acutely and significantly elevated, energy intake is slow to respond, and a negative energy balance ensues; (2) the degree of energy deficit will, if continued, compromise physical and cognitive function; (3) use of foods and beverages that are conducive to overconsumption may help limit the extent of the negative energy balance; and (4) if the energy deficit becomes severe over a longer period, appetite will again drive feeding behavior despite multiple stressors (Keys et al., 1950).
THE IMPORTANCE OF THE RECOVERY PERIOD TO INCREASED FOOD INTAKE
During acute, high levels of energy expenditure, both appetite and energy intake characteristically fail to track elevated energy expenditure (Stubbs et al., 2004a). What is remarkable about these situations is the extent of negative energy balance subjects appear able to sustain, at least in the short to medium term. One factor that is likely to account for the slow response of food intake to increased energy demands is the priority the body has given to the control of water balance. Hypohydration decreases appetite and leads to anorexia (Engell, 1995). Soldiers can lose 3 to 5 percent of body weight during deployment because of the effects of dehydration (IOM, 1995) which may reduce appetite and food intake during combat. In addition, individuals who overfeed tend to be relatively sedentary and relaxed at the time, which obviously is not the case in combat. High levels of activity that redistribute blood flow to the muscles and away from the splanchnic circulation reduce the rate of gastric emptying (Leiper et al., 2001; Mudambo et al., 1997). In addition, there is evidence that dehydration further delays gastric emptying and increases gastrointestinal distress if a person is physically active (running) (Rehrer et al., 1990). Training while consuming carbohydrate-rich beverages may lead to some adaptation in terms of increased or maintained rates of gastric emptying during exercise (Carrio et al., 1989; Rehrer et al., 1992). However, to eat more, one needs to increase to flow of blood to the gut to increase gut motility and digestion of the additional food. It is anecdotally universal that people do not exercise on a full stomach. In summary, the demands of exercise are in conflict with the demands of food intake (e.g., a relaxed meal and increased gut motility). This conflict makes it difficult for a soldier to increase the consumption and digestion of food. It is likely that as with athletes, consumption of readily digested (low-bulk and low-protein) foods and already digested (beverages) nutrients may help bypass this problem. In summary, the decreased blood flow to the gut during conditions of continuous moderate to intense activity, will decrease gut motility and increase indigestion when food is consumed. A limited capacity to increase food intake, coupled with the effects of hypohydration on appetite, results in energy intake that does not effectively track energy expenditure. To rapidly restore energy balance, a sedentary period may be most effective, not just to decrease energy expenditure, but also to facilitate higher levels of energy intake such as refeeding between missions. It is no coincidence that during the Guru-Walla overfeeding ritual, the subjects remain entirely sedentary during a period when they consumed some 6,000 to 7,000 kcal/day (Pasquet et al., 1992). The little evidence available relating to abrupt decreases in energy expenditure caused by training cessation or injury suggests that reduced activity leads to acute decreases in energy expenditure, that then leads to reduced activity marked by gain in body weight. Figure B-26 indicates that reduced energy expenditure from inactivity is very weakly linked, through
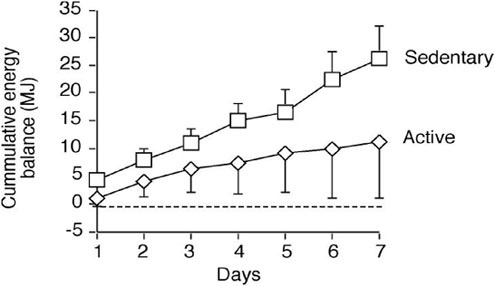
FIGURE B-26 Mean (standard error of means) cumulative energy balance (MJ) for six men who were continually resident in a whole-body indirect calorimeter for 7 days on either a sedentary or active treatment. On the sedentary treatment, daily energy expenditure was held at 1.4 × BMR (the low end of the sedentary range), and on the active treatment daily energy expenditure was held at 1.8 × BMR. Subjects were fed ad libitum throughout on a medium-fat diet of constant measurable composition.
NOTE: BMR = basal metabolic rate.
SOURCE: Stubbs et al. (2004a) Am J Physiol Regul Integr Comp Physiol used with permission.
physiological signals, to energy intake and has little effect in reducing intake (Stubbs et al., 2004a). A period of rest may promote greater levels of energy intake by providing opportunities and a physiological capacity for overconsumption. Thus, refeeding soldiers during recovery periods should be considered; it may be somewhat harder to supplement them during acute elevations of activity. In addition to these problems for appetite control, the soldiers are subjected to a variable range of stressors that do not generally occur during attend athletic and endurance events. This raises the question: Is all of the negative energy balance observed under combat conditions entirely due to the relationship between increased exercise and appetite?
EFFECTS OF ADDITIONAL COMBAT STRESSORS ON APPETITE
Stresses Associated with Combat
Hypohydration
A major stress of deployment and combat is hypohydration. This is not necessarily related to levels of energy expenditure. Soldiers appear particularly susceptible to hypohydration during deployment, in hot environments, and where they are required to wear protective clothing (IOM, 1995). The CMNR considered that a weight loss of > 3 percent and < 10 percent was principally due to an inadequate fluid intake, which would likely reduce performance (IOM, 1995). Engell (1995) has reviewed the evidence that shows inadequate water intake restricts ad libitum food intake. The converse does not appear to be true. Figure B-27 shows the effects of changes in the composition of the diet on ad libitum daily energy intake in 78 subjects self-recording their own food intake (Stubbs et al., 2000). Large variations in water intake, presumably in well-hydrated people, have little effect on energy intake. Given the likelihood of hypohydration and its effects on energy intake, it is critical that attempts are made to avoid this stress and its knock on effects for appetite and performance.
FIGURE B-27 Relationship between energy density (ED) of the diet (expressed as a daily percentage of the maximum daily ED of each subject’s diet) and energy intake (EI) in 76 subjects self-recording their food intake by weighed dietary record over 7 consecutive days. Subjects with energy intakes < 1.2 × BMR who did not lose weight during the seven days were previously excluded.
NOTE: BMR = basal metabolic rate.
SOURCE: Stubbs et al. (2000).
Sleep Deprivation
Another stress of combat is sleep deprivation. In rats sleep deprivation appears to cause an increase in locomotor behaviour, and increase in food intake, and increase in the activity of the hypothalamic-pituitary-adrenal axis (increased plasma thyroxine, norepinephrine) and increased heat loss (Rechtschaffen et al., 2002), leading to considerable loss of function and even death. These problems may relate to altered thermoregulation. In rats sleep deprivation causes malnutrition (despite the increased energy intake), which is secondary to increased energy expenditure (Everson and Wehr, 1993). Horne (1985) has noted that the extreme problems of thermoregulation, induced by sleep deprivation in rodents, do not appear to apply to humans. Many studies relevant to military situations also include periods of intense activity (e.g., Oektedalen et al., 1982; Opstad and Aakvaag, 1981; Opstad et al., 1984) and it is difficult to separate the effects of a negative energy balance induced by sleep deprivation or by exercise. Akerstedt et al. (1980) exposed twelve healthy males to 48 h of sleep deprivation under conditions where time isolation and activity and feeding were strictly controlled. They found that under these otherwise unstressed conditions adrenocortical and gonadal steroid hormones were lower or unchanged, indicating a lack of emergency stress response, due to sleep deprivation. Redwine et al. (2000) have found that mild sleep deprivation affects IL (interleukin)-6 levels, suggesting sleep deprivation may influence the integrity of immune functioning. The cognitive effects of sleep deprivation are documented elsewhere (Foo et al., 1994; Fu and Ma, 2000). It seems that sleep deprivation induces a negative energy balance (due to increased activity that causes the lack of sleep) and obviously, fatigue. In rats at least, these changes are alleviated better with supplements of energy than of protein (Everson and Wehr, 1993). There is no clear evidence that sleep deprivation decreases appetite and energy intake; on the contrary, in rats it appears to increase energy intake. However, in both rats and humans, under the disparate conditions of the studies conducted, intake does not appear to match elevated energy requirements associated with sleep deprivation. It is not clear why this is so, particularly when the limited indications are that supplemental energy intakes may alleviate some of the stress of sleep deprivation.
Anxiety
There is no doubt that the combat environment induces the most extraordinary levels of anxiety. The modernization of warfare methods increases stress, anxiety and emotional trauma, which have an acute effect at decreasing appetite. These levels of stress are rarely quantified, but they have been documented in the diaries and records of numerous soldiers throughout humankind’s continuing and ongoing conduct of various wars. In several classical accounts, especially those associated with the D-Day landings when there was a period of prebattle
anticipation, the inability to eat or even hold food down was not uncommon (see accounts at http://www.bbc.co.uk/dna/ww2/C54665). Some of these disturbances remain after the war is over as combat-stress disorder and posttraumatic stress disorder, as documented by a growing literature (Hyams et al., 1996; Pearn, 2000; Pereira, 2002; Witztum et al., 1996).
Stress-Related Mechanisms of Appetite Suppression
Stress-related mechanisms of appetite suppression are multiple, redundant, and poorly understood in humans. In rats, hypohydration-induced anorexia appears to involve corticotrophin releasing factor and neurotensin in the lateral hypothalamus (Watts, 1999). Brain stem mechanisms are also involved (Flynn et al., 1995). Sleep deprivation will clearly affect the peptide families controlling biorhythms, which may disrupt diurnal patterns of feeding. Anxiety-based responses will be mediated through the hypothalamic-pituitary-adrenal axis and inflammation/physical trauma will be mediated by the immune-inflammatory system (Newsholme and Leach, 1992). Most evidence is derived from animal models except for the growing body of psychological evidence relating to human stress. Because of the wide range of components of these responses, there is no simple stress-associated mechanism that can be alleviated by a simple nutritional or pharmacological manipulation to improve appetite. While the interactions of these different components are unknown, it is reasonable to conclude that together, they will decrease appetite under combat situations. The intuitive solution would be to minimize the perception of satiety. It is important to supplement rations with energy because as a negative energy balance proceeds, performance will deteriorate and appetite will increase, even under stressed conditions [see Keys et al. (1950) for consideration of responses to undernutrition in war].
When and When Not to Bypass Satiety
When considering strategies to bypass the decreased appetite associated with combat, one can gain insights by analogy with clinical conditions. In the clinical setting under conditions of severe stress, such as surgery and shock, the patient experiences a period of catabolism, during which inter alia plasma glucagon, coticosteriods, and catecholamines are elevated. These are all hormones that induce an acute negative nitrogen balance and raise plasma glucose, providing metabolic fuels to deal with the stress at hand (Newsholme and Leach, 1992). Under these conditions, appetite is also characteristically suppressed and there is some debate as to whether supplementation should occur ever be provided through enteral or parenteral nutrition. This is important because a number of the stresses to which soldiers are exposed on the battlefield (beyond the obvious injuries and risks) may well be similar, in extent, to those experienced during shock, trauma, or other illness in the clinical setting. Under these conditions
people are intolerant of food in the following three ways: (1) appetite suppression; (2) increased gastric stasis (decreased gastric emptying) caused by intense exercise, acute systemic injury, or postoperative stress; and (3) intolerance to specific nutrients during stress (Newsholme and Leach, 1992).
The following examples illustrate how the use of bioactive substances to improve clinical outcomes can produce unpredictable serious side-effects, including death. The first example involves injections of pharmacological doses of growth hormone (GH) in critically ill patients. It was thought that GH might be beneficial by limiting the marked nitrogen loss that frequently occurs in such patients (equivalent to overcoming a metabolic block in net protein synthesis). Growth hormone is known to stimulate protein synthesis and had previously been shown to improve nitrogen balance in a wide range of clinical conditions. However, in a large multicenter trial involving patients admitted to intensive care units, pharmacological doses of GH doubled (from about 20 percent to 40 percent) mortality as compared with the placebo group. Although the mechanisms responsible for the increased mortality are still uncertain, several suggestions have been made (Takala et al., 1999).
A second example shows how outcomes in the critically ill can be improved through the control of delivered nutrients. The importance of strict glucose homeostasis in critically ill patients was demonstrated by a large study involving about 1,500 patients who were randomized to receive insulin to control their plasma glucose concentrations strictly between 4.4 and 6.1 mmol/L, or to receive standard therapy in which a circulating concentration of 10.0 to 11.1 mmol/L was considered acceptable. In the strictly controlled group there was a four-fold reduction in mortality and a two-fold reduction in the number of transfusions and the incidence of critical care polyneuropathy (van den Berghe et al., 2001). Thus, the use of bioactive ingredients and nutrients to bypass suppressed appetite must be considered with caution under conditions of acute and unpredictable stress. The nature and extent of the stress will be important in determining tolerance to the supplement.
It is known that during military training and combat, the rate, extent, and composition of weight loss influence physical performance and cognitive function (Freidl, 1995). We know from these studies that protein is largely adequate and mineral and vitamin short-term deficits are well tolerated under field conditions (Baker-Fulco, 1995). The main purpose of supplementation is to overcome suppressed appetite and meet, as closely as possible, energy and fluid requirements. The timing of energy supplementation is likely to have a large impact on tolerability and, indirectly, on performance. Most evidence about stress and tolerability of rations is derived from noncombat conditions in which the most acute stressors are largely absent. It is perhaps necessary to ascertain under combat or similar conditions how different stressors will influence appetite and physiological and cognitive function. From a research perspective, the authors suggest it is important to: (1) quantify the likely nature and extent of the stress
under combat or similar conditions; (2) test strategies to optimize energy intake before, after, or during combat (with focus on timing and composition of the supplement); and (3) quantify the tolerability and performance response. From the perspectives of both appetite and performance, timing and composition are the two critical features of the ration that determine the benefits of supplementation. Timing is important because it will determine the most efficacious and feasible strategy of supplementation. Composition of foods influences the amount that can be ingested (Stubbs, 1998; Stubbs et al., 1995, 2001).
NUTRITIONAL INGREDIENTS OR PACKAGING TO MAINTAIN APPETITE
Diet Composition and Appetite
A number of reviews have considered the means and mechanisms by which dietary macronutrients, macronutrient substitutes, and associated nutritional parameters, such as dietary energy density influence appetite and energy balance in humans (Stubbs, 1998; Stubbs et al., 2000). As they exist in the diet, macronutrients differ in their metabolisable energy coefficients (Elia and Livesey, 1992). Average values for alcohol, protein, carbohydrate, and fat are 7, 4, 4, and 9 kcal/g. Fat and alcohol are more energy dense (energy per wet weight of food eaten) than protein and carbohydrate (Elia and Livesey, 1992).
Not all of the different macronutrients affect satiety to the same degree. There is a hierarchy in the satiating efficiency of the macronutrients such that, under a variety conditions per unit of energy ingested, protein suppresses appetite to a greater extent than carbohydrate, which has a greater effect than fat (Stubbs, 1995; Figure B-28). Caloric compensation can be defined as a unit decrease in energy intake for each unit of energy given as a particular nutrient. Protein produces supercaloric compensation, carbohydrate caloric compensation and fat ingestion leads to subcaloric compensation (Figure B-27). Thus, high-protein diets are particularly satiating and limit energy intake. Because of this, foods conducive to a high-energy intake tend to be low in protein. When ingested at the same level of energy density, protein is still the most satiating macronutrient (Johnstone et al., 1996; Stubbs et al., 1996). Under these conditions differences between carbohydrate and fat are less clear cut. Carbohydrate tends to exert a more acute effect on satiety than does fat (Johnstone et al., 1996; Stubbs et al., 1996).
Dietary energy density tends to act as a constraint on feeding behavior (Stubbs et al., 2000). A diet that has too low-an energy density will induce an energy deficit determined by the rate at which low-energy-dense foods can be digested and absorbed. There is evidence that food intake will increase in the longer term to offset this deficit. With high energy density diet, overconsumption tends to occur because the amount of food a person eats tends to be conditioned and weak against excess energy intake (Stubbs et al., 2000).
Because of its contribution to energy density, fat is often seen as the nutrient that will induce the greatest energy intake. It is possible to produce maltodextrin supplements that produce equally large elevations of energy intake (Stubbs et al., 1997, 2001). Very active subjects can also benefit from water that is stored with glycogen in a 3:1 ratio. Given that hydration is critical to troops in combat, the ability to carry water locked in glycogen may help alleviate hypohydration-induced anorexia.
Certain types of protein, carbohydrates, and fat may exert different effects on appetite (Stubbs et al., 1999) but this is a relatively new area. Current evidence suggests that sweet, wet, carbohydrate solutions induce lower caloric compensation (i.e., facilitate supplementation) than the same carbohydrates given as solid foods (diMeglio and Mattes, 2000; Stubbs et al., 2001). Women soldiers consume more when given ready access to commercial snack foods (Rose, 1989). This raises an
FIGURE B-28 Effect of increasing the energy content of macronutrient loads on Satiety Index subjectively expressed over 3.25 h.
SOURCE: Weststrate (1992).
important research issue of whether energy-dense dry foods (rich in fats and sugars) will elevate energy intake more than lower energy-dense beverages (rich in carbohydrates). In this context the hydration status of the subject is likely to be critical. Another question is raised about the effects of consumption of foods that require minimal digestion and place minimal stress on the gut, like athletes do (see above).
According to our data, large amounts of water intake in well-hydrated subjects will displace little or no energy from the diet (Figure B-27; Stubbs et al., 2001). Energy-containing beverages appear to have a supplemental effect. Stratton et al. (2003) showed that using a continuously infusing naso-gastric drip, it is possible to increment daily energy intake by approximately 1,000 kcal/day in ad libitum, free-living, well-hydrated subjects. These observations support the CMNR suggestions (IOM, 1995) that increased snacking and beverage intake may help elevate energy intake in the field.
Possible Strategies to Elevate Energy Intake and Performance in Combat
Timing
We know that loss of weight, especially as water and/or lean tissue, can be detrimental to performance in combat. Given that even in a 60-day Ranger study (Moore et al., 1992) during which subjects were in an energy deficit, approximately 1,200 kcal/day subjects who had < 10 percent body fat performed less well than slightly fatter subjects, soldiers with even relatively small amounts of extra body fat may have certain performance advantages in combat. This will be more so if the stressors are such that neither opportunity nor motivational appetite facilitate food intake. It would be valuable to compare the effects of overfeeding subjects before combat or during resting periods with the effects of supplemention during combat. One way to optimize nutritional status and hence performance in combat is to focus on supplementation during those recovery periods. A major research question is: When could energy be supplemented to achieve the best results?
Ingredients
During deployment it may be advantageous to supplement troops with carbohydrate-rich beverages to avoid hypohydration. In addition, the extra water stored with glycogen will act as a small internal water reservoir. It is our opinion that the most effective way to supplement a person that has free-living, ad libitum feeding is to trickle readily assimilated energy by using small snacks and beverages; such design also provides greater flexibility than a pack of three Meals, Ready-to-Eat (MREs). The use of flavor enhancers has had remarkable effects on soft drink sales and intakes. It is likely that soft drinks will readily replace the
fluid and carbohydrate requirement for performance if the problems of portability can be overcome (see below).
Given the high satiety value of protein and, to a lesser extent, fiber’s negative association with energy density, these components of foods are not conducive to high energy intakes. Similarly, very high-fat food items on their own might also be avoided because there is evidence that fat loads can enhance satiety at the level of the gut (Welch et al., 1988), an effect to be avoided in combat. On the other hand, except when in cold environments (Stroud et al., 1993), satiety with low food consumption is desirable to minimize gastrointestinal disturbances. Mixtures of fats and carbohydrates are particularly conducive to overconsumption as indicated by the fact that most commercial snacks are of this composition (Holland et al., 1991).
Portion Size
Previous authors have suggested that maximizing portion size will help elevate energy intake in the field setting (Rolls, 1995). In combat, however, a greater number of smaller portions will facilitate snacking and maintain energy levels. In terms of the pattern of daily intake, it is pertinent to note that meal size actually increases through the day. On average, breakfast is smaller than lunch, which is smaller than dinner (de Castro, 2000). In our experience, when offered three meals equal in size and energy content for breakfast, lunch, and dinner, subjects complain that breakfast is too large and dinner is too small. This may contribute to underconsumption of MREs because troops are unlikely to save half of one ration until later.
Packaging
A good deal of work has already been done on the way variety, packaging, and social and situational influences may help enhance intake during field training and combat (see IOM, 1995). In particular, some of the modeling approaches and marketing techniques used in commerce (Thompson, 1995) may be of considerable value in raising expectation and acceptability of rations. Companies use a variety of messages to repeatedly maintain consumer interest in their products, and it has already been shown that troops consume more if military rations are packed in a manner that resembles commercial products (Kalick, 1992; Kramer et al., 1989). There may be value in adding food-based aromas to foods to enhance their appeal given the influence of aromas in taste perceptions. After Desert Storm, many commanders expressed their belief in the importance of hot cooked food with an appealing aroma as being vital to morale (see IOM, 1995). While this luxury may not be available in combat, it should be a priority between missions.
Given the problems of hydration and food portability, the new packaging
developments are of critical importance. The new packaging provides a remarkable system whereby a variety of water sources, including a filtration of one’s own urine, can safely be ingested. A range of super-soluble, low-weight supplements could be mixed with water, thus replacing fluid, electrolytes, and glucose under conditions during which other rations cannot be consumed.
It may be valuable, in addition, to develop tools whereby soldiers can monitor their own fluid and fuel status (in that order). Dehydration can be assessed with urine sticks, and this may provide rapid feedback to soldiers who may not be experiencing the usual urges to eat and drink.
RESEARCH
Given the extreme energy deficit encountered in combat, nutritional supplementation should be the primary focus of rations for soldiers in short-term, high-intensity operations. We have provisionally recommended that research be focused on (1) better understanding of the nature and extent of stressors experienced in combat; (2) determining the optimum timing for nutritional supplementation (including between missions), bearing in mind that supplementation during unpredictable stress may not be advantageous; and (3) finding the most effective means of bypassing suppressed appetite to maintain hydration and optimize energy intake for performance in combat. More data derived from combat or similar situations on the nature of stress, types of intervention, and outcome would be valuable but difficult to obtain. Assessing how initial body composition relates to performance during extreme field training (e.g., Ranger I study) would help to derive guides for feeding before combat.
CONCLUSIONS
Three basic questions regarding the relationship of stress and appetite have been considered. First, in considering the relationship between energy intake and expenditure, it is apparent that appetite poorly tracks acute and extreme elevations of exercise-induced energy expenditure. This might be partly due to the fact that hypohydration itself induces anorexia. Behavior should be geared first to fluid ingestion and then to energy balance. In addition, intense exercise influences gastrointestinal physiology and blood flow and decreases gastric emptying. Hypohydration makes these effects more acute. Under conditions in which increased gastric emptying is needed (i.e, greater food intake), in addition to indigestion, a conflict between the demands of eating and those of exercising will occur. In the short- to medium-term, eating can limit exercise, but exercise can continue without necessarily consuming more thus intake will fail to track expenditure. The best approach to this low energy intake under these circumstances is to consume readily digestable energy and carbohydrate-rich beverages.
Second, the additional stressors of combat may exert further suppressive
effects on appetite and have been considered; however, the nature and extent of these stressors is highly unpredictable. The general rule in the clinical setting is that during periods of extreme metabolic instability, do not feed. It is critical to quantify the nature and effects of combat-related stresses and the way they affect both appetite and performance.
Third, other factors such as timing, type of ingredients, and packaging have been considered. When combat is likely to involve extreme stressors, it may be more advantageous to supplement soldiers before deployment and postmission and to maintain fluid and energy (carbohydrate) intakes during acute phases of combat. We suggest that research priorities in this area should focus on quantifying the nature and extent of stressors experienced in combat and their effects on physical and cognitive performance; in addition, assessing the effects of timing, and determing the composition of supplements to improve performance in combat should be a priority.
REFERENCES
Activity and Health Research. 1992. Allied Dunbar National Fitness Survey. London: Health Education Authority and Sports Council.
Akerstedt T, Palmblad J, de la Torre B, Marana R, Gillberg M. 1980. Adrenocortical and gonadal steroids during sleep deprivation. Sleep 3(1):23–30.
Baker-Fulco C. 1995. Overview of dietary intakes during military exercises. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 121–149.
Ballor DL, Poehlman ET. 1994. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: A meta-analytical finding. Int J Obes Relat Metab Disord 18(1):35–40.
Barr SI, Costill DL. 1992. Effect of increased training volume on nutrient intake of male collegiate swimmers. Int J Sports Med 13(1):47–51.
Black AE, Coward WA, Cole TJ, Prentice AM. 1996. Human energy expenditure in affluent societies: An analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr 50(2):72–92.
Blundell JE, King NA. 1999. Physical activity and regulation of food intake: Current evidence. Med Sci Sports Exerc 31(11 Suppl):S573–S583.
Carrio I, Estorch M, Serra-Grima R, Ginjaume M, Notivol R, Calabuig R, Vilardell F. 1989. Gastric emptying in marathon runners. Gut 30(2):152–155.
Ceesay SM, Prentice AM, Day KC, Murgatroyd PR, Goldberg GR, Scott W, Spurr GB. 1989. The use of heart rate monitoring in the estimation of energy expenditure: A validation study using indirect whole-body calorimetry. Br J Nutr 61(2):175–186.
de Castro JM. 2000. Eating behavior: Lessons from the real world of humans. Nutrition 16(10):800–813.
Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. 1992. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 56(4):641–655.
DiMeglio DP, Mattes RD. 2000. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int J Obes Relat Metab Disord 24(6):794–800.
Eden BD, Abernethy PJ. 1994. Nutritional intake during an ultraendurance running race. Int J Sport Nutr 4(2):166–174.
Elia M, Livesey G. 1992. Energy expenditure and fuel selection in biological systems: The theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev Nutr Diet 70:68–131.
Engell D. 1995. Effects of beverage consumption and hypdration status on caloric intake. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 217–237.
Everson CA, Wehr TA. 1993. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol 264(2 Pt 2):R376–R387.
Flynn FW, Curtis KS, Verbalis JG, Stricker EM. 1995. Dehydration anorexia in decerebrate rats. Behav Neurosci 109(5):1009–1012.
Foo SC, How J, Siew MG, Wong TM, Vijayan A, Kanapathy R. 1994. Effects of sleep deprivation on naval seamen: II. Short recovery sleep on performance. Ann Acad Med Singapore 23(5):676–679.
Forbes-Ewan CH, Morrissey BL, Gregg GC, Waters DR. 1989. Use of doubly labeled water technique in soldiers training for jungle warfare. J Appl Physiol 67(1):14–18.
Friedl KE. 1995. When does energy deficit affect soldier physical performance. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 253–283.
Fu ZJ, Ma RS. 2000. Effects of sleep deprivation on human performance. Space Med Med Eng (Beijing) 13(4):240–243.
Gabel KA, Aldous A, Edgington C. 1995. Dietary intake of two elite male cyclists during 10-day, 2,050-mile ride. Int J Sport Nutr 5(1):56–61.
Garrow JS, Summerbell CD. 1995. Meta-analysis: Effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr 49(1):1–10.
Holland B, Welch AA, Unwin ID, Buss DH, Paul AA, Southgate DAT. 1991. McCance and Widdowson’s The Composition of Foods. 5th ed. Cambridge: The Royal Society of Chemistry.
Horne JA. 1985. Sleep function, with particular reference to sleep deprivation. Ann Clin Res 17(5):199–208.
Hoyt RW, Jones TE, Stein TP, McAninch GW, Lieberman HR, Askew EW, Cymerman A. 1991. Doubly labeled water measurement of human energy expenditure during strenuous exercise. J Appl Physiol 71(1):16–22.
Hyams KC, Wignall FS, Roswell R. 1996. War syndromes and their evaluation: From the US Civil War to the Persian Gulf War. Ann Intern Med 125(5):398–405.
IOM (Institute of Medicine). 1995. Not Eating Enough. Washington, DC: National Academy Press.
Johnstone AM, Stubbs RJ, Harbron CG. 1996. Effect of overfeeding macronutrients on day-to-day food intake in man. Eur J Clin Nutr 50(7):418–430.
Jones PJ, Jacobs I, Morris A, Ducharme MB. 1993. Adequacy of food rations in soldiers during an arctic exercise measured by doubly labeled water. J Appl Physiol 75(4):1790–1797.
Kalick JB. 1992. The Effect of Consumer Oriented Packaging Designs on Acceptance and Consumption of Military Rations. Technical Report TR-92-034. Natick, MA: US Army Natick Research, Development and Engineering Centre.
Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. 1950. The Biology of Human Starvation. Minneapolis, MN: The University of Minnesota Press.
King NA, Tremblay A, Blundell JE. 1997. Effects of exercise on appetite control: Implications for energy balance. Med Sci Sports Exerc 29(8):1076–1089.
Kramer FM, Edinberg J, Luther S, Engell D. 1989. The impact of food packaging on food consumption and palatability. Paper presented to the Association for the Advancement of Behaviour Therapy, Washington, DC.
Leiper JB, Prentice AS, Wrightson C, Maughan RJ. 2001. Gastric emptying of a carbohydrate-electrolyte drink during a soccer match. Med Sci Sports Exerc 33(11):1932–1938.
McGowan CR, Epstein LH, Kupfer DJ, Bulik CM, Robertson RJ. 1986. The effect of exercise on non-restricted caloric intake in male joggers. Appetite 7(1):97–105.
Mela DJ. 1995. Understanding fat preference and consumption: Applications of behavioural sciences to a nutritional problem. Proc Nutr Soc 54(2):453–464.
Moore RJ, Friedl KE, Kramer TR, Martinez-Lopez LE, Hoyt RW, Tulley RE, DeLany JP, Askew EW, Vogel JA. 1992. Changes in Soldier Nutritional Status & Immune Function During the Ranger Training Course . Technical Report No. T13-92. Natick, MA: US Army Medical Research & Development Command.
Mowat F. 1975. People of the Deer. Toronto: Seal Books.
Mudambo KS, Leese GP, Rennie MJ. 1997. Gastric emptying in soldiers during and after field exercise in the heat measured with the [13C]acetate breath test method. Eur J Appl Physiol Occup Physiol 75(2):109–114.
Murgatroyd PR, Shetty PS, Prentice AM. 1993. Techniques for the measurement of human energy expenditure: A practical guide. Int J Obes Relat Metab Disord 17(10):549–568.
Newsholme EA, Leach AR. 1992. Biochemistry for the Medical Sciences. Chichester, England: John Wiley and Sons Ltd.
Norgan NG, Durnin JV. 1980. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am J Clin Nutr 33(5):978–988.
Oektedalen O, Flaten O, Opstad PK, Myren J. 1982. hPP and gastrin response to a liquid meal and oral glucose during prolonged severe exercise, caloric deficit, and sleep deprivation. Scand J Gastroenterol 17(5):619–624.
Opstad PK, Aakvaag A. 1981. The effect of a high calory diet on hormonal changes in young men during prolonged physical strain and sleep deprivation. Eur J Appl Physiol Occup Physiol 46(1):31–39.
Opstad PK, Falch D, Oktedalen O, Fonnum F, Wergeland R. 1984. The thyroid function in young men during prolonged exercise and the effect of energy and leep deprivation. Clin Endocrinol 20(6):657-669.
Pasquet P, Brigant L, Froment A, Koppert GA, Bard D, de Garine I, Apfelbaum M. 1992. Massive overfeeding and energy balance in men: The Guru Walla model. Am J Clin Nutr 56(3):483–490.
Pearn J. 2000. Traumatic stress disorders: A classification with implications for prevention and management. Mil Med 165(6):434–440.
Pereira A. 2002. Combat trauma and the diagnosis of post-traumatic stress disorder in female and male veterans. Mil Med 167(1):23–27.
Phinney SD, Bistrian BR, Evans WJ, Gervino E, Blackburn GL. 1983a. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32(8):769–776.
Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. 1983b. The human metabolic response to chronic ketosis without caloric restriction: Physical and biochemical adaptation. Metabolism 32(8):757–768.
Ravussin E, Schutz Y, Acheson KJ, Dusmet M, Bourquin L, Jequier E. 1985. Short-term, mixed-diet overfeeding in man: No evidence for “luxuskonsumption.” Am J Physiol 249(5 Pt 1):E470–E477.
Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. 2002. Sleep deprivation in the rat: X. Integration and discussion of the findings. 1989. Sleep 25(1):68–87.
Redwine L, Hauger RL, Gillin JC, Irwin M. 2000. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab 85(10):3597–3603.
Rehrer NJ, Beckers EJ, Brouns F, ten Hoor F, Saris WH. 1990. Effects of dehydration on gastric emptying and gastrointestinal distress while running. Med Sci Sports Exerc 22(6):790–795.
Rehrer NJ, Wagenmakers AJ, Beckers EJ, Halliday D, Leiper JB, Brouns F, Maughan RJ, Westerterp K, Saris WH. 1992. Gastric emptying, absorption, and carbohydrate oxidation during prolonged exercise. J Appl Physiol 72(2):468–475.
Rolls B. 1995. Effects of food quality, quantity, and variety on food intake. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 203–215.
Rose MS. 1989. Between-Meal Food Intake for Reservists Training in the Field. Technical Report T15-89. Natick, MA: US Army Research Institute of Environmental Medicine.
Saris WH. 1989. Physiological aspects of exercise in weight cycling. Am J Clin Nutr 49(5 Suppl):1099–1104.
Saris WH. 1993. The role of exercise in the dietary treatment of obesity. Int J Obes Relat Metab Disord 17 (Suppl 1):S17–S21.
Schoeller DA, van Santen E. 1982. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol 53(4):955–959.
Schoeller DA, Shay K, Kushner RF. 1997. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr 66(3):551–556.
Sjodin AM, Andersson AB, Hogberg JM, Westerterp KR. 1994. Energy balance in cross-country skiers: A study using doubly labeled water. Med Sci Sports Exerc 26(6):720–724.
Spurr GB, Prentice AM, Murgatroyd PR, Goldberg GR, Reina JC, Christman NT. 1988. Energy expenditure from minute-by-minute heart-rate recording: Comparison with indirect calorimetry. Am J Clin Nutr 48(3):552–559.
Stratton RJ, Stubbs RJ, Elia M. 2003. Short-term continuous enteral tube feeding schedules did not suppress appetite and food intake in healthy men in a placebo-controlled trial. J Nutr 133(8):2570–2576.
Stroud M. 1998. The nutritional demands of very prolonged exercise in man. Proc Nutr Soc 57(1):55–61.
Stroud MA. 1987. Nutrition and energy balance on the ‘Footsteps of Scott’ expedition 1984–86. Hum Nutr Appl Nutr 41(6):426–433.
Stroud MA, Coward WA, Sawyer MB. 1993. Measurements of energy expenditure using isotope-labelled water (2H218O) during and Arctic expedition. Eur J Apl Physiol 67(4):375–379.
Stroud MA, Ritz P, Coward WA, Sawyer MB, Constantin-Teodosiu D, Greenhaff PL, Macdonald IA. 1997. Energy expenditure using isotope-labelled water (2H218O), exercise performance, skeletal muscle enzyme activities and plasma biochemical parameters in humans during 95 days of endurance exercise with inadequate energy intake. Eur J Appl Physiol Occup Physiol 76(3):243–252.
Stubbs J, Ferres S, Horgan G. 2000. Energy density of foods: Effects on energy intake. Crit Rev Food Sci Nutr 40(6):481–515.
Stubbs RJ. 1995. Macronutrient effects on appetite. Int J Obes Relat Metab Disord 19(Suppl 5):S11–S19.
Stubbs RJ. 1998. Nutrition Society Medal Lecture. Appetite, feeding behaviour and energy balance in human subjects. Proc Nutr Soc 57(3):341–356.
Stubbs RJ, Elia M. 2001. Macronutrients and appetite control with implications for the nutritional management of the malnourished. Clin Nutr 20(Suppl 1):129–139.
Stubbs RJ, Hughes DA, Johnstone AM, Horgan GW, King N, Blundell JE. 2004a. A decrease in physical activity affects appetite, energy, and nutrient balance in lean men feeding ad libitum. Am J Clin Nutr 79(1):62–69.
Stubbs RJ, Hughes DA, Johnstone AM, Horgan GW, King N, Elia M, Blundell JE. 2003. Interactions between energy intake and expenditure in the development and treatment of obesity. In: Mederios-Neto G, Halpern A, Bouchard C, eds. Progress in Obesity Research: 9. Proceedings of the 9th International Congress on Obesity. United Kingdom: John Libbey Eurotext.
Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, Blundell J. 2004b. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol 286(2):R350–R358.
Stubbs RJ, Mazlan N, Whybrow S. 2001. Carbohydrates, appetite and feeding behavior in humans. J Nutr 131(10):2775S–2781S.
Stubbs RJ, Prentice AM, James WP. 1997. Carbohydrates and energy balance. Ann N Y Acad Sci 819:44–69.
Stubbs RJ, Raben AR, Westerterp-Plantenga MS. 1999. Macronutrient metabolism and appetite. In: Westerterp-Plantenga S, Steffens AB, Tremblay A, eds. Regulation of Food Intake and Energy Expenditure. Milan: Edra Press. Pp. 59–84.
Stubbs RJ, Ritz P, Coward WA, Prentice AM. 1995. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: Effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr 62(2):330–337.
Stubbs RJ, van Wyk MC, Johnstone AM, Harbron CG. 1996. Breakfasts high in protein, fat or carbohydrate: Effect on within-day appetite and energy balance. Eur J Clin Nutr 50(7):409–417.
Sum CF, Wang KW, Choo DC, Tan CE, Fok AC, Tan EH. 1994. The effect of a 5-month supervised program of physical activity on anthropometric indices, fat-free mass, and resting energy expenditure in obese male military recruits. Metabolism 43(9):1148–1152.
Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. 1999. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341(11):785–792.
Thompson EG. 1995. Industry approaches to food research. In: Marriott BM, ed. Not Eating Enough. Washington, DC: National Academy Press. Pp. 239–250.
van Baak MA. 1999. Physical activity and energy balance. Public Health Nutr 2(3A):335–339.
van den Berghe G, Wooters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasserlaers D, Ferdinande P, Lauwers P, Bouillon R. 2001. Intensive insulin therapy in critically ill patients. N Engl J Med 345(19):1359–1367.
van Etten LM, Westerterp KR, Verstappen FT, Boon BJ, Saris WH. 1997. Effect of an 18-wk weight-training program on energy expenditure and physical activity. J Appl Physiol 82(1): 298–304.
Watts AG. 1999. Dehydration-associated anorexia: Development and rapid reversal. Physiol Behav 65(4–5):871–878.
Welch IM, Sepple CP, Read NW. 1988. Comparisons of the effects on satiety and eating behaviour of infusion of lipid into the different regions of the small intestine. Gut 29(3):306–311.
Westerterp KR. 1998. Alterations in energy balance with exercise. Am J Clin Nutr 68(4):970S–974S.
Westerterp KR, Saris WH, van Es M, ten Hoor F. 1986. Use of the doubly labeled water technique in humans during heavy sustained exercise. J Appl Physiol 61(6):2162–2167.
Weststrate JA. 1992. Effects of nutrients on the regulation of food intake. Unilever Research, Vlaardingen, the Netherlands.
Witztum E, Levy A, Solomon Z. 1996. Therapeutic response to combat stress reaction during Israel’s wars. Isr J Psychiatry Relat Sci 33(2):77–78.
Optimization of Immune Function in Military Personnel
Simin Nikbin Meydani and Faria Eksir, Tufts University
INTRODUCTION
Although moderate amount of exercise could result in improving the immune function, it has been shown that strenuous physical activity can suppress the immune response (Hoffman-Goetz and Pedersen, 1994). Studies have reported that during combat as well as during times of rigorous training, soldiers are
exposed to many types of stress factors that can affect different components of the immune system (Moore et al., 1992). Lack of sleep, low energy food ration, and psychological stress are among those factors that contribute to a low immune response. The fact that during military work, soldiers are often exposed to long durations of strenuous physical activity, makes the danger even more serious. The duration of physical stress as well as the low energy intake that is associated with military life can cause severe alterations in the immune response and increase risk of infection among soldiers. Many studies have been conducted on the effects of individual stress factors on the immune function. A few studies have shown that the combined effect of the stress factors can be even more damaging to the immune response than the effect of each individual factor (Booth et al., 2003; Kramer et al., 1997).
Immunological studies conducted on military personnel have reported several unfavorable consequences resulting from stresses associated with military life. A major concern has to do with the low-calorie food ration provided for soldiers in combat. Studies have well documented the impact of dietary intake on physical performance and on the immune response (Booth, 2003; Montain and Young, 2003). Undernutrition and/or malnutrition, especially during stressful times, can result in suppression of the immune response, which can lead to increased susceptibility to infection (particularly respiratory infection) and diarrheal diseases often experienced by combat personnel. In fact, it has been documented that during combat, diseases account for more inactive days than either combat wounds or nonbattle injury cases among military personnel. The question would be: What measures can be taken to ameliorate the situation for our military personnel? Clearly, reducing the level of stress would be an effective course. However, given the nature of the job, in all likelihood, that is also the most unattainable option. A more feasible choice would be to conduct vaccinations against various possible diseases, a practice that already has been taken up by the military sectors. Still, available vaccinations only protect against certain diseases and do not provide protection against new pathogens to which the soldiers would likely be exposed. In addition, the vaccines are less effective in immuno-suppressed individuals. An effective and more promising means of protecting the immune function during combat would be through an optimal nutrition plan, which is the focus of this paper. The specific questions that this paper addresses are the following:
-
What links are known to exist between nutritional factors and the optimization of the immune system? Specifically which nutritional factors would help reduce occurrence of infections or enhance disease resistance?
-
Are there consequences on immune function from a short-term hypocaloric diet?
-
Are there nutrients that might improve resistance to infection despite a hypocaloric diet?
NUTRITIONAL STRATEGIES TO IMPROVE THE IMMUNE RESPONSE
Most nutrient deficiencies result in the impairment of the host defense system and increase susceptibility to infectious diseases (Chandra and Sarchielli, 1993; Keusch et al., 1983). Additionally, recent studies have shown that malnutrition, such as deficiency of selenium and vitamin E, plays a major role in increasing the pathogenicity of microbes and surfacing of unusual viral infections (Beck and Levander, 1998). This is quite important in the case of soldiers at combat in foreign lands, because they frequently encounter pathogens that they have not previously been exposed to. A strong host defense system is critical in the ability of the body to fight off novel pathogens and develop an immune response for future attacks. Supplying the soldiers with adequate nutrients could reduce the risk of a suppressed immune response. Furthermore, providing more than adequate levels of some nutrients can strengthen the immune function and protect against infection and disease.
Using animal models, a number of studies have demonstrated the role of micronutrients (vitamins and trace minerals) in enhancing the immune function. However, studies of micronutrients and immune function in human subjects have been limited and very few have looked at the effect of nutrients on disease and susceptibility to infection. Recently, however, a few studies have examined the relationship between nutritional deficit and/or supplementation and disease in the elderly population.
It should be noted that in spite of the chronological age difference between the elderly population and the military personnel (mostly consisting of a young population), there are similarities in their immune systems. In both groups, the elderly and the military personnel in combat, immunosuppression is evident and characterized by a decrease in cell-mediated immunity and changes in the ability of lymphocytes to proliferate. Notably, the elderly, as a consequence of immunological changes, have a higher susceptibility to, and a greater morbidity and mortality from, infectious diseases, particularly respiratory infections and diarrheal diseases, which are some of the major concerns for soldiers in combat as well. Thus, in the absence of data for military combat personnel, results from a recent clinical trial on vitamin E and respiratory infections will be reviewed as an example of how nutrient intervention could be used to optimize the immune response and reduce risk of infection in an immunocompromised individual.
VITAMIN E AND INFECTIOUS DISEASES
The immunostimulatory effect of vitamin E has been shown to be associated with resistance to infections. Most of the animal studies that investigated the effects of vitamin E on infectious diseases reported a protective effect despite the variations in the dose and duration of the supplementation, infectious organisms involved, and route of administration as reviewed by Meydani et al. (2001).
Vitamin E supplementation in old mice resulted in significantly lower viral titer and preserved antioxidant nutrient status following influenza virus infection (Hayek et al., 1997). This protective effect of vitamin E against influenza infection seems to be partly due to enhancement of Th1 (T helper 1) response, increased IL (interleukin)-2 and IFN-γ production (Han et al., 2000).
VITAMIN E AND RESPIRATORY INFECTIONS IN THE ELDERLY
Only a limited number of studies have investigated the effect of vitamin E on resistance against infections in humans. The subjects in these studies were mainly elderly persons. Infections, particularly respiratory infections (RI), are common in the elderly, resulting in decreased daily activity, prolonged recovery times, increased health care service use, and more frequent complications that may lead to death (Alvarez et al., 1988; Crossley and Peterson, 1996; Farber et al., 1984; Garibaldi et al., 1981; Gugliotti, 1987; Hasley et al., 1993; Jackson et al., 1992; Mehr et al., 1992; Nicolle et al., 1984; Plewa, 1990; Schneider, 1983). Contributing to the increased incidence of infection with age is the well-described decline in immune response (Siskind, 1980). For example, there are higher morbidity and mortality from cancer, pneumonia, and postoperative complications in those who have a diminished delayed-type hypersensitivity (DTH) skin test response (Christou et al., 1989; Cohn et al., 1983; Wayne et al., 1990).
Nutritional status is an important determinant of immune function (Chandra, 1990; Keusch et al., 1983), and nutritional supplementation has been shown to enhance older subjects’ immune response (Chandra, 1992; Meydani and Blumberg, 1989). In our earlier placebo-controlled, double-blind trials in elderly persons, vitamin E supplementation improved immune response, including DTH and response to vaccines (Meydani et al., 1990, 1997). In this study we also reported a nonsignificant (p < 0.09) 30 percent lower incidence of self-reported infections among the groups supplemented with vitamin E (60, 200, or 800 mg/day for 235 days) compared with the placebo group (Meydani et al., 1997). Because infection was not the primary outcome, the study did not have enough power to detect significant differences in the incidence of infections. To overcome these limitations, we conducted a large double-blind, placebo-controlled trial to determine the effect of one-year supplementation with vitamin E on objectively recorded respiratory illnesses in elderly nursing home residents (Meydani et al., 2004).
In this randomized, double-blind study, 617 people older than 65 and residing at 33 nursing homes in the Boston area who met the study’s eligibility criteria received either a placebo or 200 IU of vitamin E (dl-α-tocopherol) daily for one year. All participants received a capsule containing half the recommended daily allowance of essential vitamins and minerals. The main outcomes of the study were incidence of respiratory tract infections, number of persons and number of days with RIs (upper and lower), and number of new antibiotic prescriptions for RIs among all randomized participants and those who completed the study (Meydani et al., 2004).
Supplementation with vitamin E had no significant effect on the incidence or duration of all RIs taken together, or on upper or lower respiratory tract infections measured separately. However, fewer vitamin E-supplemented subjects acquired one or more RIs (65 percent versus 74 percent, RR = 0.87, CI = 0.73–0.99, p = 0.035), or upper RIs (50 percent versus 62 percent, RR = 0.81, CI = 0.65–0.96, p = 0.015). Further analysis on the foremost RI, the common cold, indicated that the vitamin E group had a lower incidence of common colds (0.66 versus 0.83 per subject-year, RR = 0.80, CI = 0.64–0.98, p = 0.046) and fewer subjects in the vitamin E group acquired one or more common colds (46 percent versus 57 percent, RR = 0.79, CI = 0.63–0.96, p = 0.016) (Meydani et al., 2004). The vitamin E-treated group also had fewer days with common cold per person-year compared with those in the placebo group, but the difference did not reach statistical significance (22 percent less, p = 0.11).
In conclusion, the results of this clinical trial show that vitamin E supplementation significantly reduces the risk of acquiring upper respiratory infections, particularly the common cold.
CONCLUSIONS
Several investigations have demonstrated that vitamin E significantly enhances immune functions in humans, especially in the elderly. Animal studies as well as recently completed clinical trials strongly suggest that this effect of vitamin E is associated with reduced risk of acquiring infections, particularly upper respiratory infections in the elderly. Given that, similar to the elderly, military personnel in combat are immunosuppressed, these studies strongly suggest that they might also benefit from consuming additional level of vitamin E. Studies by Lee and Wan (2000), in which they demonstrated vitamin E to be effective in improving the immune response of young adults, further supports this recommendation. Thus, studies to demonstrate efficacy of increasing the vitamin E level in military combat ration are warranted.
ACKNOWLEDGMENTS
The work of the authors was supported by NIA, National Institute of Health Grant 1 R01-AG13975, and US Department of Agriculture agreement 58-1950-9-001, and National Institute of Health Grant 2R01 AG009140-10A1 and a gift for preparation of supplement from Roche, Inc.
REFERENCES
Alvarez S, Shell CG, Woolley TW, Berk SL, Smith JK. 1988. Nosocomial infections in long-term facilities. J Gerontol 43(1):M9–M17.
Beck MA, Levander OA. 1998. Dietary oxidative stress and the potentiation of viral infection. Annu Rev Nutr 18:93–116.
Booth CK. 2003. Combat rations and military performance. Do soldiers on active service eat enough? Asia Pac J Clin Nutr 12(Suppl):S2.
Booth CK, Coad RA, Forbes-Ewan CH, Thomson GF, Niro PJ. 2003. The physiological and psychological effects of combat ration feeding during a 12-day training exercise in the tropics. Mil Med 168(1):63–70.
Chandra RK. 1990. Nutrition is an important determinant of immunity in old age. In: Prinsley DM, Sandstead HH, eds. Nutrition and Aging. Proceedings of the 1988 International Conference on Nutrition and Aging, held in Galveston, Texas, October 5-7, 1988. New York: Alan R. Liss, Inc. Pp. 321–334.
Chandra RK. 1992. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet 340(8828):1124–1127.
Chandra RK, Sarchielli P. 1993. Nutritional status and immune responses. Clin Lab Med 13(2):455–461.
Christou NV, Tellado-Rodriguez J, Chartrand L, Giannas B, Kapadia B, Meakins J, Rode H, Gordon J. 1989. Estimating mortality risk in preoperative patients using immunologic, nutritional, and acute-phase response variables. Ann Surg 210(1):69–77.
Cohn JR, Hohl CA, Buckley CE 3rd. 1983. The relationship between cutaneous cellular immune responsiveness and mortality in a nursing home population. J Am Geriatr Soc 31(12):808–809.
Crossley KB, Peterson PK. 1996. Infections in the elderly. Clin Infect Dis 22(2):209–215.
Farber BF, Brennen C, Puntereri AJ, Brody JP. 1984. A prospective study of nosocomial infections in a chronic care facility. J Am Geriatr Soc 32(7):499–502.
Garibaldi RA, Brodine S, Matsumiya S. 1981. Infections among patients in nursing homes: Policies, prevalence, problems. N Engl J Med 305(13):731–735.
Gugliotti R. 1987. The incidence of nosocomial infections in a skilled nursing facility. Conn Med 51(5):287–290.
Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BS, Meydani SN. 2000. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology 100(4):487–493.
Hasley PB, Brancati FL, Rogers J, Hanusa BH, Kapoor WN. 1993. Measuring functional change in community-acquired pneumonia. A preliminary study using the Sickness Impact Profile. Med Care 31(7):649–657.
Hayek MG, Taylor SF, Bender BS, Han SN, Meydani M, Smith DE, Eghtesada S, Meydani SN. 1997. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis 176(1):273–276.
Hoffman-Goetz L, Pedersen BK. 1994. Exercise and the immune system: A model of the stress response? Immunol Today 15(8):382–387.
Jackson MM, Fierer J, Barrett-Connor E, Fraser D, Klauber MR, Hatch R, Burkhart B, Jones M. 1992. Intensive surveillance for infections in a three-year study of nursing home patients. Am J Epidemiol 135(6):685–696.
Keusch GT, Wilson CS, Waksal SD. 1983. Nutrition, host defenses, and the lymphoid system. Adv Host Def Mech 2:275–306.
Kramer TR, Moore RJ, Shippee RL, Friedl KE, Martinez-Lopez L, Chan MM, Askew EW. 1997. Effects of food restriction in military training on T-lymphocyte responses. Int J Sports Med 18(Suppl 1):S84–S90.
Lee CY, Man-Fan Wan J. 2000. Vitamin E supplementation improves cell-mediated immunity and oxidative stress of Asian men and women. J Nutr 130(12):2932–2937.
Mehr DR, Foxman B, Colombo P. 1992. Risk factors for mortality from lower respiratory infections in nursing home patients. J Fam Pract 34(5):585–591.
Meydani SN, Blumberg JB. 1989. Nutrition and the immune function in the elderly. In: Munro H, Danforth A, eds. Human Nutrition: A Comprehensive Treatise. Vol. VII. New York: Plenum Press. Pp. 61–87.
Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. 1990. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr 52(3):557–563.
Meydani SN, Fawzi WW, Han SN. 2001. The effect of vitamin deficiencies (E and A) and supplementation on infection and immune response. In: Suskind RM, Tontisirin K, eds. Nutrition, Immunity, and Infection in Infants and Children. Vol. 45. Philadelphia, PA: Lippincott Williams & Wilkins. Pp. 213–241.
Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. 2004. Vitamin E and respiratory tract infections in elderly nursing home residents: A randomized controlled trial. J Am Med Assoc 292(7):828–836.
Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. 1997. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. J Am Med Assoc 277(17):1380–1386.
Montain SJ, Young AJ. 2003. Diet and physical performance. Appetite 40(3):255–267.
Moore RJ, Friedl KE, Kramer TR, Martinez-Lopez LE, Hoyt RW, Tulley RE, DeLany JP, Askew EW, Vogel JA. 1992. Changes in Soldier Nutritional Status & Immune Function During the Ranger Training Course. Technical Report No. T13-92. Natick, MA: US Army Medical Research & Development Command.
Nicolle LE, McIntyre M, Zacharias H, MacDonell JA. 1984. Twelve-month surveillance of infections in institutionalized elderly men. J Am Geriatr Soc 32(7):513–519.
Plewa MC. 1990. Altered host response and special infections in the elderly. Emerg Med Clin North Am 8(2):193–206.
Schneider EL. 1983. Infectious diseases in the elderly. Ann Intern Med 98(3):395–400.
Siskind GW. 1980. Immunological aspects of aging: An overview. In: Schimke RT, ed. Biological Mechanism in Aging. Bethesda, MD: US Department of Health and Human Services. Pp. 455–467.
Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. 1990. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol 45(2):M45–M48.
Optimization of Nutrient Composition for Assault Rations: Interaction of Stress with Immune Function
Ronenn Roubenoff, Millenium Pharmaceuticals
INTRODUCTION
Both military missions and military training cause major stress to the body on a number of levels: dehydration, whole-body inflammation and immune modulation, muscle catabolism, oxidative stress, and increased energy requirements are the most readily demonstrable effects of these activities. During high-tempo, stressful combat missions, it is expected that soldiers will not be able to carry adequate calories and nutrients to meet the heightened requirements of their environment and activity. Therefore, the goal of a model military ration must be to reduce the effects of these stresses in the sure foreknowledge that they
cannot be avoided or fully countered under combat or rigorous training conditions. The goal of this discussion is to answer the following questions:
-
What theoretical constructs should be considered in evaluating the interplay of stress, diet, exercise, and immune function in determining functional capacity?
-
What dietary factors can affect muscle injury and immune function?
-
What biomarkers could be used to assess the effects of dietary interventions?
-
What more do we need to know to enhance the benefits of military rations?
The body’s stress response is multifaceted, with endocrine, immune, and neuropsychiatric systems participating in a complex interaction that remains poorly understood (Marsland et al., 2002; Stowell et al., 2001; Webster et al., 2002). The stress response invokes cellular, humoral, and neurological responses (see Figure B-29). The ability of nutritional elements to modulate these responses varies by both nutrient and system (Venkatraman and Pendergast, 2002). Although, as athletes and coaches have known for years, poor nutrition can increase the risk of injury and be detrimental to performance, the expectaction that nutrition can markedly improve performance may be unrealistic (Venkatraman and Pendergast, 2002). Nutritionists face a challenge to improve the immune system responses to the multiple stresses in a combat situation through changing the nutritional composition of the combat ration. Nevertheless some aspects of the stress response are most amenable to dietary manipulations and include:
-
Modulating the immune function with signal-transducing fatty acids, prostaglandins, leukotrienes, and cytokines.
-
Decreasing of total energy deficit caused by low-caloric intake.
-
Reducing oxidative stress by way of antioxidant intake.
-
Limiting muscle damage exacerbation caused by inadequate energy and protein intake.
-
Modulating emotional response to stress by offering hedonic foods (e.g., chocolate, caffeine).
An important consideration in devising optimal meal content for supporting intensive physical activity (Rasmussen et al., 2000; Tipton et al., 2001). Several researchers have suggested that there is an “open window” of immune suppression after an exercise bout, and its severity correlates with the intensity and duration of exercise (Figure B-30) (Lakier Smith, 2003; Nieman, 2003). This concept has not been fully explored and limited data support it; however, if it is correct, then dietary intake during the open window should be considered as a potential route of delivery of immune-supportive nutrients (see below). Further investigation of this possibility is warranted.
FIGURE B-29 Selected elements of the stress response: left panel, central nervous system; right panel, cytokine secretion.
NOTE: AVP = arginine vasopressin; CRH = corticotrophin-releasing hormone; GABA = γ-aminobutyric acid; IL = interleukin; POMC = proopiomelanocortin; TNF = tumor necrosis factor.
SOURCE: Chrousos (1995), used with permission. Copyright © 1995 Massachusetts Medical Society. All Rights Reserved.
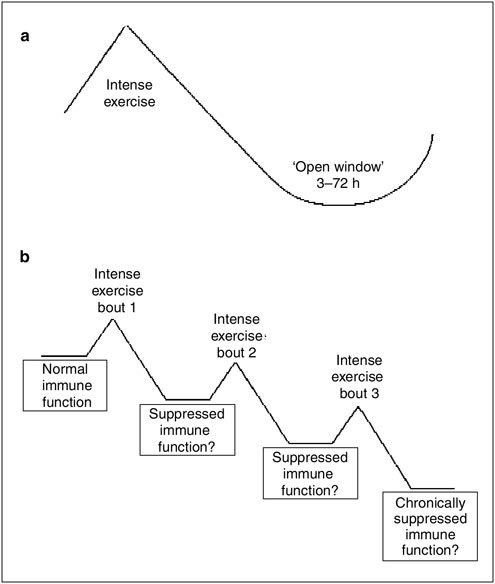
FIGURE B-30 (a) “Open Window” theory of the (b) cumulative effects of intense exercise on immune function.
SOURCE: Lakier Smith (2003).
DIETARY FACTORS THAT AFFECT MUSCLE INJURY AND IMMUNE FUNCTION
The physical stress of combat and high-intensity training causes (1) damage to skeletal muscle, (2) a systemic inflammatory response, and (3) alterations in immune function (Zoico and Roubenoff, 2002). A variety of nutrients may, to some extent, affect aspects of these responses to stress. For example, fatty acids, such as omega-3 fatty acids, that suppress inflammation may reduce inflamma-
tion at the site of injury, although this benefit to soldiers under combat situations remain to be proven (Sethi, 2002). Similarly, amino acids such as arginine and glutamine may be important for combat situations and should be considered for enrichment in soldiers’ diets (Nieman, 2001; Nieves and Langkamp-Henken, 2002). In addition, high-intensity physical activity (and perhaps mental stress as well) increase insulin resistance, which could be exacerbated by diets high in simple sugars. Finally, antioxidant nutrients—both vitamins (such as vitamins E and C) and nonvitamin antioxidants (such as catechins found in tea), as well as anthocyanins found in blueberries and other highly colored fruits—can have protective effects on both muscle and systemic inflammatory responses (Nieman, 2001; Nieves and Langkamp-Henken, 2002).
POTENTIAL BIOMARKERS OF DIETARY INTERVENTIONS
A key factor limiting the ability to evaluate the effectiveness of nutritional and pharmacological interventions in combat situations is the limited number of biomarkers of response that are currently known. It is crucial to be able to evaluate the effects of such interventions by using simple blood or functional tests that can be implemented in the field. In the future, it should be feasible to assess any soldier’s level of stress during combat or training in relation to his baseline and, ideally, alter his dietary intake or prescribe medications that could mitigate downstream injury.
At this time, the best candidates for such biomarkers include indicators of inflammation such as C-reactive protein, cytokines such as interleukin-1 and -6, tumor necrosis factor-α, and their antagonists or receptors (Bosch et al., 2003; Lakier Smith, 2003; Zoico and Roubenoff, 2002). However, these biomarkers will need to be validated in real-life situations and then converted into portable, environmentally stable, easily interpretable tests for field use.
FUTURE DIRECTIONS
The study of how military rations can maintain troops’ fitness for combat is in its infancy. Further studies are needed to evaluate the results of various diets on immune function and on other effects of exercise and stress. Although studies of some individual nutrients have been undertaken, more are needed with more sophisticated outcome measures, evaluation of biomarkers, and application to real-life situations. However, the real work must come in evaluating combinations of nutrients in true diets. This will obviously take a great deal of time and effort to achieve, but the benefits to troop functionality and ability to recover from intense exertion are potentially great. As the cost and time needed to train soldiers for the modern battlefield increase, dietary interventions offer a cost-effective and exciting way to maximize the troops’ effectiveness in the field.
REFERENCES
Bosch JA, de Geus EJ, Veerman EC, Hoogstraten J, Nieuw Amerongen AV. 2003. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med 65(2):245–258.
Chrousos GP. 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332(20):1351–1362.
Lakier Smith L. 2003. Overtraining, excessive exercise, and altered immunity: Is this a T helper-1 versus T helper-2 lymphocyte response? Sports Med 33(5):347–364.
Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. 2002. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav 77(4-5):711–716.
Nieman DC. 2001. Exercise immunology: Nutritional countermeasures. Can J Appl Physiol 26(Suppl):S45–S55.
Nieman DC. 2003. Current perspective on exercise immunology. Curr Sports Med Rep 2(5):239–242.
Nieves C Jr, Langkamp-Henken B. 2002. Arginine and immunity: A unique perspective. Biomed Pharmacother 56(10):471–482.
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. 2000. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88(2):386–392.
Sethi S. 2002. Inhibition of leukocyte-endothelial interactions by oxidized omega-3 fatty acids: A novel mechanism for the anti-inflammatory effects of omega-3 fatty acids in fish oil. Redox Rep 7(6):369–378.
Stowell JR, Kiecolt-Glaser JK, Glaser R. 2001. Perceived stress and cellular immunity: When coping counts. J Behav Med 24(4):323–339.
Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. 2001. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metabol 281(2):E197–E206.
Venkatraman JT, Pendergast DR. 2002. Effect of dietary intake on immune function in athletes. Sports Med 32(5):323-337.
Webster JI, Tonelli L, Sternberg EM. 2002. Neuroendocrine regulation of immunity. Ann Rev Immunol 20:125–163.
Zoico E, Roubenoff R. 2002. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev 60(2):39–51.
The Potential Impact of Probiotics and Prebiotics on Gastrointestinal and Immune Health of Combat Soldiers
Mary Ellen Sanders, Food Culture Technologies
Joshua Korzenik, Harvard Medical School
INTRODUCTION
This paper evaluates the health benefits associated with pro- and prebiotics and their potential utility as a component of combat rations. Combat rations are intended for short-term (three to seven days) use by soldiers during high-intensity, stressful, repetitive combat missions. Desired benefits from this ration, which may potentially be derived from pro- or prebiotics, include prevention of diarrhea;
prevention of infections; reduction of infection-caused morbidity; enhancement of the immune system function; and prevention of kidney stones.
A group of experts assembled by the Food and Agriculture Organization/World Health Organization (FAO/WHO) defined probiotics as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO, 2002). To meet this definition, probiotics must remain alive. Some immune modulatory effects have been documented for killed cells or bacterial cell components, but this is beyond the scope of this paper. Probiotics are not synonymous with our native, beneficial microbiota, although they are often derived from this source. Prebiotics are food ingredients that provide selective stimulation of the growth or activity of beneficial native bacteria (Gibson and Roberfroid, 1995). They are generally complex carbohydrates, such as fructooligosaccharides (FOS) and inulin, which are synthesized from food components or isolated from plant materials.
Some technological issues must be considered for the incorporation of pro-and prebiotics in combat rations for healthy soldiers. For example, the effects for probiotics are strain specific and microorganisms must remain viable in the chosen delivery vehicle. Also, probiotics would be considered a food ingredient, and therefore, any particular strain or combination of strains chosen must have documented safety and efficacy. Furthermore, the dose chosen must be documented to elicit the desired physiological effects. Likewise, prebiotics must be chosen based on documentation of efficacy and used at doses which elicit specific microbiota responses and do not cause any gastrointestinal side effects.
Although a body of supportive data exist that document the role of certain prebiotics and strains of probiotics in enhancing gastrointestinal health, pro- and prebiotics have not been tested specifically on healthy, hypocaloric young adults under stress. Furthermore, data documenting physiological benefits of pro- and prebiotics after short-term (three to seven days) administration are rare. To take advantage of pro- or prebiotic-induced benefits, a more effective strategy may be to supplement the soldier’s diet in the weeks or months before and during the period of combat ration. The use of these compounds for longer time may allow these physiologic effects to be harnessed in a way that would be beneficial during the period of combat stress. In addition, pro- or prebiotic use should be considered as a possible nutritional supplement for soldiers taking antibiotics, and as enteral nutrition during surgical procedures or when exposure to pathogens is likely. Demonstrated actions of pro- and prebiotics are potentially highly relevant to the design of combat rations. However, existing research, while promising, does not convincingly support their inclusion in combat rations administered for a short term. More research is needed to understand how best to take advantage of the utility of these supplements.
Ensuring optimal nutritional status is the primary goal of combat rations. Advances in nutritional science suggest that adequate nutrition means much more than providing macronutrients and preventing nutrient deficiency diseases. A
host of food components have been shown to play roles both in reducing the risk of acute and chronic illnesses and optimizing physiological performance. Pro-and prebiotics are being studied as agents that can play a role in enhancing human health via infection prevention and immune enhancement. The rationale for including these components in combat rations stems from data demonstrating the physiologic effects these substances caused which could potentially benefit intestinal immunity, prevent enteric infections, decrease recovery time from enteric infections and improve bowel habits. Other theoretical, but unproven, benefits of pro- and prebiotics that could be of use include optimizing intestinal function by maximizing nutrient utilization and improving stamina. Unfortunately many of the randomized, controlled studies on probiotics are in pediatric populations or in adults having specific health concerns and these data cannot be directly extrapolated to a healthier population.
This review will consider the human studies that may be applicable to improving the nutritional status of combat troops and explore technological issues associated with inclusion of probiotics or prebiotics in combat rations.
The specific questions that are addressed in this paper are:
-
What are the current applications for pro- and prebiotics in preventing or protection from infections/disease?
-
What are the types and levels of pro- and prebiotics that could be added to such rations of presumably healthy soldiers to optimize their immune system, which may best sustain physical and cognitive performance and prevent possible adverse health consequences with a hypocaloric diet?
EFFECTS OF PROBIOTICS AND PREBIOTICS
A huge and diverse range of bacterial species colonize the human body. Internally, the microbiota extend from mouth to anus, and, for women, into the vaginal tract. Externally they reside on the skin surface. Many lines of research have demonstrated the significant role of the microbiota in human physiology. The microbiota are involved in the development of the immune system, prevention of infection from pathogenic or opportunistic microbes, and maintenance of intestinal barrier function. Recently, the role of commensal microbes in adiposity was established (Backhed et al., 2004). Conventional mice were found to have higher percent body fat, greater feed consumption and higher oxygen utilization than their germ-free counterparts. For a variety of reasons, normal native bacteria may not always perform these functions optimally. The use of pro- and prebiotics to alter native microbiota and thus improve these functions has been studied.
The effects of the components of the gut microbiota may range from potentially pathogenic to health promoting. Lactic acid producing genera such as Bifidobacterium or Lactobacillus have a long-established association with health. These bacteria can be increased either by feeding appropriate strains as a probiotic
or appropriate prebiotic substrates which selectively stimulate the growth or function of beneficial microbiota.
Research into the physiological benefits of probiotics use in the normal population is extensive. Published effects include regulation of immune function and prevention of infection (Bengmark, 2003; Gill, 2003); possible reduction of the risk of relapse in inflammatory bowel conditions (Gionchetti et al., 2003; Ishikawa et al., 2003; Tamboli et al., 2003); reduction of duration of infectious diarrhea in infants (Van Niel et al., 2002); reduction of the onset of atopic dermatitis in high-risk infants (Kalliomaki et al., 2001, 2003); and control of symptoms associated with lactose intolerance (de Vrese et al., 2001). Less dramatic, albeit statistically significant, results have shown the ability of certain probiotic bacteria to decrease the incidence of dental caries (Nase et al., 2001), antibiotic use (Hatakka et al., 2001), respiratory infections (Hatakka et al., 2001), and antibiotic-associated diarrhea (Cremonini et al., 2002). There is emerging research in the areas of probiotics reducing the risk of oxalate kidney stone formation (Duncan et al., 2002; Sidhu et al., 2001); and decreasing stomach colonization by Helicobacter pylori (Cruchet et al., 2003; Linsalata et al., 2004).
The proposed benefits of prebiotics are focused on their effects on the colon and include: (1) increasing colonic bifidobacteria (Buddington et al., 1996); (2) increasing intestinal levels of lactate and short chain fatty acids, especially acetate, butyrate, and propionate (Campbell et al., 1997); (3) decreasing fecal pH (Campbell et al., 1997); (4) altering activities of certain fecal enzymes (Gudiel-Urbano and Goni, 2002); (5) reducing constipation (Kleessen et al., 1997); and (6) increasing mineral absorption (Roberfroid et al., 2002). Most of these effects have been most convincingly demonstrated with prebiotics such as inulin or oligofructose. The effects of prebiotics on reducing the incidence of infectious enteric disease or on immune function has not been well documented.
The relevance of many of these studies to short-term use (three to seven days) of pre- or probiotics in healthy (albeit stressed and hypocaloric) soldiers is questionable because most of these studies focus on longer term administration to subgroups of at-risk individuals such as preterm infants, infants and children with infectious diarrhea, individuals with chronic illnesses, or the elderly. However, it has been noted that a high incidence of noninfectious diarrhea occurs in deployed soldiers. In this case, some value to short-term administration of prebiotics may be realized via prebiotic-mediated osmotic effects. There is some evidence that may be applicable to support a role of pro- or prebiotics in combat rations although benefits may require longer term administration than three to seven days.
STRAIN-DEPENDENCY AND DOSE-DEPENDENCY OF PROBIOTIC AND PREBIOTICS EFFECTS
The benefits reported for probiotics should be considered specific to the strain or strains used and the levels of viable microbes consumed. Although few
studies have evaluated strain-dependent clinical effects (due to the high cost of conducting human studies), numerous in vitro and animal studies have demonstrated that different strains of the same probiotic species can have different or opposing effects. For example, in mouse models of inflammation, different strains have been shown to stimulate expression of different classes of cytokines, which may result in anti- or pro-inflammatory effects (personal communication, B. Pot, Institut Pasteur de Lille, France). In time, however, it may be possible to generalize that certain effects are associated with specific species or even genera of bacteria but the limited body of results from controlled human studies precludes this conclusion at present. Consequently, the target for intervention and the probiotic component must be carefully selected.
Few controlled studies have evaluated the effecst of pro- or prebiotic dose in humans. Doses achieving statistically significant results in a sample of clinical targets are listed in Table B-20. One meta-analysis of studies on Lactobacillus and infectious diarrhea in infants demonstrated a direct correlation between dose and effect (see Figure B-31, Van Niel et al., 2002). Using the endpoint of fecal recovery of an orally administered probiotic, Saxelin and coworkers (1991) demonstrated that there was no recovery of L. rhamnosus GG in feces at feeding levels of 106–108 colony forming units (cfu)/day. However, at 109 cfu/day, two of seven volunteers showed L. rhamnosus GG in feces. At 1010 cells/day all were colonized. Generally speaking, studies using higher doses of probiotics are likely to have positive effects. Therefore, using the highest dose possible, considering
TABLE B-20 Daily Doses Used in Different Studies Documenting Clinical Effects
|
Clinical Effect |
References |
Dose (cfu/day) |
|
↓Day care center antibiotic use/infections/dental caries |
Hatakka, et al. 2001; Nase et al. 2001 |
108 |
|
↓Incidence of antibiotic associated diarrhea |
Vanderhoof et al., 1999 |
1–2 × 1010 |
|
↑Fecal lactobacilli (L. rhamnosus GG) |
Saxelin et al., 1991 |
109 |
|
↑Fecal L. reuteri |
Valeur et al., 2004 |
108-9 |
|
↓Incidence atopic eczema |
Kalliomaki et al., 2001 |
1010 |
|
↑Lactose digestion |
de Vrese et al., 2001 |
1010* |
|
↓IBS abdominal pain |
Niedzielin et al., 2001 |
2 × 1010 |
|
↓Duration of infant diarrhea |
Majamaa et al., 1995 |
1011 |
|
↑Remission of pouchitis |
Mimura et al., 2004 |
1012 |
|
NOTE: cfu = colony-forming units; IBS = irritable bowel syndrome. *Dose in this case more closely tied to quantity of lactose ingested than in daily amount. In general, symptom relief caused by the lactose present in one serving of milk or yogurt can be significantly reduced by consumption of 1010 cfu live cultures. |
||
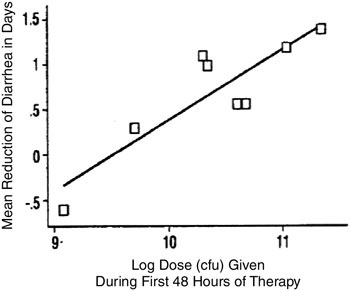
FIGURE B-31 Relationship between Lactobacillus dose and reduction of diarrhea in children.
NOTE: cfu = colony-forming units.
SOURCE: Van Niel et al. (2002), used with permission of Pedatrics.
the product format and cost constraints, is a reasonable approach to formulation. In general, it is important to be mindful of the strain(s) and dose(s) used in studies of clinical documentation and not generalize these results to other strains and doses.
PHYSIOLOGICAL EFFECTS OF PROBIOTICS AND PREBIOTICS
Effects on Fecal Microbiota
One of the most thoroughly documented effects of pro- and prebiotics is their ability to influence fecal microbiota. Limitations of this work, however, stem from assumptions about the composition of an “ideal” microbiota, methodologies for quantification of the diverse microbial communities in feces, and ability to use composition of fecal microbiota as an indicator of microbiota upstream in the intestinal tract. Nonetheless, prebiotics and probiotics comprised of lactobacilli and bifidobacteria have been shown to increase levels of those bacteria in the feces (Bouhnik et al., 1999). Although effects have been shown to be dose-dependent, it should be noted that in the case of subjects with already high bifidobacteria counts, prebiotic feeding has little to no measurable effect. In
FIGURE B-32 Temporal temperature gradient gel electrophoresis of 16S rDNA amplicons of the dominant fecal flora in a healthy control subject sampled over a two-year period. S1, fecal sample collected in 1997; S2-S5, samples collected in 1999 on days 1, 23, 58, and 78, respectively. The dendrogram gives a statistically optimal representation of similarities between temporal temperature gradient gel electrophoresis profiles.
SOURCE: Seksik et al. (2003), used with permission from the BMJ Publishing Group.
some cases, changes in enzymatic (Buddington et al., 1996), physical, or biochemical (Campbell et al., 1997) parameters of the feces also have been documented.
Significantly altering the intestinal microbiota is a difficult task. Normal gut microbiota have their own intrinsic stability, which is poorly understood (see Figure B-32). In addition, the body has its own defenses against bacteria that must be traversed before they reach the colon. Both gastric acidity and bile act as barriers to the survival of transiting bacteria. Once they reach the colon, probiotic bacteria enter a complex environment with more than 450 different species and as many as 1012 cfu per gram of stool. An effective probiotic will increase the number of the particular species being added but usually has little effect on other species. Prebiotics are more consistently delivered as they are largely unaltered by their transit through the gastrointestinal tract until they reach the colon, where they serve as a substrate for particular bacteria. In addition to increasing the numbers of certain bacteria, many prebiotics have a potentially broader effect on the colonic microbiota by altering the pH and making the colonic environment less hospitable for certain species, decreasing their concentration.
Effects on the Gut Barrier
Elements of the intestinal barrier which protect the host from a potential microbial threat include the mucous layer or biofilm adjacent to the epithelial lining, other secreted products [such as immunoglobulin A (IgA)], the epithelial cell layer itself and circulating immune cells. Probiotics have been demonstrated to influence each of these components. Research using cell culture systems, animal
models, and humans suggests that probiotics may have a role, along with commensal bacteria, in maintaining intestinal barrier integrity. A recent paper (Resta-Lenert and Barrett, 2003) examined the ability of live and killed L. acidophilus ATCC4356 and Steptococcus thermophilus ATCC19258 to interfere with the infection of human intestinal epithelial cell lines (HT29 and Caco-2) by enteroinvasive Escherichia coli (EIEC). The live probiotic blend interfered with EIEC adhesion and invasion, protected against physiological disruption caused by EIEC, and increased transepithelial resistance (a marker for improved epithelial barrier function). Antibiotic killed blend did not significantly effect EIEC adhesion or protect against physiological disruption. This model suggests that L. acidophilus and S. thermophilus could protect the cell lines from EIEC and enhance barrier function. A variety of other mechanisms demonstrated by probiotics may be important for an anti-infective capacity, including enhancement of mucus secretion, competitive binding of mucosal cells, displacement of pathogenic microbes, production of bacteriocins (compounds with bacterial antibiotic activity) and reduction of bacterial translocation from the gut lumen (Eijsink et al., 2002; Garcia-Lafuente et al., 2001; Mack et al., 2003; Resta-Lenert and Barrett, 2003).
Effects on Immune System
Probiotic bacteria have been demonstrated to bolster the immune defense against potential bacterial threats. Studies have indicated that certain probiotics can modulate nonspecific and specific aspects of immune responses. According to Gill (1999), these findings suggest that probiotics “could be used as valuable dietary adjuncts for optimizing immunocompetence” in population groups with less than optimal immune systems.
Probiotics appear to alter the activity of phagocytic cells and natural killer (NK) cells, both important against infections and integral to the non-specific immune network. In humans, increases in peripheral blood phagocytic activity and/or NK cell function with L. acidophilus La1 (Schiffrin et al., 1997); Bifidobacterium lactis Bb12 (Schiffrin et al., 1997); L. rhamnosus HN001 (Gill et al., 2001b; Sheih et al., 2001); B. lactis HN019 (Arunachalam et al., 2000; Chiang et al., 2000; Gill et al., 2001b); and L. casei Shirota (Nagao et al., 2000) have been observed.
The components of the specific immune response, humoral- (mediated by antibodies) and cell-mediated (mediated by T-lymphocytes), have also been shown to be affected by probiotics in animal models and, to a lesser extent, in healthy human subjects. Lactobacillus rhamnosus GG stimulated antibody response during rotavirus infection (Kaila et al., 1992; Majamaa et al., 1995). Increased antibody response to vaccines has been observed with a mixture of L. acidophilus La1 and B. lactis BB12 (Link-Amster et al., 1994) and L. rhamnosus GG (Isolauri et al., 1995). In humans, cell-mediated responses to probiotics include
B. lactis HN019-induced (Gill et al., 2001b) and L. brevis-induced production of interferon α (Kishi et al., 1996). Also, B. lactis HN019 has been shown to increase proportions of total, helper (CD4+) and activated (CD25+) T-lymphocytes in the blood of elderly persons (Gill et al., 2001c). Effects seem to be most clearly demonstrable in human subjects with compromised immune function (Gill et al., 2001a; Nagao et al., 2000). Dendritic cell function, pivotal cells for T-cell immune response, is stimulated by lactobacilli; interferon γ production is increased in these cells (Halpern et al., 1991); and systemic and mucosal IgA production is increased as well (Kaila et al., 1992)—these effects were also demonstrated by Bifidobacterium bifidum and B. breve (Roller et al., 2004; Yasui et al., 1999). These effects may be clinically important in the viral immune response, as demonstrated by increasing IgA production to rotaviruses in children with rotavirus diarrhea (Majamaa et al., 1995). In addition, numerous studies have also demonstrated a probiotic effect at limiting aberrant, excessive intestinal inflammation seen in inflammatory bowel disease through modulating the T-cell-driven process (Pavan et al., 2003; Rachmilewitz et al., 2004).
Effects on Protection from Pathogenic Infections
A simple study provides some insight into the potential of certain probiotics to interfere with intestinal pathogens. Shu and colleagues (2000) documented the ability of B. lactis HN019 to protect conventionally colonized mice from lethality caused by Salmonella typhimurium (see Figure B-33). In addition to their improved survival, a higher post-challenge food intake and weight gain and a reduced pathogen translocation to spleen and liver was observed. These results were correlated with improved immune parameters. Similar results were observed using a synbiotic (i.e., a mixture of prebiotic and probiotic) product (Asahara et al., 2001). Using a Shiga toxin-producing E. coli (STEC) model of infection in mice, B. breve strain Yakult also decreased mortality (Asahara et al., 2004). Interestingly, neither B. bifidum ATCC 15696 nor B. catenulatum ATCC 27539 had the ability to reduce STEC-induced mortality in this model, perhaps due to the fact that the B. breve strain produced a high quantity of acetic acid in the feces, but the B. bifidum or B. cantenulatum strains did not.
The clinical relevance of these effects is less certain. A 4 to 7 day (until discharged from the hospital) treatment of preterm infants with L. rhamnosus GG failed to prevent urinary tract infections, necrotizing enterocolitis, or sepsis (Dani et al., 2002). This same strain failed to prevent urinary tract infections in women with a history of previous infections (Kontiokari et al., 2001). A mixture of five probiotics failed to eradicate H. pylori infection, although some suppression was demonstrated. In another study, L. plantarum 299V was administered in a juice drink for one week before and after elective surgery. No differences in gastric colonization (assessed by nasogastric aspirates); bacterial translocation (assessed by culture of a mesenteric lymph node and serosal scraping at laparotomy);
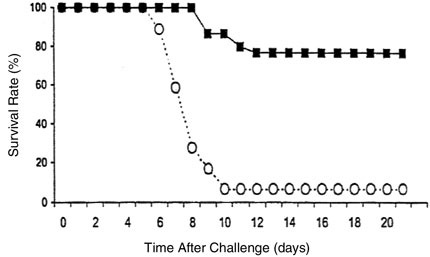
FIGURE B-33 Survival rate of conventionally colonized mice fed B. lactis DR10 or no supplement following a single challenge with Salmonella typhimurium (![]() , control mice; ■, mice fed B. lactis).
, control mice; ■, mice fed B. lactis).
SOURCE: Shu et al. (2000).
or septic complications were observed as compared with the control group (McNaught et al., 2002). A second study with similar endpoints was conducted on patients undergoing elective abdominal surgery. Two weeks before their surgery, 72 patients were given either a placebo or a synbiotic preparation containing L. acidophilus La5, B. lactis Bb12, S. thermophilus, L. bulgaricus, and oligofructose. The resulting lack of differences in bacterial translocation, systemic inflammation, or septic complications questions the benefit of this synbiotic intervention (Anderson et al., 2004).
The results of studies using probiotics and/or prebiotics to prevent travelers’ diarrhea or minimize its symptoms are mixed. One study followed British soldiers deployed to Belize. Soldiers were given one of three preparations (L. fermentum KLD, L. acidophilus LA, or placebo). Although the probiotic was administered at a high dose (2 × 1011/day), no improvement in the incidence of diarrhea was observed after three weeks administration (Katelaris et al., 1995). FOS (10 g/day) was not shown to improve symptoms in a randomized, double-blind, placebo-controlled study of 244 healthy subjects traveling to different destinations and, in fact, increased flatulence (Cummings et al., 2001). Studies using probiotics to prevent travelers diarrhea have generated both positive and negative results (de dios Pozo-Olano et al., 1978; Oksanen et al., 1990).
Effects on Decreasing Duration of Enteric Infections
Pro- and prebiotics have been investigated as a means of decreasing symptoms from enteric infections. Of potentially direct relevance to the population of interest in combat nutrition is a study that demonstrated a benefit in acute amebiasis. In a study of 57 adults, using Saccharomyces boulardii as an adjunct to antibiotic therapy in the treatment of acute amebiasis decreased the duration of clinical symptoms and cyst passage (Mansour-Ghanaei et al., 2003).
Supplementation with prebiotics may be of benefit in decreasing symptoms from diarrhea as well, perhaps by mechanisms that may not only alter gut microbiota but also enhance fluid reabsorbtion through the colon. This may be accomplished by the conversion of complex carbohydrates to short chain fatty acids by bacteria and subsequent use by a short chain fatty acid-sodium transporter in colonocytes. Ramakrishna and coworkers (2000) supplemented oral rehydration solution with amylase-resistant starch in a study of adults and adolescents with cholera. They found that fecal weight and duration of diarrhea were reduced by the addition of this prebiotic.
Probiotics have been studied extensively with the aim of decreasing duration of symptoms in children with acute illness. A meta-analysis of 18 studies of coadministration of probiotics with oral rehydration demonstrated a shorter duration of the disease (Huang et al., 2002). Supplementation of infant cereal with a prebiotic in two randomized controlled trials did not demonstrate any difference in infant diarrhea, frequency of use of medical resources or response to immunizations. However, these trials were done in infants who also were being breastfed, and breast milk contains significant amounts of complex carbohydrates with prebiotic properties (Duggan et al., 2003).
A fermented milk product containing a probiotic strain of L. casei DN-114001 did not prevent gastrointestinal or respiratory infection in an elderly population (Turchet et al., 2003), but it did reduce the duration of infections.
The infection rate observed in brain injury patients was lower (50 percent compared with 100 percent) and the length of hospitalization was shorter (10 compared with 22 days) in a group receiving a probiotic- and glutamine-supplemented enteral diet than in a control group receiving the unsupplemented enteral diet (Falcao de Arruda and de Aguilar-Nascimento, 2004). This study, however, evaluated only 10 patients per group.
The effects of probiotics on the prevention and treatment of symptoms associated with consumption of antibiotics have been evaluated in several studies. The majority of these studies were conducted with a yeast, Saccharomyces boulardii, which has been shown to have a specific ability to decrease recurrence of Clostridium difficile infection (Surawicz, 2003). A study of a probiotic and prebiotic treatment was effective in reducing antibiotic associated diarrhea (AAD) in children (La Rosa et al., 2003). The probiotic used in this study was listed as a “Lactobacillus sporogenes.” This is not a recognized species of Lacto-
bacillus, but is more likely a species of Bacillus (Sanders et al., 2001). A meta-analysis concluded that S. boulardii and lactobacilli have the potential to reduce frequency of AAD incidence; however, benefits from probiotic treatment remain to be proven (D’Souza et al., 2002). This conclusion was challenged (Beckly and Lewis, 2002), and several reports documenting probiotic administration having no effect on AAD have been published (Lewis et al., 1998; Takanow et al., 1990; Thomas et al., 2001). Furthermore, the biological basis for conducting a meta-analysis on studies using such different probiotic preparations as a yeast and bacterium is questionable.
Effects on Colitis
Inflammation can lead to a breakdown of the gut barrier, leaving the host more susceptible to the translocation of infectious agents and autoimmune responses. The manner in which the immune components manage to discriminate (and respond) between foreign microbes and the diverse microbe population of the intestinal tract (approximately 2 kg) is gradually being deciphered. Tolerance towards the intestinal microbe population may derive from the reaction of commensal microbiota with immune components and consequently from prevention of innate immune responses. For example, an important class of transmembrane proteins, various toll-like receptors (TLRs), react specifically with bacteria (both commensal and pathogenic) and their components (Rakoff-Nahoum et al., 2004).
Rodent (Pavan et al., 2003; Rachmilewitz et al., 2004) and human (Gionchetti et al., 2003) models of gut inflammation have been used to assess the ability of different probiotic bacteria or blends of bacteria to downregulate the inflammatory response. For example, a rat model was used to demonstrate the effects of L. farciminis, a producer of nitric oxide, on colitis. In colitic rats, L. farciminis treatment reduced several markers of inflammation (macroscopic damage scores, myeloperoxidase and nitric oxide synthase activities) as compared with controls. Hemoglobin, used as a nitric oxide scavenger, negated the anti-inflammatory effect if infused colonically, suggesting the role of nitric oxide in mediating this anti-inflammatory effect. Oral administration of nitric oxide-producing L. farciminis improved 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Nitric oxide is a smooth muscle dilator produced in situ by intestinal bacteria. However, no evidence has been published about its ability to provide relief from intestinal cramping.
In humans, the most compelling data come from studies conducted on subjects with pouchitis (inflammation of the internal mucosa of a pouch, part of the small intestine created as a neo-rectum in postcolectomy patients having had ulcerative colitis). Administration of high levels (9 × 1011 viable lyophilized bacteria/day) of a commercial probiotic product, VSL#3 (comprising eight different species of Streptococcus, Lactobacillus, and Bifidobacterium) extended remission (Gionchetti et al., 2003). None of the 20 patients receiving the placebo
maintained remission, while 17 of 20 patients receiving active treatment maintained remission for at least nine months.
While these studies are important in showing the distinct and dramatic effects of probiotics on gut inflammation, these results may not be directly relevant to formulation of combat rations because they were conducted with ill subjects.
EFFECTS ON DEVELOPMENT OF KIDNEY STONES
The gut microbiota have been hypothesized to play a role in oxalate accumulation in the urine. The absence of Oxalobacter formigenes from fecal microbiota has been shown to be a risk factor in the development of kidney stones (Mittal et al., 2003). Manipulation of the gut microbiota has been proposed to reduce the risk of kidney stones. No studies in humans have documented that probiotic administration reduces the incidence of kidney stones. However, animal and human studies have documented that O. formigenes can establish itself in the gut and reduce the urinary oxalate concentration (Duncan et al., 2002; Sidhu et al., 2001).
EFFECTS ON ALLERGIC RESPONSES
Although the best studies on allergy are with infants and the prevention of atopic dermatitis, one study does show the ability of viable yogurt bacteria to reduce the symptoms associated with allergies in healthy college students. Trapp and colleagues (1993) studied the effects of the long-term consumption (12 months) of 200 g yogurt daily compared with pasteurized yogurt or no yogurt on self-reported symptoms and immune parameters in young (n = 42) and senior (n = 56) adults. This study was a follow-up to a previous study showing that consumption of yogurt for four months led to a 4 fold increase in γ-interferon in adults (Halpern et al., 1991). In this study, 200 g of daily yogurt (Y), pasteurized yogurt (PY), or no yogurt (NY) was consumed by the two age groups. The number of subjects in each of the six resulting groups was not disclosed in the paper. The paper also did not clarify the microbiological content of the yogurt, so it is likely that the yogurt (“similar to commercially available low-fat yogurt”) only contained L. bulgaricus and S. thermophilus at levels > 108 cfu/gram. Health questionnaires that were completed weekly provided data on the incidence of a variety of gastrointestinal, respiratory, and skin symptoms. Blood samples yielded results on blood cell count and chemistry (including blood lipids and γ-glutamyl transpeptidase [GTT]). Interferon, immunoglobulin E (IgE), and antibody titers to a pneumococcal vaccine were also assessed. The young-adult Y group recorded a significantly lower frequency of allergies and itching as compared with both the PY and NY groups. The senior Y group recorded a significant decrease in allergies over time. Interestingly, the senior NY group recorded a steady increase in total cholesterol and LDL (6.4 percent increase), which did not occur in the Y
or PY groups. Gamma-glutamyl transpeptidase, a liver enzyme associated with fat and/or alcohol or drug metabolism, decreased only in the Y or PY groups. In general the groups consuming yogurt rated their overall health as better than that in the NY group. Total serum IgE was lower for senior subjects in the Y or PY groups. No other statistically significant differences were seen among groups.
PRACTICAL CONSIDERATIONS FOR USE
Several issues should be considered before the use of a pro- or prebiotic in any product, including combat rations.
Probiotic Strain Selection and Prebiotic Selection
There are many probiotic strains commercially available that have been studied for efficacy in humans. Despite the wealth of information available on probiotics, it can be difficult to determine the best strains for a particular situation, such as for healthy soldiers under short-term, high-stress combat situations. Strains such as B. lactis HN019, B. lactis BB12, L. rhamnosus GG, and L. plantarum 299V have a solid basis of scientific support and might be good strains to consider initially. It may be prudent to restrict probiotic strains to species of lactobacilli or bifidobacteria to minimize any safety risk. The best candidate prebiotics for this application would be fructooligosaccharides, including inulin, or galactooligosaccharides. Careful review of scientific documentation for commercial strains of probiotics and prebiotics under consideration with attention to the desired effect must be the basis for strain selection for combat rations.
Safety and Efficacy
The use of pro- and prebiotics in the human diet is not new. Many species of probiotics have been tested for tolerance in humans and have been sold in the supplement and food market for years. However, many other species have not been tested or may carry unnecessary risk. Generally, the pathogenic potential of probiotic species of lactobacilli and bifidobacteria is considered to be quite low (Boriello et al., 2003). If the product developed is in the “drug” category, then the concept of risk versus benefit should be considered. Lactobacilli and bifidobacteria have a very low pathogenic potential, a conclusion supported by several international groups (Borriello et al., 2003; FAO/WHO, 2002). However, probiotics of other genera (e.g., Enterococcus, Escherichia) should be carefully screened for characteristics that may present risks of antibiotic transfer or infectivity.
Prebiotics must be used at levels that provide selective stimulation of the growth or activity of beneficial native bacteria but at the same time do not result
in unpleasant side effects. The level used must be determined for the specific prebiotic under consideration. Side effects can be minimized through a gradual increase in dose.
Strains of probiotics and types of prebiotics should be chosen using science-based evidence for efficacy in humans, ideally in the subjects of interest. Unfortunately, there are no published reports on the efficacy of any prebiotics or probiotic strains to be used for combat troops.
The dose of a pro- or prebiotic is an important consideration, and it should be based on studies showing a physiological effect and lack of significant safety risks. Generally, the higher the level of probiotics, the greater effect observed. With prebiotics, however, if the dose is too high, there is a risk of adverse gastrointestinal effects (diarrhea, gas) or loss of specificity of the effect. Most research studies have used a dose of 5 to 12 g/day, which is a reasonable target.
Stability
For probiotics, stability of the microorganisms is critical for effectiveness but its maintenance can present challenges. For example, viability depends on many factors, including temperature, moisture, atmosphere, pH, and the surrounding matrix. Typically, cell counts can be maintained for one year at room temperature in a freeze-dried state in unopened packages. However, when they are added to food, their stability might be altered and should be tested in the food matrix. For prebiotics, however, stability as supplements or in food matrices is not a concern.
SUMMARY
Existing data are insufficient to make definite strong recommendations for inclusion of pro- or prebiotics as part of a combat ration provided to soldiers for three to seven days. Adequate studies both on this specific population and on the duration of consumption have not been performed. While immune and intestinal benefits have been demonstrated in a variety of settings and populations, some assumptions are required to extrapolate these results to this particular application. Nevertheless, a strong potential exists in using pro- or prebiotics to prevent or decrease symptoms from enteric illnesses. To take advantage of these benefits, we recommend that pro- and prebiotics be evaluated for administration to individuals in the weeks or months before and during the period of combat ration use. The use of these compounds for a longer period may result in beneficial effects during the period of combat stress.
Stability challenges of keeping probiotics alive under the conditions of storage may preclude their incorporation into soldiers’ rations, even if efficacy data
strongly supported their inclusion. However, this is not a limitation of prebiotics. Although there are limits to the evidence supporting the efficacy of prebiotics in this specific application, it should be recognized that a basal level of prebiotics (approximately 5 g/day) is consumed as part of the normal American diet. Even this low level is likely not present in the soldiers’ rations. Therefore, supplementing the rations with this minimal level may provide some support for intestinal function that could be beneficial with essentially no risk. Nourishment of the gut with fiber in the form of these prebiotics could be very worthwhile.
Research priorities that would help advance this field and allow for the possible practical application of pro- and prebiotics to combat rations belong to two broad categories: questions regarding the human microbial biome and more specific issues concerning pro- and prebiotics in the combat population: (1) defining the complete complement or biome (populations and activities) of normal intestinal microbes; (2) understanding the stability of normal intestinal microbiota to resist perturbations and infection; (3) detailing the response of that complement of microbes to diet, stress, drugs, and daily lifestyle fluctuations; and (4) understanding the mechanisms of communication between microbial and host cells.
Before prebiotics or probiotics can be applied to the military population and conditions, specific studies need to be developed to address (1) preventing gastrointestinal illness in soldiers in field conditions; (2) decreasing morbidity and enhancing recovery from a variety of noncombat related illnesses; and (3) using pro- and prebiotics in other areas of interest to this population, such as increase in nutrient utilization; effects on stamina and fatigue; and using of bioengineered probiotics for the delivery of other compounds such as those that may be important for dealing with fatigue resistance or wound healing. Furthermore, to assess the potential benefits for soldiers, more information is needed about noncombat morbidity and risk factors for soldiers to develop infections or other health problems.
In summary, pro- and prebiotics may provide a very low risk intervention for supporting gastrointestinal and immune function. They are quite compatible in dairy-based formats (drinkable yogurt, for example) or as nutritional supplements. Key questions on the extent of these ingredients’ effects on healthy populations, their mechanisms of action, and the parameters for their optimal use remain to be researched. With current knowledge, it is reasonable to recommend a basal level of prebiotic supplementation of combat rations. However, additional research on soldier populations should be conducted before the inclusion of probiotics in combat rations can be recommended.
ACKNOWLEDGMENTS
Thanks to Dominique Brassart, Lorenzo Morelli, and Glenn Gibson for their perspectives on this topic.
REFERENCES
Anderson AD, McNaught CE, Jain PK, MacFie J. 2004. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 53(2):241–245.
Arunachalam K, Gill HS, Chandra RK. 2000. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (Hn019). Eur J Clin Nutr 54(3):263–267.
Asahara T, Nomoto K, Shimizu K, Watanuki M, Tanaka R. 2001. Increased resistance of mice to Salmonella enterica serovar typhimurium infection by synbiotic administration of Bifidobacteria and transgalactosylated oligosaccharides. J Appl Microbiol 91(6):985–996.
Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. 2004. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun 72(4):2240–2247.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101(44):15718–15723.
Beckly J, Lewis S. 2002. The case for probiotics ramains unproven. Br Med J 325(7350):902.
Bengmark S. 2003. Use of some pre-, pro- and synbiotics in critically ill patients. Best Pract Res Clin Gastroenterol 17(5):833–848.
Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, Valtonen V. 2003. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 36(6):775–780.
Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P, Marteau P, Flourie B, Bornet F, Rambaud JC. 1999. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J Nutr 129(1):113–116.
Buddington RK, Williams CH, Chen SC, Witherly SA. 1996. Dietary supplement of neosugar alters the fecal flora and decreases activities of aome reductive enzymes in human subjects. Am J Clin Nutr 63(5):709–716.
Campbell JM, Fahey GC Jr, Wolf BW. 1997. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127(1):130–136.
Chiang BL, Sheih YH, Wang LH, Liao CK, Gill HS. 2000. Enhancing immunity by dietary consumption of a probiotic lactic acid bacterium (Bifidobacterium lactis HN019): Optimization and definition of cellular immune responses. Eur J Clin Nutr 54(11):849–855.
Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. 2002. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: A parallel group, triple blind, placebo-controlled study. Am J Gastroenterol 97(11):2744–2749.
Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M. 2003. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 19(9):716-721.
Cummings JH, Christie S, Cole TJ. 2001. A study of fructo oligosaccharides in the prevention of travellers’ diarrhoea. Aliment Pharmacol Ther 15(8):1139–1145.
Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. 2002. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 82(2):103–108.
de dios Pozo-Olano J, Warram JH Jr, Gomez RG, Cavazos MG. 1978. Effect of a lactobacilli preparation on traveler’s diarrhea. A randomized, double blind clinical trial. Gastroenterology 74(5 Pt 1):829–830.
de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. 2001. Probiotics—Compensation for lactase insufficiency. Am J Clin Nutr 73(2 Suppl):421S–429S.
D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. 2002. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. Br Med J 324(7350):1361–1364.
Duggan C, Penny ME, Hibberd P, Gil A, Huapaya A, Cooper A, Coletta F, Emenhiser C, Kleinman RE. 2003. Oligofructose-supplemented infant cereal: 2 randomized, blinded, community-based trials in peruvian infants. Am J Clin Nutr 77(4):937–942.
Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. 2002. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol 68(8):3841–3847.
Eijsink VG, Axelsson L, Diep DB, Havarstein LS, Holo H, Nes IF. 2002. Production of class II bacteriocins by lactic acid bacteria. An example of biological warfare and communication. Antonie Van Leeuwenhoek 81(1–4):639–654.
Falcao de Arruda IS, de Aguilar-Nascimento JE. 2004. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci 106(3):287–292.
FAO/WHO (Food and Agriculture Organization/World Health Organization). 2002. Guidelines for the Evaluation of Probiotics in Food. Geneva, Switzerland: FAO/WHO.
Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Malagelada JR. 2001. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 48(4):503–507.
Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 125(6):1401–1412.
Gill HS. 1999. Potential of using dietary lactic acid bacteria for enhancement of immunity. Dialogue 32:6–11. New Zealand Dairy Advisory Bureau.
Gill HS. 2003. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol 17(5):755–773.
Gill HS, Darragh AJ, Cross ML. 2001a. Optimizing immunity and gut function in the elderly. J Nutr Health Aging 5(2):80–91.
Gill HS, Rutherfurd KJ, Cross ML. 2001b. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J Clin Immunol 21(4):264–271.
Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. 2001c. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr 74(6):833–839.
Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. 2003. Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology 124(5):1202–1209.
Gudiel-Urbano M, Goni I. 2002. Effect of short-chain fructooligosaccharides and cellulose on cecal enzyme activities in rats. Ann Nutr Metab 46(6):254–258.
Halpern GM, Vruwink KG, Van de Water J, Keen CL, Gershwin ME. 1991. Influence of long-term yoghurt consumption in young adults. Int J Immunotherapy 7(4):205–210.
Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. 2001. Effect of long term consumption of probiotic milk on infections in children attending day care centres: Double blind, randomised trial. Br Med J 322(7298):1327–1329.
Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ. 2002. Efficacy of probiotic use in acute diarrhea in children: A meta-analysis. Dig Dis Sci 47(11):2625–2634.
Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. 2003. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr 22(1):56–63.
Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. 1995. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 13(3):310–312.
Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. 1992. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res 32(2):141–144.
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. 2001. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 357(9262):1076–1079.
Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361(9372):1869–1871.
Katelaris PH, Salam I, Farthing MJ. 1995. Lactobacilli to prevent traveler’s diarrhea? N Engl J Med 333(20):1360–1361.
Kishi A, Uno K, Matsubara Y, Okuda C, Kishida T. 1996. Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-alpha producing capacity in humans. J Am Coll Nutr 15(4):408–412.
Kleessen B, Sykura B, Zunft HJ, Blaut M. 1997. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am J Clin Nutr 65(5):1397–1402.
Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. 2001. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. Br Med J 322(7302):1571–1575.
La Rosa M, Bottaro G, Gulino N, Gambuzza F, Di Forti F, Ini G, Tornambe E. 2003. Prevention of antibiotic-associated diarrhea with Lactobacillus spororgens and furcto-oligosaccharides in children. A multicentric double-blind vs placebo study. Minerva Pediatr 55(5):447–452.
Lewis SJ, Potts LF, Barry RE. 1998. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect 36(2):171–174.
Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. 1994. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol 10(1):55–63.
Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, Simone CD, Ierardi E, Di Leo A. 2004. The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter 9(2):165–172.
Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. 2003. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52(6):827–833.
Majamaa H, Isolauri E, Saxelin M, Vesikari T. 1995. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Ped Gastroenterol Nutr 20(3):333–338.
Mansour-Ghanaei F, Dehbashi N, Yazdanparast K, Shafaghi A. 2003. Efficacy of Saccharomyces boulardii with antibiotics in acute amoebiasis. World J Gastroenterol 9(8):1832–1833.
McNaught CE, Woodcock NP, MacFie J, Mitchell CJ. 2002. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 51(6):827–831.
Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. 2004. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53(1):108–114.
Mittal RD, Kumar R, Mittal B, Prasad R, Bhandari M. 2003. Stone composition, metabolic profile and the presence of the gut-inhabiting bacterium Oxalobacter formigenes as risk factors for renal stone formation. Med Princ Pract 12(4):208–213.
Nagao F, Nakayama M, Muto T, Okumura K. 2000. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci Biotechnol Biochem 64(12):2706–2708.
Nase L, Hatakka K, Savilahti E, Saxelin M, Ponka A, Poussa T, Korpela R, Meurman JH. 2001. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res 35(6):412–420.
Niedzielin K, Kordecki H, Birkenfeld B. 2001. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 13(10):1143–1147.
Oksanen PJ, Salminen S, Saxelin M, Hamalainen P, Ihantola-Vormisto A, Muurasniemi-Isoviita L, Nikkari S, Oksanen T, Porsti I, Salminen E. 1990. Prevention of travellers’ diarrhoea by Lactobacillus GG. Ann Med 22(1):53–56.
Pavan S, Desreumaux P, Mercenier A. 2003. Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria. Clin Diagn Lab Immunol 10(4):696–701.
Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. 2004. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126(2):520–528.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118(2):229–241.
Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. 2000. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med 342(5):308–313.
Resta-Lenert S, Barrett KE. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52(7):988–997.
Roberfroid MB, Cumps J, Devogelaer JP. 2002. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J Nutr 132(12):3599–3602.
Roller M, Rechkemmer G, Watzl B. 2004. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J Nutr 134(1):153–156.
Sanders ME, Morelli L, Bush S. 2001. Lactobacillus sporogenes is not a Lactobacillus probiotic. ASM News 67(8):385–386.
Saxelin M, Elo S, Salminen S, Vapaatalo H. 1991. Dose response colonization of faeces after oral administration of Lactobacillus casei strain GG. Microbial Ecol Health Dis 4:209–214.
Schiffrin EJ, Brassart D, Servin AL, Rochat F, Donnet-Hughes A. 1997. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am J Clin Nutr 66(2):515S–520S.
Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. 2003. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 52(2):237–242.
Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. 2001. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr 20(2 Suppl):149–156.
Shu Q, Lin H, Rutherfurd KJ, Fenwick SG, Prasad J, Gopal PK, Gill HS. 2000. Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice. Microbiol Immunol 44(4):213–222.
Sidhu H, Allison MJ, Chow JM, Clark A, Peck AB. 2001. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J Urol 166(4):1487–1491.
Surawicz CM. 2003. Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pract Res Clin Gastroenterol 17(5):775–783.
Takanow RM, Ross MB, Eretel IN, Dickinson DG, McCormick LS Garfinkel JF. 1990. A double-blind, placebo-controlled study of the efficacy of lactinex in the prophylaxis of amoxicillin-induced diarrhea. DECP Ann Pharmacother 224:(38)2–4.
Tamboli CP, Caucheteux C, Cortot A, Colombel JF, Desreumaux P. 2003. Probiotics in inflammatory bowel disease: A critical review. Best Pract Res Clin Gastroenterol 17(5):805–820.
Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. 2001. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: A randomized, placebo-controlled trial. Mayo Clin Proc 76(9):883–889.
Trapp DL, Chang CC, Halpern GM, Keen CL, Gershwin ME. 1993. The influence of chronic yogurt consumption on populations of young and elderly adults. Int J Immunotherapy IX:53–64.
Turchet P, Laurenzano M, Auboiron S, Antoine JM. 2003. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114 001 on winter infections in free-living elderly subjects: A randomised, controlled pilot study. J Nutr Health Aging 7(2):75–77.
Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. 2004. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 70(2):1176–1181.
Van Niel CW, Feudtner C, Garrison MM, Christakis DA. 2002. Lactobacillus therapy for acute infectious diarrhea in children: A meta-analysis. Pediatrics 109(4):678–684.
Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. 1999. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 135(5):564–568.
Yasui H, Shida K, Matsuzaki T, Yokokura T. 1999. Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek 76(1–4):383–389.
Developing a Low Residue Diet
Joanne L. Slavin, University of Minnesota
LOW-RESIDUE DIETS
A low-residue (low-fiber) diet1 is for people who need to care for their intestinal tracts. This diet limits the amount of food waste that moves through the large intestine, which should help control diarrhea and abdominal cramping. Low-residue diets are also recommended for diverticulitis, after some types of intestinal surgery such as colostomy or ileostomy, and in the acute phases of certain inflammatory bowel conditions such as ulcerative colitis or Crohn’s disease. Diet therapy texts suggest that patients on a low-residue diet avoid foods made with seeds, nuts, or raw or dried fruit; with whole grains; and with raw fruits and vegetables. In addition, they should remove fruit and vegetable skins before cooking, limit dairy consumption to two cups per day, limit fats because they increase stool bulk, and avoid tough, fibrous meats with gristle.
Low-residue diets have been around for a long time, but there is little research in support for their use or effectiveness. Kember and coworkers (1995), in a blind prospective trial of low-residue diet versus normal diet in preparation for barium enema, found no difference in fecal residue between the groups. They recommended that patients just consume their usual diet, rather than the low-residue one before the procedure.
WHAT IS NORMAL LAXATION?
The term “laxation” describes a wide range of gastrointestinal effects, including stool weight, transit time, bloating and distention, flatus, and constipation, and diarrhea. Digestive problems are one of the most common medical complaints, with 40.5 percent of individuals reporting one or more adverse digestive symptoms in a recent survey (Sandler et al., 2000). Many of these complaints are associated with the consumption of high-fiber foods, such as beans, cabbage, and onions, or with malabsorption of carbohydrates such as lactose. Diarrhea is a potentially life-threatening condition, and although it is associated with a high intake of nondigestible carbohydrates, it is more likely related to the consumption of bacteria-contaminated food, viral infection, irritable bowel syndrome, Crohn’s disease, or colonic tumors (Schiller, 2004). Commonly accepted criteria for clinical diarrhea are (1) elevated stool output (> 250 g/day); (2) watery, difficult-to-control bowel movements; and (3) elevated frequency of bowel movements (> 3/day) (McRorie et al., 2000).
In general, stools are about 75 percent water although this is highly variable and increases greatly with diarrhea. Of the remaining stool, about 33 percent is dead bacteria, 33 percent is undigested carbohydrate, and the remaining 33 percent is protein, fat, mucus, dead cells, and inorganic material. Consumption of highly digestible foods and a limited intake of dietary fiber, dairy products, and other poorly digested material will produce a small stool. Although dairy products are typically listed as foods to avoid on a low-residue diet, presumably because of their lactose content, clinical studies that document dairy foods producing more residue are lacking.
Other factors that affect stool size are often noted in studies, but are not well supported by research trials. Stress associated with exams or competition can speed intestinal transit. Exercise is known to speed intestinal transit as well (Oettle, 1991), although data on this topic are conflicting (Bingham and Cummings, 1989). Bingham and Cummings (1989) found that on a controlled dietary intake, transit time increased in nine subjects and decreased in five after participating in a nine-week exercise program. The exercise program did not change other measures of bowel function, stool weight, or fecal frequency. Patients often relate the importance of that morning cup of coffee or smoking on regular bowel habit. Drugs, both laxatives designed to speed transit and others, alter bowel function and fecal composition (Lembo and Camilleri, 2003). Even on rigidly controlled diets of the same composition, subjects had a large variation in daily stool weights. Tucker and colleagues (1981) examined the predictors of stool weight when completely controlled diets were fed to normal volunteers. They found that personality was a better predictor of stool weight than was dietary fiber intake, with outgoing subjects more likely to produce higher stool weights.
DEFINING DIETARY FIBER
Consumption of high-fiber, carbohydrate-based diets protects against life-threatening illnesses such as heart disease, diabetes, obesity, and cancer. Since the mid-1970s, attention has focused on dietary fiber as the active component in this protection. Dietary fiber is generally divided into soluble and insoluble fiber to reflect differences in physiological effects. These descriptors, however, have serious limitations because the effects of dietary fiber in whole foods are often greater than that found with isolated fiber fractions.
The Panel on Dietary Reference Intakes for Macronutrients reviewed the research on dietary fiber and disease prevention to decide whether to set a recommended intake level for dietary fiber. Before their report, there was no Recommended Dietary Allowance (RDA) for dietary fiber. The panel also found in its deliberations that there was no official definition of dietary fiber. Thus, an Institute of Medicine Panel on the Definition of Dietary Fiber was formed to review the existing literature on dietary fiber and to determine its best scientific definition (IOM, 2001). Dietary fiber consists of nondigestible carbohydrates and lignin that are intrinsic and intact in plants. Functional Fiber consists of isolated nondigestible carbohydrates that have beneficial physiological effects in humans. Total fiber is the sum of dietary fiber and functional fiber.
The DRI committee used the updated definitions from the Panel on the Definition of Dietary Fiber for dietary, functional, and total fiber in their report (IOM, 2002). Additionally, they set an Adequate Intake (AI) for total fiber from foods at 38 g and 25 g/day for young men and women, respectively, based on intake levels observed to protect against coronary heart disease (CHD). Adequate Intake is the recommended average daily intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people; AI is used when an RDA cannot be determined. The committee concluded that a recommended intake level for total fiber based on the prevention of CHD should be sufficient to reduce constipation in most normal people given an adequacy of hydration of the large bowel. There was insufficient evidence to set a Tolerable Upper Intake Level (UL) for dietary fiber and functional fiber. The usual intake of dietary fiber in the United States is only about 14 grams per day (Martlett et al., 2002). Thus, there is a large fiber gap to fill between usual intake of dietary fiber and recommended intakes.
DEFINING AND MEASURING THE PHYSIOLOGICAL EFFECTS OF FIBER
The physiological effects of fibers differ and scientists have attempted to categorize fibers according to function, methods of extraction, or solubility. In an attempt to assign physiological effects to chemical types of fiber, scientists
classified fibers into soluble and insoluble fiber. Unfortunately, scientific support that soluble fibers can lower serum cholesterol while insoluble fibers can increase stool size is inconsistent at best. A meta-analysis testing the effects of pectin, oat bran, guar gum, and psyllium on blood lipid concentrations found that 2 to 10 g/day of viscous fiber (e.g., oat bran and psyllium) was associated with small, but significant, decreases in total and low-density lipoprotein cholesterol concentrations (Brown et al., 1999). Oat bran lowers serum lipids, but wheat bran does not (Anderson et al., 1991). Resistant starch, generally a soluble fiber, does not affect serum lipids (Jenkins et al., 1998). Thus, not all soluble fibers are hypocholesterolemic agents, and other traits, such as viscosity of fiber, play a role and must be considered.
The insoluble fiber association with laxation also is inconsistent. Fecal weight increases 5.4 g per gram of wheat bran fiber (mostly insoluble), 4.7 g per gram of fruits and vegetables (soluble and insoluble), 3.5 g per gram of isolated cellulose (insoluble), and 1.2 g per gram of isolated pectin (soluble) consumed (Table B-21) (Cummings, 1993). When subjects were fed 15, 30, or 42 g/day of dietary fiber from a mixed diet, there was a significant increase in stool weight on all diets. Most of the increased stool weight was from undigested dietary fiber, although the mid-range of fiber intake was also associated with an increase in bacterial mass (Kurasawa et al., 2000).
Many fiber sources (e.g., oat bran and psyllium) are mostly soluble but still increase stool weight. Not all insoluble fibers (e.g., isolated cellulose) are particularly good at relieving constipation. The disparities between the amounts of soluble and insoluble fiber measured chemically and the magnitude of their physiological effects led the Institute of Medicine Panel on the Definition of Dietary Fiber to recommend that with fiber, the terms “soluble” and “insoluble” gradually be eliminated and replaced by specific beneficial physiological effects of a fiber, perhaps viscosity (the ability of a liquid to resist flow under shear-
TABLE B-21 Average Increase in Fecal Weight Per Gram of Fiber Consumed
|
Source |
Increased Fecal Weight (g/g fiber fed) |
|
Wheat |
5.4 |
|
Fruits and vegetables |
4.7 |
|
Gums and mucilages |
3.7 |
|
Cellulose |
3.5 |
|
Oats |
3.4 |
|
Corn |
3.3 |
|
Legumes |
2.2 |
|
Pectin |
1.2 |
|
SOURCE: Cummings (1993), reprinted with permission from CRC Press, Boca Raton, Florida. |
|
stress) and fermentability (ability of a carbohydrate to be converted to alcohol, acids, or other compounds (IOM, 2001).
Perhaps less invasive measures of bowel function could be used to evaluate functional fibers. Bowel movement frequency would not require stool collection and appears to be linked to higher fiber intakes (Sanjoaquin et al., 2004). Another approach would be to model systems for bowel function attributes. A fecal bulking index has been described for rats (Monro, 2004). Other animal models or model systems could be devised standardized, and included in regulations. Non-invasive methods are available to estimate fiber fermentation in humans (Symonds et al., 2004).
SUMMARY
Many of the diseases of public health significance—obesity, cardiovascular disease, type 2 diabetes—as well as the less prevalent, but no less significant, diseases—colonic diverticulosis, constipation, and inflammatory bowel conditions such as ulcerative colitis or Crohn’s disease—can be prevented or treated by increasing the consumption of a variety of foods containing various fibers. Promotion of such a food plan by the dietetics professional and implementation by the adult population should increase fiber intakes across the life cycle (Martlett et al., 2002). Since the assault rations are meant for short-term use (three to seven days for up to a month), the prevention of chronic diseases is not a factor considered when recommending dietary fiber.
More relevant to the case of soldiers in military operations are the dietary fiber effects on the prevention of constipation and diarrhea and the maintenance of proper gastrointestinal function. Also, decreasing stool weight and volume would be an advantage for soldiers deployed to these missions. To decrease stool weight, less dietary fiber is advised. The lower limit of dietary fiber intake for small stool size, yet normal bowel function, is difficult to define. With functional fibers, many possibilities are available, shorter or longer chain length, different chemical composition, increasing or decreasing fiber fermentation, and increasing or decreasing fiber viscosity. The choice of fiber will also depend on the delivery system. For example, soluble fibers (such as oligosaccharides and arabinogalactan) are easier to use in liquid systems, and others are easier to use in food, such as grain fibers. In general, shorter chain nondigestible carbohydrates may be less well tolerated physiologically, but they are easy to incorporate into liquids. A mixture of isolated fibers might be better tolerated than any one fiber source.
REFERENCES
Anderson JW, Gilinsky NH, Deakins DA, Smith SF, O’Neal DS, Dillon DW, Oeltgen PR. 1991. Lipid responses of hypercholesterolemic men to oat-bran and wheat-bran intake. Am J Clin Nutr 54(4):678–683.
Bingham SA, Cummings JH. 1989. Effect of exercise and physical fitness on large intestinal function. Gastroenterology 97(6):1389–1399.
Brown L, Rosner B, Willett WW, Sacks FM. 1999. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am J Clin Nutr 69(1):30–42.
Cummings JH. 1993. The effect of dietary fiber on fecal weight and composition. In: Spiller GA, ed. CRC Handbook of Dietary Fiber in Human Nutrition. 2nd ed. Boca Raton, FL: CRC Press. Pp. 263–349.
Jenkins DJ, Vuksan V, Kendall CW, Wursch P, Jeffcoat R, Waring S, Mehling CC, Vidgen E, Augustin LS, Wong E. 1998. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr 17:609–616.
IOM (Institute of Medicine). 2001. Dietary Reference Intakes: Proposed Definition of Dietary Fiber. Washington, DC: National Academy Press.
IOM. 2002. Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: The National Academies Press.
Kember PG, McBride KD, Tweed CS, Collins MC. 1995. A blinded prospective trial of low-residue versus normal diet in preparation for barium enema. Br J Radiol 68(806):128–129.
Kurasawa S, Haack VS, Marlett JA. 2000. Plant residue and bacteria as bases for increased stool weight accompanying consumption of higher dietary fiber diets. J Am Coll Nutr 19(4):426–433.
Lembo A, Camilleri M. 2003. Current concepts: Chronic constipation. N Engl J Med 349(14):1360–1368.
Martlett JA, McBurney MI, Slavin JL. 2002. Position of the American Dietetic Association: Health implications of dietary fiber. J Am Diet Assoc 102(7):993–1000.
McRorie J, Zorich N, Riccardi K, Bishop L, Filloon T, Wason S, Giannella R. 2000. Effects of olestra and sorbitol consumption on objective measures of diarrhea: Impact of stool viscosity on common gastrointestinal symptoms. Regul Toxicol Pharmacol 31(1):59–67.
Munro JA. 2004. Adequate intake values for dietary fibre based on faecal bulking indexes of 66 foods. Eur J Clin Nutr 58(1):32–39.
Oettle GJ. 1991. Effect of moderate exercise on bowel habit. Gut 32(8):941–944.
Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. 2000. Abdominal pain, bloating, and diarrhea in the United States: Prevalence and impact. Dig Dis Sci 45(6):1166–1171.
Sanjoaquin MA, Appleby PN, Spencer EA, Key TJ. 2004. Nutrition and lifestyle in relation to bowel movement frequency: A cross-sectional study of 20630 men and women in EPIC-Oxford. Public Health Nutr 7(1):77–83.
Schiller LR. 2004. Chronic diarrhea. Gastroenterology 127(1):287–293.
Symonds EL, Kritas S, Omari TI, Butler RN. 2004. A combined 13CO2/H2 breath test can be used to assess starch digestion and fermentation in humans. J Nutr 134(5):1193–1196.
Tucker DM, Sandstead HH, Logan GM Jr, Klevay LM, Mahalko J, Johnson LK, Inman L, Inglett GE. 1981. Dietary fiber and personality factors as determinants of stool output. Gastroenterology 81(5):879–883.
Diet and Kidney Stones: Optimizing Military Field Rations
Linda K. Massey, Washington State University
INTRODUCTION
More than 90 percent of kidney stones in the adult US population are made of calcium oxalate, calcium phosphate, urate salts, or a combination of these.
The risk of developing any of these types of stones varies with urinary composition, which can be improved by appropriate dietary choices. The components of urinary composition affecting stone formation include volume, oxalate, calcium, citrate, magnesium, phosphate, and pH. The relationship of these compounds to saturation levels of the salt forms (e.g., calcium oxalate, calcium phosphate) has been summarized by Tiselius (1997). In addition, sodium and potassium concentrations affect the salt form of urate, which in turn affects solubility (Moe et al., 2002). Urate salts can often form the core for calcium stone formation, enhance calcium stone growth, and form stones directly (Coe, 1978).
Two mechanisms have been proposed for stone formation and growth. In the classic paradigm, stones grow slowly with layers of calcium oxalate or calcium phosphate building on a core, frequently urate. When sectioned, these stones have the concentric layers that can also be seen in the candy known as a “jawbreaker.” Recently, another model has been proposed to explain rapidly forming stones. In accordance with hydraulic models, urine flows more slowly along the outside of the urethral lumen. When urines are supersaturated with potential stone-forming salts, which frequently happens when urine production is limited, stones may form rapidly (within hours) in concentrated urines. In this model, aggregation of small crystals into large conglomerates occurs. These stones frequently have the appearance of a raspberry. Even if not expelled, they may bind to the epithelium, forming the core for a new stone.
Many “normal” urines contain small calcium oxalate crystals, but the crystals do not form stones. Inhibitory proteins may keep these crystals from aggregating or accumulating as additional layers on a stone attached to the epithelium. Variations in these proteins cannot yet be measured clinically.
This paper addresses the types and levels of nutritional factors that could be added to assault rations of a presumably healthy soldier to lower the risk of kidney stones formation. These factors should be included in a ration which may also best sustain physical and cognitive performance and prevent possible adverse health consequences of soldiers under stress and consuming a hypocaloric diet.
RISK OF URINARY STONE FORMATION UNDER COMBAT MISSIONS
Combat conditions that affect urinary composition associated with stone risk include extreme physical activity, impaired caloric intake, increased sweating, and dehydration. First, urinary volume decreases. The causes include increased sweat loss accompanying physical exertion, protective clothing retaining body heat, and inadequate fluid intake. In the only study on physical exercise and stone risk, Sakhaee and colleagues (1987) found that moderate physical exercise, six hours on a bicycle ergometer at 70 to 75 percent of maximum oxygen consumption, increased stone risk by decreasing urinary volume from 847 to 290 mL. Even though total renal excretion of stone-forming constituents decreased, the
reduction in volume resulted in higher saturation of calcium oxalate and uric acid. In addition, the pH dropped from 6.35 to 5.79. A decreased pH will increase urinary calcium.
The pH will also drop if energy intake is not sufficient to meet increased energy use. Reddy and colleagues (2002) reported that two weeks’ consumption of a high-protein, low-carbohydrate weight reduction diet (1,930 versus 2,314 kcal usual energy) decreased urinary pH from 6.1 to 5.5 and increased urinary calcium from 160 to 258 mg/day. During this study, hydration was maintained as participants maintained urinary outputs of 2.4 L/day for all study phases, but even with the relatively modest hypocaloric status in this study, stone risk indices increased.
Studies have shown that diet can be modified in nonmilitary situations to reduce the risk of supersaturated urines. Interventions include maintaining adequate water intake and hydration. A clinical trial by Borghi and colleagues (1996) reported that stone recurrence was reduced significantly by even modest increases in urinary volume. Hydration is considered adequate if urine production is 2 L/day (Borghi et al., 1999).
Restriction of several dietary components may also be effective in reducing stone risk. Avoiding high-oxalate foods is the highest priority.The foods highest in oxalate per serving are listed in Table B-22. For example, soy food consumption can increase urinary oxalate significantly (Massey et al., 2002). To complicate the issue, soy protein concentrates contain high but variable amounts of oxalate depending on cultivar, growing conditions, and processing methods, so each soy product would have to be checked for oxalate content. Caffeine increased urinary calcium for several hours after its consumption (Massey and Sutton, 2004), but there is no adaptation to the hypercalciuric effects of caffeine as there is with mental stimulatory effects. Reduction of caffeine may not be possible because soldiers need to be mentally alert under combat conditions. Urinary pH is affected by protein intake as well, in particular by the sulfur content of the proteins (Frassetto et al., 1998; Massey, 2003). Both animal and
TABLE B-22 Foods Highest in Oxalate, Recommended to Avoid
|
Food |
|
Parsley Rhubarb Spinach Beets, both roots and leaves Legumes, including beans, soy, and peanuts Tea, green or black, regular not herbal Chocolate Tree nuts, almonds, walnuts, pecans Concentrated brans, especially wheat |
TABLE B-23 Potential Acid as Sulfate from Sulfur-Containing Amino Acids
|
Food |
Potential Acid as Sulfate (mEq per 100 g of protein) |
|
Oatmeal |
82 |
|
Egg |
80 |
|
Walnuts |
74 |
|
Pork |
73 |
|
Wheat |
69 |
|
White Rice |
68 |
|
Barley |
68 |
|
Tuna |
65 |
|
Chicken |
65 |
|
Corn |
61 |
|
Beef |
59 |
|
Milk |
59 |
|
Cheddar |
46 |
|
Soy |
40 |
|
Peanuts |
40 |
|
Millet |
31 |
|
Almonds |
23 |
|
Potato |
23 |
|
SOURCE: Massey (2003). |
|
plant protein sources have the potential to increase sulfuric acid excretion, thereby decreasing pH (Table B-23). Substitution of plant protein for an equivalent amount of beef resulted in no change in risk as shown in a metabolic study (Massey and Kynast-Gales, 2001). High-purine foods should be restricted, but these are not commonly found in field rations (e.g., gravies and meat extracts; organ meats: kidneys, liver, sweetbreads; anchovies; herring, sardines). In addition, dietary purine catabolism to uric acid only contributes about one-third of the daily uric acid excretion.
Dietary components to be enhanced include potassium, citrate, and magnesium. Potassium reduces urinary calcium excretion (Lemann et al., 1991), and potassium urate is more soluble than sodium urate and, therefore, presents a lower risk of stone formation (Maalouf et al., 2004).
Higher urinary potassium is associated with decreased stone growth after shockwave lithotripsy (Pierratos et al., 2000). Finally food potassium is associated with basic compounds that counteract the acidic effects of protein (Sebastian et al., 1994). Good sources of potassium include milk legumes and most fruits and vegetables. Citrate is also beneficial because citrate competes with oxalate for binding calcium, but calcium citrate is more soluble. Citrus fruits and juices are good sources of citrate, and citrate salts are used in some combat foods.
Finally, higher urinary magnesium from increased magnesium intakes is beneficial because magnesium competes with calcium to bind oxalate, and magnesium oxalate is 100 times more soluble than calcium oxalate. Whole grains and green vegetables are good sources of magnesium. Because food intake may be less than optimal, consider using a potassium–magnesium–citrate supplement. Ettinger and colleagues (1997) showed that 60 mEq/day of citrate as the combined potassium–magnesium salt reduced stone recurrence by 85 percent in a three-year clinical trial.
Two nutrients should be controlled to amounts appropriate for combat situations: calcium and salt (sodium chloride). Calcium intakes of 1,000 mg/day are appropriate for individuals with normal calciuria, while 600 mg/day is appropriate for individuals with diagnosed hypercalciuria. Because populations contain with individuals with both of these conditions, an intake of 800 mg/day is recommended. This level of calcium will reduce minimize the risk of hypercalciuria and will reduce urinary oxalate by binding dietary oxalate in the gut lumen (Massey and Kynast-Gales, 1998). Salt intake in excess of amounts needed to replace sweat losses increases urinary calcium losses by 1 mmol of calcium (40 mg) for each 100 mmol of salt (5,844 mg) excreted. Individuals typically show a range of responses, from virtually none to more than twice the average amounts of calcium excreted (Massey and Whiting, 1996). The need of the soldier for adequate salt to replace sweat losses is more important than reducing risk of kidney stones.
Ten milligrams per day of urinary oxalate comes from endogenous synthesis (Holmes, 2000), and ascorbate is one precursor. Massey and colleagues (2005) recently reported that 40 percent of both stone formers and nonstone formers increased urinary oxalate after taking 2 g/day supplements of ascorbate. Supplementation amounts as low as 500 mg/day have been reported to increase urinary oxalate significantly (Massey, 2000). Therefore, supplementation of any amount of vitamin C above the RDA (e.g., men 19–30 years is 75 mg/day) should be avoided. The hyperoxaluric effect of ascorbate was seen primarily within two to six hours after supplementation, and urinary oxalate levels declined to non-supplementation levels within three days.
SUMMARY
A combination of factors during combat situations might increase the risk of stone formation during combat situations. For instance, the increased sweat volumes that occur during high-intensity physical activity combined with a fluid restriction may result in dehydration, a risk factor for stone formation. Furthermore, changes in pH that may occur as a result of diet changes (decrease in pH due to inadequate caloric intake to meet energy expenditure) might also alter saturation levels of certain salts and result also in an increased risk of stone formation.
Provision of adequate amounts of water is critical when soldiers are deployed to high-intensity combat missions, where high sweat volumes can induce dehydration. Adequate hydration is the most important factor to reduce the risk of stone formation but it is difficult to maintain in field combat conditions. Adequate caloric intake to meet energy needs would be beneficial to maintain the urinary pH at normal levels; this, however, is also unlikely to occur.
A more practical approach to lessening concerns of stone formation might be the simultaneous modification of some nutrients and dietary components along with food fortification with potassium–magnesium–citrate salts that may reduce the risk of urinary crystallization (Pak et al., 2003). For example, food developers should avoid the inclusion of foods high in oxalate in the ration, such as soy protein sources. Calcium intakes of 800 mg/day are appropriate for individuals when genetic predisposition to hypercalciuria is not known. In addition, supplementation of any amount of vitamin C above the RDA for adult men (75 mg/day) should also be avoided.
REFERENCES
Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. 1996. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol 155(3):839–843.
Borghi L, Meschi T, Schianchi T, Briganti A, Guerra A, Allegri F, Novarini A. 1999. Urine volume: Stone risk factor and preventive measure. Nephron 81(Suppl 1):31–37.
Coe FL. 1978. Hyperuricosuric calcium oxalate nephrolithiasis. Kidney Int 13(5):418–426.
Ettinger B, Pak CY, Citron JT, Thomas C, Adams-Huet B, Vangessel A. 1997. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 158(6):2069–2073.
Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. 1998. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68(3):576–583.
Holmes RP. 2000. Oxalate synthesis in humans: Assumptions, problems, and unresolved issues. Mol Urol 4(4):329–332.
Lemann J Jr, Pleuss JA, Gray RW, Hoffmann RG. 1991. Potassium administration reduces and potassium deprivation increases urinary calcium excretion in healthy adults. Kidney Int 39(5):973–983.
Maalouf NM, Cameron MA, Moe OW, Sakhaee K. 2004. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens 13(2):181–189.
Massey LK. 2000. Effects of ascorbate supplements on urinary oxalate and risk of kidney stones. J Am Diet Assoc 100(5):516.
Massey LK. 2003. Dietary animal and plant protein and human bone health: A whole foods approach. J Nutr 133(3):862S–865S.
Massey LK, Kynast-Gales SA. 1998. Substituting milk for apple juice does not increase kidney stone risk in most normocalciuric adults who form calcium oxalate stones. J Am Diet Assoc 98(3):303–308.
Massey LK, Kynast-Gales SA. 2001. Diets with either beef or plant proteins reduce risk of calcium oxalate precipitation in patients with a history of calcium kidney stones. J Am Diet Assoc 101(3):326–331.
Massey LK, Sutton RA. 2004. Acute caffeine effects on urine composition and calcium kidney stone risk in calcium stone formers. J Urol 172(2):555–558.
Massey LK, Whiting SJ. 1996. Dietary salt, urinary calcium, and bone loss. J Bone Miner Res 11(6):731–736.
Massey LK, Grentz LM, Horner HT, Palmer RG. 2002. Soybean and soyfood consumption increase oxalate excretion. Top Clin Nutr 17(2):49–59.
Massey LK, Kynast-Gales SA, Liebman M. 2005. Ascorbate increases human oxaluria and kidney stone risk. J Nutr 135: (in press)
Moe OW, Abate N, Sakhaee K. 2002. Pathophysiology of uric acid nephrolithiasis. Endocrinol Metab Clin North Am 31(4):895–914.
Pak CY, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. 2003. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol 169(2):465–469.
Pierratos A, Dharamsi N, Carr LK, Ibanez D, Jewett MA, Honey RJ. 2000. Higher urinary potassium is associated with decreased stone growth after shock wave lithotripsy. J Urol 164(5):1486–1489.
Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. 2002. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40(2):265–274.
Sakhaee K, Nigam S, Snell P, Hsu MC, Pak CY. 1987. Assessment of the pathogenetic role of physical exercise in renal stone formation. J Clin Endocrinol Metab 65(5):974–979.
Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC Jr. 1994. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 330(25):1776–1781.
Tiselius HG. 1997. Risk formulas in calcium oxalate urolithiasis. World J Urol 15(3):176–185.
Assault Rations: Organoleptic, Satiability, and Engineering Challenges
Dennis H. Passe, Scout Consulting, LLC
INTRODUCTION
The sensory and engineering challenges of developing an assault ration are complex but surmountable. The fundamentals that should be addressed are (1) the basics of sensory palatability (both the positive and negative) and the interactions that can occur between physical stress/exercise levels and hedonic (pleasure) levels; (2) the current statistical design methods for optimizing formulas and production processes; (3) the basics of packaging engineering; and (4) the psychological and behavioral aspects of the soldiers. Although most of the basics are reasonably well understood, an essential, but challenging, aspect to develop the best possible assault ration is to integrate these areas of understanding with a team approach.
The following sections focus primarily on beverages, but much of the material applies to solid foods as well. The following questions are addressed in this section:
-
What are the challenges of developing nutritionally enhanced food products, especially beverages, for a special group of people, specifically people under physical, cognitive, and environmental stress?
-
What are nutrients that have been added to food products with the purpose of enhancing the immune function, hydration status, or gastrointestinal processes; performance; or other physiological or cognitive functions?
-
What are specific metabolic concerns, for example, interactions with other nutrients, bioavailability, and absorption?
-
What are the issues to consider when such a nutritionally enhanced food product is developed, in terms of sensory, palatability, and storage/stability?
-
What strategies have worked to maximize the consumption of such food product (or beverages) by soldiers under stress? What strategies have not worked?
THE IMPORTANCE OF PALATABILITY
Flavor and Fluid Intake
Flavor is an important palatability element in encouraging voluntary fluid intake. The preponderance of research shows that a flavored beverage is consumed in greater quantities than water (Table B-24).
TABLE B-24 Percentage of Increase in Voluntary Fluid Intake of a Flavored Drink Versus Water. Values in parentheses indicate a decrease in Voluntary Fluid Intake
|
Increase (%) |
Reference |
|
10–79 |
Szylyk et al., 1989 |
|
13–19 |
Minehan et al., 2002 |
|
24 |
Passe et al., 2004 |
|
31 |
Spioch and Nowara, 1980 |
|
32 |
Rivera-Brown et al., 1999 |
|
36 |
Clapp et al., 1999 |
|
41–83 |
Hubbard et al., 1984 |
|
42 |
Maughan et al., 1997 |
|
45–90 |
Wilk and Bar-Or, 1996 |
|
54–59 (5–6) |
Wilmore et al., 1998 |
|
99–143 |
Rolls et al., 1980 |
|
110–115 |
Passe et al., 2000 |
|
233–318 |
Passe et al., 2000 |
|
247 |
Sohar et al., 1962 |
In early observations of military personnel, Rothstein and colleagues (1947) indicated that providing some flavoring resulted in enhanced palatability. In subsequent controlled field (Engell and Hirsch, 1991; Minehan et al., 2002; Sohar et al., 1962; Spioch and Nowara, 1980) and laboratory studies (Clapp et al., 1999; Hubbard et al., 1984; Maughan et al., 1997; Passe et al., 2004; Szlyk et al., 1987, 1989) with adults under varying degrees of physical stress, beverage palatability and voluntary fluid intake increased when a flavored drink was offered. Conversely, the least popular drinks were those containing carbonation (Sohar et al., 1962).
In addition, providing a choice of flavors can further enhance fluid intake. In a study of the effects of flavor variety, Rolls and colleagues (1980) reported that the addition of one flavor increased voluntary fluid intake by 99 percent in comparison with water intake. Adding three flavors increased voluntary fluid intake over water (control) by 143 percent.
Exercise and Palatability
In experiments taking a more systematic look at flavor palatability and voluntary fluid intake, Passe and colleagues (2000) investigated the effects of high-versus low-palatability beverages on intake during exercise. After athletes first screened beverages for palatability, both the most and least acceptable flavors for each subject and water were subsequently made available ad libitum during three hours of aerobic exercise. During screening, the most acceptable flavor scored higher than water (p < 0.01), which scored higher than the least acceptable flavor (p < 0.01) (Figure B-34). During exercise, however, the palatability of the least acceptable flavor increased and exceeded that of water (p < 0.01) (Figure B-35). Furthermore, in a replication experiment with the same flavors and a similar aerobic exercise protocol, but using a two-choice procedure, the flavored sports beverage, whether originally identified as most acceptable or least acceptable, was consumed in significantly greater quantities than water, even after only 15 minutes of exercise (Figures B-36 and B-37).
These findings highlight the importance of flavor in contributing to voluntary fluid intake when exercising. They also suggest that a powerful association exists between flavor palatability of a sports beverage and exercise status. That is, a beverage with low palatability during at-rest conditions can dramatically increase in palatability during exercise (Figure B-38) (Passe et al., 2000). This association is consistent with the findings of Horio and Kawamura (1998) who reported that a shift in the hedonic value of a beverage can occur as a function of exercise.
While the effect of flavor (and beverage temperature; see section below) may be a key driver of voluntary fluid intake during exercise, there appears to be a relationship between exercise and hedonic experience. That is, when considering the addition of flavors to a beverage, the hedonic experience of the flavor being
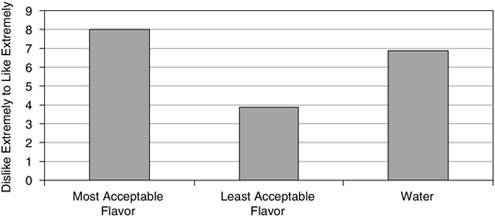
FIGURE B-34 Overall acceptance (liking) for most acceptable flavor, least acceptable flavor, and water during sedentary (at rest) conditions.
NOTE: All differences are significant at p < 0.001.
SOURCE: Data obtained from Passe et al. (2000).
FIGURE B-35 Cumulative amount consumed (grams) of most acceptable flavor, least acceptable flavor, and water during three hours of exercise. Single-choice procedure.
NOTE: MAF = most acceptable flavor; LAF = least acceptable flavor. a = MAF > (LAF, Water), LAF = Water; b = MAF > Water, LAF = (MAF, Water); c = (MAF = LAF) > Water. a, b, c, significantly different p < 0.05.
SOURCE: Passe et al. (2000), used with permission from Elsevier.
FIGURE B-36 Cumulative amount consumed of most acceptable flavor and water during three hours of exercise. Two-choice procedure.
NOTE: MAF = most acceptable flavor. Most acceptable flavor compared to water, p < 0.05.
SOURCE: Adapted from Passe et al. (2000), used with permission from Elsevier.
FIGURE B-37 Cumulative amount consumed of least acceptable flavor and water during three hours of exercise. Two-choice procedure.
NOTE: LAF = least acceptable flavor. Least acceptable flavor compared to water, p < 0.05.
SOURCE: Adapted from Passe et al. (2000), used with permission from Elsevier.
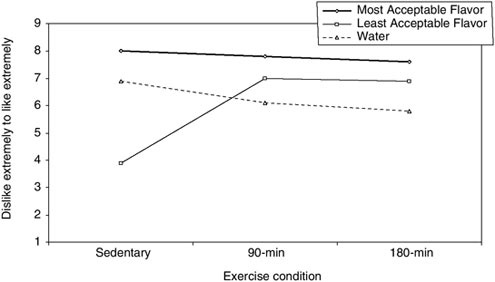
FIGURE B-38 Overall acceptance (liking) for most acceptable flavor, least acceptable flavor, and water during sedentary (at rest) conditions and after 90 minutes and 180 minutes of exercise. Overall acceptance scale 1–9: 1 (dislike extremely) through 9 (like extremely).
NOTE: Acceptability of least preferred flavor increased significantly from sedentary to exercise conditions. All drink comparisons at least p < 0.05.
SOURCE: Passe et al. (2000), used with permission from Elsevier.
tasted should be tested during or after exercise and rated for acceptability. Lluch and colleagues (1998) demonstrated in females that the appeal of a low-fat meal increased after exercise relative to the same meal consumed after rest, but not for males (King and Blundell, 1995; King et al., 1996). Lluch and colleagues (1998) also observed that the increase in the hedonic value of foods following exercise occurred predominantly for the high-carbohydrate (low-fat) items in the menu, consistent with the need for carbohydrate-related glycogen use during exercise.
To date, there have been few, if any, published reports of systematic investigations of flavor components that could increase or decrease the palatability of beverages during physical stress or exercise. In a series of experiments, Passe (2001) investigated the dose–response relationships between key flavor components (sweetness, saltiness, tartness, flavor strength) and palatability measured immediately following exercise. Healthy adults exercised for 30 minutes, after which they had ad libitum access to a beverage that they then evaluated for palatability and perceived intensity as a function of the key flavor components. Results indicated critical breakpoints for all of these sensory attributes (Figures B-39 through B-42). Together these studies suggest, at least for major flavor components in a sports beverage, that there are optima that must be achieved to
fully enhance beverage flavor quality and that deviation from these optima, with either higher or lower concentrations, can reduce palatability.
The results from the sodium palatability study are consistent with those of Wemple and colleagues (1997), who investigated the effects of 25 and 50 mmol/L of sodium in flavored, six-percent carbohydrate drinks on voluntary fluid intake following dehydration. Fluid intake was significantly enhanced for the 25 mmol/L beverage over water but not for the 50 mmol/L beverage. Similarly, Leshem and colleagues (1999) have reported an increase in salt preference in soup following exercise. In this study subjects (21 male students, 24 years old) engaged in “routine exercise” for one hour after which they were allowed to self-adjust the salt level in tomato soup. Relative to baseline measurements and a control group that did not exercise, subjects increased the level of salt in the soup by about 50 percent (from 1.3 to 2 percent NaCl). A similar study investigated the effects of carbonation on beverage palatability in exercising subjects. In this case, the acceptability of a sports beverage drops precipitously with carbonation (Figure B-43) (Passe et al., 1997).
Negative Sensory Characteristics
In addition to increasing the benefits that the correct combination of beverage characteristics can have on hedonic experiences and voluntary fluid intake, it is also important to understand how negative characteristics can adversely affect palatability during physical stress or exercise. Failure to eliminate or reduce unacceptable beverage characteristics may produce unwanted reactions—this has been observed when water is tainted by off-flavors (Rothstein et al., 1947; Szlyk et al., 1987, 1989), when the beverage type is mismatched for the exercise occasion (Sohar et al., 1962), or when the temperature is too warm (Armstrong et al., 1985; Boulze et al., 1983; Hubbard et al., 1984; Sandick et al., 1984; Szlyk et al., 1987, 1989).
In a study investigating the potential acceptability of various beverage characteristics (Passe, 2001), adult subjects consumed, ad libitum, a variety of beverages (lemon-lime flavored 6 percent carbohydrate electrolyte sports beverage, apple juice, mixed-vegetable juice, bottled water, and a cola) after 30 minutes of aerobic activity. At the end of the study, subjects were asked to complete a questionnaire which probed how “annoyed” they would be by certain beverage characteristics if they occurred in a beverage. The most salient negative beverage characteristics for this group were related to feelings of nausea or bloating, objectionable mouthfeel, perceived viscosity, objectionable flavor, and excessive sweetness (Table B-25). The negative characteristics identified as being least important were low saltiness, low sweetness, low tartness, and high artificial color appearance. A replicate study was subsequently conducted with results that closely paralleled those of the first study (Table B-25).
Consistent with the above results, Pelchat and Rozin (1982) observed in a
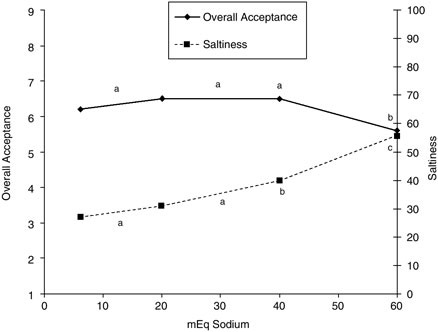
FIGURE B-39 Overall acceptance (liking) and perceived beverage saltiness after 30 minutes of exercise.
NOTE: For each curve, values not sharing a letter (a, b, c) in common are significantly different (p ≤ 0.05). Overall acceptance scale 1–9: 1 (dislike extremely) through 9 (like extremely). Saltiness scale 0–100:0 (not salty) through 100 (very salty).
SOURCE: Passe (2001).
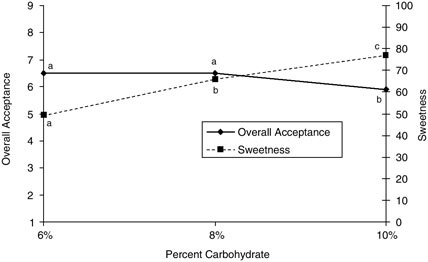
FIGURE B-40 Overall acceptance (liking) and perceived beverage sweetness after 30 minutes of exercise.
NOTE: For each curve, values not sharing a letter in common are significantly different (p ≤ 0.05). Overall acceptance scale 1–9: 1 (dislike extremely) through 9 (like extremely). Sweetness scale 0–100:0 (not sweet) through 100 (very sweet).
SOURCE: Passe (2001).
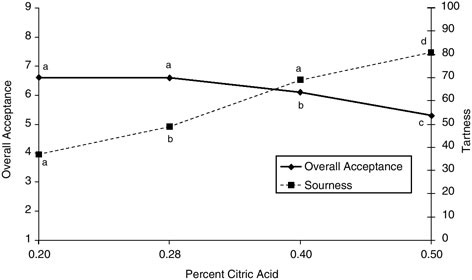
FIGURE B-41 Overall acceptance (liking) and perceived beverage sourness/tartness after 30 minutes of exercise.
NOTE: For each curve, values not sharing a letter in common are significantly different (p ≤ 0.05). Overall acceptance scale 1–9: 1 (dislike extremely) through 9 (like extremely). Sourness scale 0–100:0 (not tart) through 100 (very tart).
SOURCE: Passe (2001).

FIGURE B-42 Overall acceptance (liking) and perceived beverage flavor strength after 30 minutes of exercise.
NOTE: For each curve, values not sharing a letter in common are significantly different (p ≤ 0.05). Overall acceptance scale 1–9: 1 (dislike extremely) through 9 (like extremely). Flavor scale 0–100:0 (weak flavor) through 100 (strong flavor).
SOURCE: Passe (2001).

FIGURE B-43 Overall acceptance (liking) and perceived beverage carbonation after 30 minutes of exercise.
NOTE: For each curve, values not sharing a letter in common are significantly different (p ≤ 0.05). Overall acceptance scale 1–9: 1 (dislike extremely) through 9 (like extremely). Carbonation scale 0–100:0 (weak carbonation) through 100 (strong carbonation). CO2 = carbon dioxide.
SOURCE: Passe et al. (2000), used with permission from Elsevier.
survey study that nausea following ingestion of a food was the most potent correlate of acquired dislike of the taste of food. The effects of postingestion gastrointestinal distress can be powerful and are a central theme in the area of conditioned taste-aversion learning. The reader is referred to Garcia and colleagues (1974) and Rozin and Kalat (1971) for discussion.
BEVERAGE TEMPERATURE
One of the most effective ways to improve the palatability of a beverage to heat-stressed or exercising subjects is to chill it (Armstrong et al., 1985; Boulze et al., 1983; Hubbard et al., 1984; Passe, 2001; Rothstein et al., 1947; Sandick et al., 1984; Sohar et al., 1962; Szlyk et al., 1987, 1989). Research over the years has consistently shown that cooling a drink enhances its palatability and increases voluntary fluid intake (Table B-26).
In addition, there appears to be a perception-changing effect of beverage temperature in relation to flavor on voluntary fluid intake. Hubbard and colleagues (1984) reported a 50 percent increase in water consumption when water
TABLE B-25 Level of Annoyancea with Various Potential Beverage Flaws
TABLE B-26 Percentage of Increase in Voluntary Fluid Intake of a Chilled Drink over One at “Ambient” Temperature
|
Increase Intake (%) |
Reference |
|
23; 79 |
Sandick et al., 1984 |
|
87 |
Hubbard et al., 1984 |
|
88 |
Szlyk et al., 1989 |
|
127 |
Armstrong et al., 1985 |
|
393; 114 |
Boulze et al., 1983 |
was chilled, a 40 percent increase when it was flavored, and an 80-percent increase when it was both chilled and flavored.
In the case of sweetened beverages, temperature may exert an effect on palatability by modifying perceived sweetness intensity. Calvino (1986) generated psychophysical sweetness intensity curves at beverage temperatures (7°C, 37°C, and 50°C). Solutions at 37°C (99 °F) and 50°C (122 °F) were perceived as
being sweeter than at 7°C (45°F). This study also confirm what others had reported before, that is, that this temperature effect diminishes with higher sucrose concentrations and becomes negligible at higher concentrations of approximately 0.4–0.5 M sucrose. In addition, the slopes of the sweetness functions at the higher temperatures were lower than at 7°C (45°F); that is, the sweetness function was steeper at 7°C (45°F) than at the warmer temperatures, which suggests an improved ability to discriminate sweetness differences with lower temperatures.
EXERCISE AND SALT APPETITE
Following exercise-induced dehydration, sodium in a rehydration beverage is important because it restores and maintains body fluid levels. Therefore, the influence of exercise on the preference for foods or high salt concentrations (salt appetite) is of special interest for soldiers under stress due to the loss of sodium during exercise. To date, however, there are only a few reports directly investigating the effect of exercise on salt appetite or salt preference in humans (Horio and Kawamura, 1998; Leshem et al., 1999; Takamata et al., 1994; Wald and Leshem, 2003).
Takamata and colleagues (1994) investigated the effect of exercise-induced water and sodium depletion on the palatability of various aqueous solutions of sodium chloride. Over a 23-hour rehydration period, the unpleasant taste of the higher salt solutions became less so. The perceived intensity of 0.3 M sodium chloride increased after exercise and remained elevated throughout most of the rehydration period.
In a study investigating the effect of exercise on preference for salt with a group of male university students who exercised versus a control group, Leshem and colleagues (1999) compared the acceptability of salt in soup between the two groups. The preferred concentration of salt in soup increased by 50 percent, relative to the concentration preferred by the control group, immediately after exercise (p < 0.02). Furthermore, on the morning after, the exercising group still preferred a higher level of salt in soup than the controls did (p < 0.05). A comparison of groups on the intake of salty snacks failed to find any significant differences; however, Leshem and colleagues (1999) concluded that there is not yet evidence demonstrating that increased salt preference results in an increase in intake. This is an area of research requiring additional attention.
Horio and Kawamura (1998), unlike Leshem and colleagues (1999) and Takamata and coworkers (1994), did not observe an increase in preference for salty taste following exercise in 58 university-student volunteers. Results indicated that the hedonic rating of the salt solutions and detection thresholds did not change following exercise. While Horio and Kawamura (1998) and Takamata and colleagues (1994) both investigated saltiness preference in aqueous solutions using sip-and-spit techniques, a potentially important methodological difference
existed in that the exercise for the former was of 30 minute duration and was of moderate intensity compared with a six-hour exercise protocol designed to deplete the subjects of body water and sodium for the latter. It is possible that the exercise-induced sodium depletion (3.29 mEq/kg) contributed to the increased palatability ratings observed in Takamata and colleagues (1994). Support for such a mechanism has been suggested by Wald and Leshem (2003), who reported that following exercise, greater postingestive flavor conditioning of preference for a novel beverage (via pairing with salt capsules) occurred under conditions of greater sweating. The increase in physiological well-being from the sodium supplementation may be facilitating the acquisition of the flavor preference.
Wemple and colleagues (1997) investigated the effects of two levels of sodium chloride (25 mmol/L and 50 mmol/L) in a flavored, 6 percent sucrose beverage versus artificially sweetened and flavored water (no added sodium) in exercise subjects who lost three percent of their body weights through dehydration, after which they were allowed to rehydrate freely for three hours. The beverage with the added 25 mmol/L of sodium chloride was preferred. In support of these findings, Kanarek and colleagues (1995) have reported that preference for salted popcorn is greater for women who exercise more than three hours per week than for those who exercise less.
In summary, the role of sodium in a rehydration beverage is important because of its necessary role in restoring and maintaining body fluid levels following exercise-induced dehydration. Substantial amounts can be lost in sweat and must be replaced to maintain physiological functions. Although preliminary evidence suggests that the desire for saltiness may increase with exercise; getting sufficient quantities of sodium in a properly formulated rehydration beverage may be a sensory and palatability challenge. The research presented above suggests that a relatively low level of sodium, 25 mmol/L, may enhance beverage palatability. Research in the area of sodium palatability in sports beverages is in its infancy, and additional research is necessary to achieve acceptable palatability with higher levels of sodium.
PRODUCT DEVELOPMENT CHALLENGES
The challenges of developing an assault ration for a target population expending large amounts of energy are analogous to those faced by the developers of medicinal foods for patient populations also requiring high caloric intake. The most efficient way to achieve high caloric density is through fat, recognizing that we must also meet other nutritional goals.
The product, whether a solid or fluid, must also look and taste like “real” food—a hurdle that requires at least some basic understanding of our target population’s food preferences. Our final product could be a solid food or a liquid or a combination of both. In addition it is helpful to identify palatability gold standards that we wish to achieve for a particular product. In this sense, obtain-
ing the services of a chef during this phase can be helpful (e.g., to intensify the flavor profile of a high-energy beef stroganoff).
Cost is also important to consider in the early phases of our planning. Even though we may initially approach a project with a “no-cost-constraint” philosophy, experience tells us that we very quickly lose decision-making efficiency if we have to back track and reengineer our decisions later on the basis of cost. Perhaps the most efficient mode is to solve the energy density, palatability, and cost equations simultaneously. Once the type of product and the target are identified there are other requirements that must be established and will affect the nature of the final product. A key element of product development success is simultaneously improving the formula-component levels. For example, the following text outlines a simple approach (currently used by the food industry) to develop a shelf-stable, low-calorie electrolyte drink for high-stress environments to reduce the risk of hyponatremia:
-
The product formula can be addressed by a response surface design incorporating the following key ingredients: water, iodine or another microstabilizer, electrolyte blend, sweetener, flavor.
-
Cost is included in the model, so cost options must be identified as well.
-
Sensory testing of the design points and subsequent statistical modeling will identify the best palatability.
-
Microbiological stability should be checked through standard screening.
-
The validity of the final product should be established by field testing.
SENSORY STORAGE TESTING
Sensory storage testing, while sometimes an afterthought, is critical to product survival. Such testing establishes that sensory quality is maintained at acceptable levels throughout the time when the product is available for use. Microbiological testing is typically done in parallel but is not covered in detail in this discussion.
There are two basic approaches to establishing shelf life. The first identifies whether the product is acceptable at a specified date. This is a simple approach that only requires two tests: initial and final. If we are fairly confident of the product’s ability to maintain its sensory integrity, we use this kind of test as confirmatory of sensory integrity.
The second approach identifies when the product shifts in quality, if any shift occurs. This approach requires periodic testing between the initial and the final tests for time (above). To save time and cost, a feature that can and should be incorporated into the testing protocol is to include an accelerated portion of the test that mimics long-term storage studies by using elevated temperatures or other intentional challenges to the food. Most product development teams elect to conduct some variation of this second approach.
PROCESS AND PACKAGING ENGINEERING CHALLENGES
The first process engineering challenge is to determine whether the product can, in fact, be produced. Microbiological stability, production reliability (operating within production standards) and efficiency (cost effectiveness) are central for processing and packaging the product.
Processing usually damages flavor to some degree. Achieving the target flavor and texture profile while maintaining microbiological stability is usually a matter of trade-offs. Heat, pressure, shear, and the presence of preservatives usually conspire to degrade the final sensory experience. The process engineer’s challenge is to get to the best compromise.
Packaging engineering takes up where process engineering leaves off. The key challenges here will be to create the most efficacious moisture, oxygen, microbial, and, possibly, ultraviolet barriers. If the product is inherently microbiologically stable (perhaps due to low water activity and low pH), the packaging options are simpler and less costly. However, if the product characteristics require a formula and process that leave it more vulnerable either in a sensory or microbiological way, the packaging can compensate by an enhanced barrier support. Hermetically sealing a product will provide protection against microbe ingress to a product that has been rendered safe but not stable. Oxygen-impermeable barriers will enhance sensory shelf life. Although more complex and more expensive, these approaches sometimes help to achieve the best value relative to achieving our food-target goal.
SPECIAL NUTRITIONAL ISSUES
Over the three to seven days that an assault ration will be used, the nutritional focus is likely to be on the macronutrient profile. Specifically, dietary components that help regulate the body’s insulin response and maintain the correct glucose levels are crucial. As insulin lowers blood sugar levels and selectively drives amino acids into muscle tissue, tryptophan, normally competing with the branch-chain amino acids to cross the blood–brain barrier, can more freely cross into the brain. The increased tryptophan levels may facilitate an increase in serotonin levels, which can affect performance, cognitive function, alertness, mood, and appetite. Consequently, to maintain hormone and glucose balance, an assault ration may be improved from some augmented valine, leucine, and isoleucine levels in combination with some fiber, amylase-resistant starch, fat, and sufficient carbohydrate.
Important micronutrients to include are an electrolyte blend to encourage voluntary fluid intake. Although the science may be limited with respect to the benefits of antioxidants in performance, their inclusion may help protect healthy tissue from inflammation during muscle breakdown and repair.
SUMMARY
Flavor and temperature are the two most important sensory attributes at our disposal to optimize beverage quality. A high level of acceptability can be achieved by simultaneously improving the flavor, texture, and appearance profile and reducing any negative sensory and postingestive effects.
The most efficient and cost effective approach to improve complex formulas and processes is the use of advanced response surface statistical designs. These methods are, fortunately, intuitive and easily implemented.
In addition, storage testing is critical to ensure the field success of product quality. If possible, accelerated-temperature designs to facilitate an early read on potential problems should be conducted.
Finally, decisions should be made by an integrated team of experienced professionals that include experts on physiology, nutrition, product development, food processing, and sensory qualities of food.
ACKNOWLEDGMENTS
I gratefully acknowledge the valuable insights and suggestions from Steve Ink, Ph.D., Director of Agricultural Technology, Northern Illinois University, DeKalb; John Konieczka, JBK Consulting, LLC; Paula Manoski, Quality R&D Partners; Bob Murray, Ph.D., Gatorade Sports Science Institute.
REFERENCES
Armstrong LE, Hubbard RW, Szlyk PC, Matthew WT, Sils IV. 1985. Voluntary dehydration and electrolyte losses during prolonged exercise in the heat. Aviat Space Environ Med 56(8):765–770.
Boulze D, Montastruc P, Cabanac M. 1983. Water intake, pleasure and water temperature in humans. Physiol Behav 30(1):97–102.
Calvino AM. 1986. Perception of sweetness: The effects of concentration and temperature. Physiol Behav 36(6):1021–1028.
Clapp AJ, Bishop PA, Walker JL. 1999. Fluid replacement preferences in heat-exposed workers. Am Ind Hyg Assoc J 60(6):747–751.
Engell D, Hirsch E. 1991. Environmental and sensory modulation of fluid intake in humans. In: Ramsey DJ, Booth D, eds. Thirst: Physiological and Psychological Aspects. Berlin: Springer-Verlag. Pp. 382–402.
Garcia J, Hankins WG, Rusiniak KW. 1974. Behavioral regulation of the milieu interne in man and rat. Science 185(4154):824–831.
Horio T, Kawamura Y. 1998. Influence of physical exercise on human preferences for various taste solutions. Chem Senses 23(4):417–421.
Hubbard RW, Sandick BL, Matthew WT, Francesconi RP, Sampson JB, Durkot MJ, Maller O, Engell DB. 1984. Voluntary dehydration and alliesthesia for water. J Appl Physiol 57(3):868–873.
Kanarek RB, Ryu M, Przypek J. 1995. Preferences for foods with varying levels of salt and fat differ as a function of dietary restraint and exercise but not menstrual cycle. Physiol Behav 57(5):821–826.
King NA, Blundell JE. 1995. High-fat foods overcome the energy expenditure induced by high-intensity cycling or running. Eur J Clin Nutr 49(2):114–123.
King NA, Snell L, Smith RD, Blundell JE. 1996. Effects of short-term exercise on appetite responses in unrestrained females. Eur J Clin Nutr 50(10):663–667.
Leshem M, Abutbul A, Eilon R. 1999. Exercise increases the preference for salt in humans. Appetite 32(2):251–260.
Lluch A, King NA, Blundell JE. 1998. Exercise in dietary restrained women: No effect on energy intake but change in hedonic ratings. Eur J Clin Nutr 52(4):300–307.
Maughan RJ, Leiper JB, Shirreffs SM. 1997. Factors influencing the restoration of fluid and electrolyte balance after exercise in the heat. Br J Sports Med 31(3):175–182.
Minehan MR, Riley MD, Burke LM. 2002. Effect of flavor and awareness of kilojoule content of drinks on preference and fluid balance in team sports. Int J Sport Nutr Exerc Metab 12(1):81–92.
Passe DH. 2001. Physiological and psychological determinants of fluid intake. In: Maughan RJ, Murray R, eds. Sports Drinks. Basic Science and Practical Aspects. Boca Raton, FL: CRC Press. Pp. 45–87.
Passe DH, Horn M, Murray R. 1997. The effects of beverage carbonation on sensory responses and voluntary fluid intake following exercise. Int J Sport Nutr 7(4):286–297.
Passe DH, Horn M, Murray R. 2000. Impact of beverage acceptability on fluid intake during exercise. Appetite 35(3):219–229.
Passe DH, Horn M, Stofan J, Murray R. 2004. Palatability and voluntary intake of sports beverages, diluted orange juice, and water during exercise. Int J Sport Nutr Exerc Metab 14(3):272–284.
Pelchat ML, Rozin P. 1982. The special role of nausea in the acquisition of food dislikes by humans. Appetite 3(4):341–351.
Rivera-Brown AM, Gutierrez R, Gutierrez JC, Frontera WR, Bar-Or O. 1999. Drink composition, voluntary drinking, and fluid balance in exercising, trained, heat-acclimatized boys. J Appl Physiol 86(1):78–84.
Rolls BJ, Wood RJ, Rolls ET. 1980. Thirst: The initiation, maintenance, and termination of drinking. In: Sprague JM, Epstein AN, eds. Progress in Psychobiology and Physiological Psychology. New York: Academic Press. Vol. 9. Pp. 263–321.
Rothstein A, Adolph EF, Wills JH. 1947. Voluntary dehydration. In: Adolph EF, ed. Physiology of Man in the Desert. New York: Interscience Publishers. Pp. 254–270.
Rozin P, Kalat JW. 1971. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev 78(6):459–486.
Sandick BL, Engell DB, Maller O. 1984. Perception of drinking water temperature and effects for humans after exercise. Physiol Behav 32(5):851–855.
Sohar E, Kaly J, Adar R. 1962 The prevention of voluntary dehydration. In: Symposium on Environmental Physiology and Psychology in Arid Conditions. Proceedings of the Lucknow Symposium. Paris: United Nations Educational Scientific and Cultural Organization. Pp. 129–135.
Spioch FM, Nowara M. 1980. Voluntary dehydration in men working in heat. Int Arch Occup Environ Health 46(3):233–239.
Szlyk PC, Hubbard RW, Matthew WT, Armstrong LE, Kerstein MD. 1987. Mechanisms of voluntary dehydration among troops in the field. Mil Med 152(8):405–407.
Szlyk PC, Sils IV, Francesconi RP, Hubbard RW, Armstrong LE. 1989. Effects of water temperature and flavoring on voluntary dehydration in men. Physiol Behav 45(3):639–647.
Takamata A, Mack GW, Gillen CM, Nadel ER. 1994. Sodium appetite, thirst, and body fluid regulation in humans during rehydration without sodium replacement. Am J Physiol 266(5 Pt 2):R1493–R1502.
Wald N, Leshem M. 2003. Salt conditions a flavor preference or aversion after exercise depending on NaCl dose and sweat loss. Appetite 40(3):277–284.
Wemple RD, Morocco TS, Mack GW. 1997. Influence of sodium replacement on fluid ingestion following exercise-induced dehydration. Int J Sport Nutr 7(2):104–116.
Wilk B, Bar-Or O. 1996. Effect of drink flavor and NaCL on voluntary drinking and hydration in boys exercising in the heat. J Appl Physiol 80(4):1112–1117.
Wilmore JH, Morton AR, Gilbey HJ, Wood RJ. 1998. Role of taste preference on fluid intake during and after 90 min of running at 60% of VO2max in the heat. Med Sci Sports Exerc 30(4):587–395.
Foods for People under Stress: Special Considerations
Steven M. Wood, Abbott Laboratories
INTRODUCTION
Developing foods for people under stress requires the examination of several critical issues. First, a product concept should be developed based on scientific evidence that includes identification of stress-induced changes and the rationale for using specific nutrients to modulate the identifiable changes. Second, food technology parameters, including physical stability, standardization of ingredients, and product sterility, need to be ensured. Third, to establish efficacy and bioavailability of nutrients, testing of the foods should be conducted in the intended population. Last, in the retail environment, regulatory issues and pathways that include product forms, claims, and labeling should be addressed. This section addresses the rationale for using specific nutrients, illustrated by examples in which supplemental nutrition has been studied and found to modulate physiological processes or physical condition (efficacy) in people under stress. In addition, the progression of a series of studies in trauma stress, military training, and senior populations is presented. These studies identified significant immunologic benefits of a novel nutritional formula shown to modulate stress-induced immune dysregulation.
The following are specific questions that this review attempts to answer:
-
What are the challenges of developing nutritionally enhanced food products for a special group of people, specifically people under physical, cognitive, and environmental stress? Stress under military operations can be from exercise, extreme environmental temperature, dehydration, heat exhaustion, threat to personal safety, sleep deprivation, and other operational demands.
-
Are there nutrients that could be added to these foods to minimize oxidative stress and therefore deterioration of performance?
-
Are there other nutrients that could be added to these foods to optimize the immune system, nutrient absorption, hydration, or other physiological functions?
-
What are nutrient specific metabolic concerns; for example, interaction with other nutrients, bioavailability, and absorption or others?
-
Will nutrient addition affect the organoleptic qualities of a food as to make it less appealing? If so, how would you improve the palatability in order to maximize the consumption of the food product?
-
If these nutrients where to be added to a food, what are the issues to consider in terms of packaging and shelf-life considerations?
EXAMPLE OF ACUTE STRESS AND NUTRITIONAL MODULATION
Acute stress, such as trauma, can cause increased nutritional requirements; furthermore, nutrients may be supplied to modulate stress-induced changes such as inflammation and lung function. Acute respiratory distress syndrome (ARDS) was described by Ashbaugh and coworkers (1967), who reported respiratory failure in association with sepsis, trauma, and drug overdoses. This respiratory syndrome, characterized by pulmonary infiltration, poor lung compliance, hypoxemia, and poor gas exchange, can cause significant mortality (Bigatello and Zapol, 1996). Although many factors are associated with the development of ARDS, adverse lung function changes can be attributed primarily to inflammatory cells concentrated within the lung (Fulkerson et al., 1996; Goodman et al., 1996). These specialized cells secrete mediators that typically help control pathogens; however, uncontrolled levels of mediators may ultimately damage healthy tissues.
Dietary lipids or fats have many functions within the body, and specific fatty acids can modulate inflammation. Metabolism of omega-3 fatty acids such as eicosapentaenoic acid (EPA) and γ-linolenic acid (GLA) can alter the arachidonic acid pathway, ultimately affecting potent neutrophil chemotactic factors and proinflammatory mediators such as prostanoids and leukotrienes. EPA shifts production of cellular metabolites to those that are less proinflammatory (thromboxane A3 and prostaglandin I3), while GLA inhibits arachidonic acid precursors. GLA can also be converted to dihomo-γ-linoleic acid (DGLA), a precursor of prostaglandin E1 that has anti-inflammatory properties (Fan and Chapkin, 1998). Animal and human studies have documented that feeding a specialized nutritional formula containing EPA and GLA, along with antioxidants, can rapidly change the phospholipid fatty acid composition of inflammatory cell membranes. This alteration can have the following benefits: (1) reduction of the synthesis of proinflammatory eicosanoids; (2) attenuation of endotoxin-induced increases in pulmonary neutrophil recruitment; (3) reduction of microvascular protein permeability; (4) reduction of time on a ventilator as well as days in the intensive care unit; and (5) increase in cardiopulmonary hemodynamics and respiratory gas exchange (Gadek et al., 1999; Mancuso et al., 1997a, 1997b; Murray et al., 1995, 2000; Palombo et al., 1996, 1997, 1999). These data demonstrate the potential of nutritional modulation to significantly reduce the consequences of acute stress on respiratory function.
MILITARY TRAINING-INDUCED STRESS AND NUTRITIONAL INTERVENTION STUDIES
The inability to successfully adapt to stress as measured by immune function has been observed in military and nonmilitary groups (Bernton et al., 1995; Bonneau et al., 1990; Gray et al., 1997, 1999, 2001; Herbert and Cohen, 1993; Kasl et al., 1979; Kiecolt-Glaser et al., 2003; Kramer et al., 1997; Lee et al., 1992; Marsland et al., 1997; Martinez-Lopez et al., 1993; Segerstrom and Miller, 2004). Several studies have found an increased risk of infectious diseases (e.g., respiratory diseases and cellulitis) or decline in immunity and immune function as a result of military training-induced stress (Bernton et al., 1995; Gray et al., 1997, 1999, 2001; Kasl et al., 1979; Kramer et al., 1997; Lee et al., 1992; Martinez-Lopez et al., 1993). Immune function appears to be a sensitive indicator of health and stress. One in vivo immune marker that predicts susceptibility of infection and mortality is delayed-type skin hypersensitivity (DTH). Christou and coworkers (1995) studied hospitalized patients and found those who responded to a DTH test were less likely to become infected; furthermore, those who were already infected and nonresponsive had a significant elevation in mortality. Bernton and coworkers (1995) found that as Ranger Training progressed, there was a decline in total DTH induration (i.e., immune reaction causing reddening and hardening of skin tissue) diameter with a concomitant increase in the number of subjects not responding (anergy) to any antigens (see Figure B-44). Thus, it appears that training-induced stress reduces immune responsiveness and places soldiers at increased risk of infection.
Our laboratory, in collaboration with other colleagues, has studied stress-induced immune cell changes and antioxidant status changes that occur during military training. In prospective, randomized, double-blind control studies, we determined whether these factors could be attenuated by nutritional intervention (Chao et al., 1999; Pfeiffer et al., 1999; Shippee et al., 1995). Subjects from three military training courses were studied: Special Forces Selection and Assessment School (SFAS), 21 days in duration; Ranger Training (RT), 62 days in duration; and Marine Mountain Warfare Training (MMWT), 28 days in duration. These studies provided uniform stress factors and measurements of nutritional intake to evaluate immune modulation through nutritional supplementation.
Studies focusing on single nutrient deficiencies or nutrient supplementation, and their influence on immune function have provided valuable insights; however, multinutrient, rather than single nutrient, deficiencies typically occur. To meet the increased requirements of stress or lack of intake, the best approach would be to provide a multinutrient combination. Furthermore, there may also be intake or metabolic differences among soldiers and a multinutrient formula may be required to meet all of the nutrient requirements of a diverse group. A strategy (described below) was developed to identify specific nutrients and to supply adequate nutrition for optimal immune function. The implementation of this strategy led to the testing of a multinutrient formula that provided immunological
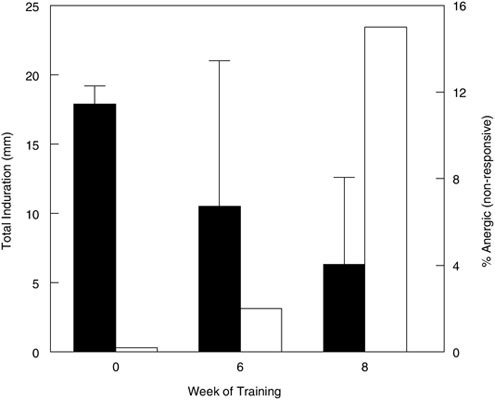
FIGURE B-44 Delayed-type skin hypersensitivity of soldiers participating in Ranger Training. Solid bars represent the mean total induration in milliliters ± standard error of the means) while the open bars are the percentage of soldiers that do not respond (anergic) to any antigen tested at the baseline and at six and eight weeks of Ranger Training.
SOURCE: Data obtained from Bernton et al. (1995).
support to soldiers. The immune responsiveness to senior citizens was also tested (see next section).
The goal of the first study was to determine whether supplemental glutamine (15 g/day, a nutrient shown to decline with stress but important for immune function) could alter plasma amino acid profiles and immune function during the 21-day SFAS course (Shippee et al., 1995). SFAS assesses a soldier’s physical, emotional, and mental stamina to predict success in Special Forces training. Soldiers consumed primarily Meals, Ready-to-Eat (MREs) and were given a drink containing glutamine (experimental) or glycine (control) twice, daily. Plasma glutamine and immune function were measured at baseline and at end of training. There was no difference in plasma glutamine levels between the control and experimental groups; both groups experienced an increase in plasma glutamine levels. There was a 20 percent decline in lymphocyte proliferation to phytohemagglutinin (PHA) from baseline to the end of stressful training in both
groups, and 40 percent of the soldiers were anergic to the DTH test at the end of the study (Shippee et al., 1995). Weight loss was approximately six pounds per soldier during the course, and all nutrient intakes exceeded the military-recommended dietary allowances, except for folic acid (approximately 80 percent). It appeared that there was little immune benefit from glutamine supplementation.
Two additional studies were conducted with soldiers completing MMWT and SFAS courses to evaluate the effects of the antioxidants, vitamin E, vitamin C, β-carotene, and selenium either individually or in combination. Soldiers exposed to moderate altitude and cold weather training during MMWT had elevated levels of markers of oxidative stress (i.e., breath pentane, lipid peroxides, and urinary 8-hydroxydeoxyguanosine). The rise in breath pentane, a measure of oxidative stress, was only attenuated in the soldiers consuming the combination of antioxidants. There were no differences among the control group and the groups consuming individual antioxidants supplements (Figure B-45, Chao et al., 1999). Soldiers completing SFAS courses were then studied.
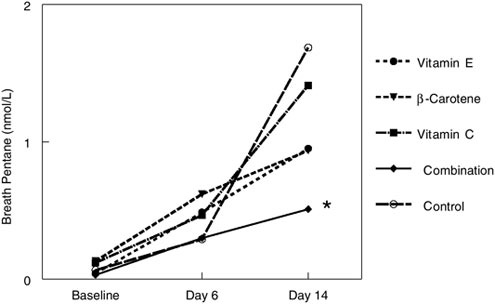
FIGURE B-45 Breath pentane concentrations (nmol/L) of marines in Marine Mountain Warfare Training consuming supplements [vitamin E (440 α-tocopherol equivalents); β-carotene (2,000 retinol equivalents); vitamin C (500 mg of ascorbic acid); and a combination of other supplements including 100 µg of selenium and 30 mg of zinc (the control was a supplement of 1,000 mg of oyster shell calcium)]. Baseline samples were collected immediately before field training, day 6 (during field training), and day 14 immediately after the end of field training.
NOTE: *Siginifcantly different from control (p < 0.05) at day 14.
SOURCE: Data obtained from Chao et al. (1999).
MRE diets were supplemented with a drink containing a similar level of the antioxidant combination described above (400 IU of vitamin E, 500 mg of vitamin C, 16 mg of β-carotene, 100 µg of selenium) or with an isonitrogenous–isoenergetic control drink. There were differences in serum vitamin E and vitamin C concentrations between the groups at the end of SFAS courses (p < 0.05). Even though markers of oxidative stress (plasma total radical antioxidant parameter, and lipid peroxide) pointed to substantial oxidative stress occurring during training, no differences in these markers were found between the two groups. At the end of the course, immune function declined in the control group, as seen previously in the glutamine study [lymphocyte proliferation from PHA stimulation (21 percent decline) and DTH (39 percent anergic decline)], while antioxidant supplementation attenuated the decline of lymphocyte proliferation to PHA stimulation (6 percent decline) and DTH (24 percent anergic decline). Weight loss (approximately six pounds) was similar between groups and comparable to the previous glutamine study.
It appears that a combination of antioxidants provided some benefit; therefore, a multinutrient formula was tested. The strategy, discussed in the following section, was to provide a novel nutritional immune formulation that contained energy (54 percent of kilocalories as carbohydrates, including a fermentable carbohydrate [fructooligosacchrarides] and 30 percent as fats, including structured lipids to provide a novel energy substrate as well as improved absorption of fat-soluble nutrients), protein (14 percent of kilocalories), antioxidants, and a mix of vitamins, minerals, and amino acids to sustain immune function for people under stress.
Nutritional Components for a Novel Immune Formula
Nutrition has been linked to cellular pathways essential for effective immune function (Beisel et al., 1981; Scrimshaw and SanGiovanni, 1997), and administration of selected supplemental nutrition may attenuate stress-induced immune changes. Several reviews have summarized the effects of individual nutrient deficiencies and their influence on immune function (Beisel et al., 1981; Corman, 1985; Santos et al., 1983). For example, zinc deficiency reduced effective immune function, while supplemental zinc improved effective immune function (Fraker et al., 2000). Plasma zinc levels decline in soldiers participating in intensive military training (Singh et al., 1989) as a result of elevated urinary excretion, although the decline is not classified as an “overt” nutrient deficiency (Miyamura et al., 1987). Because of the demands of increased metabolism or minor nutrient deficiencies, soldiers may benefit from selected supplemental nutrition resulting in optimal immune function, thereby improving soldiers’ readiness.
During training, soldiers experience oxidative stress (Chao et al., 1999; Pfeiffer et al., 1999). As a result, free radical accumulation can result in tissue injury, delayed wound healing, and increased susceptibility to infectious dis-
eases (Sandstead et al., 1982). Immune cells modulate free radical oxidation by using concentrated antioxidants (Moser, 1987). Antioxidants play a role in the proper functioning of immune cells (i.e., neutrophils) and help prevent oxidative stress and immune function decline (Greenman et al., 1988; Kanter et al., 1993; Prasad, 1980). Exercise and muscle stress rely on both fats and carbohydrates as fuel sources to meet energy demands. However, lipids have the advantage over carbohydrates in energy density. A factor contributing to muscle fatigue is muscle glycogen depletion and carbohydrate oxidation; therefore, researchers have attempted to maintain muscle glycogen through the delivery of lipids, e.g. medium-chain triacylglycerols (MCT). MCTs diffuse across enterocytes quickly and are absorbed through mitochondria independently of transport mechanisms and are oxidized as rapidly as glucose (Decombaz et al., 1983; Johnson et al., 1990), but few MCTs are available to the muscle because they are first absorbed through the portal vein and metabolized in the liver. Ingestion of large amounts of MCTs can also cause gastrointestinal (GI) discomfort. Studies have not detected any benefit in muscle performance resulting from supplemental MCTs as compared with supplemental carbohydrate during exercise (Horowitz et al., 2000; Jeukendrup et al., 1998). Other studies, however, have shown significant benefits in muscle performance when MCTs are consumed with carbohydrates (Jeukendrup et al., 1995; Van Zyl et al., 1996). For example, Van Zyl and coworkers (1996) compared cycling performance (two-hour ride at 60 percent of VO2max followed by a simulated 40-kilometer race) of subjects consuming a 10-percent glucose polymer solution, an isocaloric 4.3-percent MCT emulsion, or a mixture of carbohydrate and MCT. The mixture of carbohydrate and MCT provided the best performance (p < 0.05) (see Figure B-46, Van Zyl et al., 1996).
A novel approach would be to include structured lipids in the formulation. Structured lipids have been shown to attenuate the hypermetabolic and protein catabolic responses of trauma and stress (DeMichele et al., 1988; Kenler et al., 1996; Mok et al., 1984; Selleck et al., 1994). Random re-esterification of MCT with long-chain fatty acids yields structured triacylglycerols or structured lipids that retain desirable characteristics of both types of fatty acids (DeMichele and Bistrian, 2001). Feeding fish oil–derived structured triacylglycerol containing MCT and long-chain fatty acids to patients undergoing surgery resulted in 50 percent fewer GI complications and infections in this susceptible population (Kenler et al., 1996).
Stress also influences GI health. Structured triacylglycerols (structured lipids) have been shown to increase the absorption of both lipids and fat-soluble nutrients important for immune function in models of GI injury (Tso et al., 1999, 2001). Furthermore, there was a two- to three-fold increase in decanoic acid and EPA in the lymphatic system (Figure B-47, Tso et al., 1999). Thus, structured lipid fatty acids, even those of medium-chain length, are absorbed through the lymphatic system and delivered to the blood stream, and are available as an energy source for active muscles. Similarly, researchers have determined that
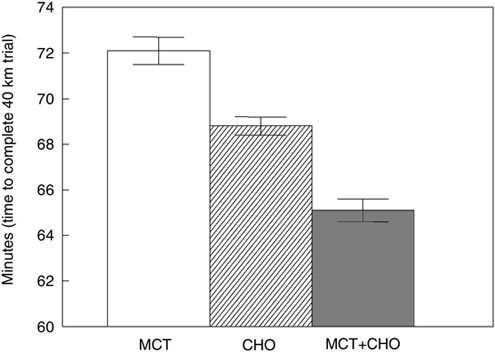
FIGURE B-46 Time needed to complete a 40 km simulated time-trial (performance). Subjects ingested either medium-chain triacylglyceride (MCT), carbohydrate (CHO), or MCT plus CHO.
NOTE: All groups are significantly diffferet from each other (p < 0.05).
SOURCE: Van Zyl et al. (1996), used with permission.
some nutrients (e.g., vitamin E) are absorbed better when given with structured lipids in normal and malabsorptive states (Tso et al., 2001). Therefore, dietary structured lipids may influence soldiers’ physical readiness by providing a unique energy source to meet increased physical demands. By aiding in the absorption of fat-soluble nutrients necessary for optimal immune responses, they may also influence immune function (Calder et al., 2002).
In addition to alterations in absorption, nutrients can influence the immune cells in the GI tract. For example, fermentable carbohydrates are substrates for bifidobacteria and other beneficial bacteria (Barrangou et al., 2003). Mounting evidence suggests that fermentable carbohydrates are also beneficial to the immune system and general GI health (Pierre et al., 1999; Schley and Field, 2002).

FIGURE B-47 Lymphatic output in Sprague-Dawley rats following an overnight fast. The intestinal lymph duct was cannulated and lymph was analyzed for decanoic acid (10:0 from medium chain triacylglycerols) and eicosapentaenoic acid 20:5 (long-chain ω-3 fatty acid) from intragastrically infusing a physical mix (PM) or structured lipid (STG) in normal (Control) and GI injured (Ischemia/Reperfusion–I/R) models. Measurements were made before lipid infusion (time 0) and at two, four, six, and eight hours after lipid feeding. Mean fasting lymph flow increased as a result of lipid infusion (peaking after three hours and reaching a steady output by the eighth hour) and was similar for all groups.
SOURCE: Tso et al. (1999), used with permission.
Testing the Novel Nutritional Immune Formula (NNIF) During Military Training
Soldiers participating in SFAS courses experienced similar weight loss (approximately six pounds per soldier) in both the control and NNIF groups even though more energy was offered along with the typical MRE diet due to the addition of either the control or NNIF supplements. Even with weight loss, however, a significant difference was observed between the control and NNIF groups with respect to DTH (41 percent versus 22 percent anergic; in addition, a trend towards increased whole blood lymphocyte proliferation to concanvalin A (–6 percent versus 17 percent for control and NNIF groups, respectively) was observed (Wood et al., in press). Furthermore, the consumption of NNIF attenuated stress-induced immune cell changes and reduced the number of soldiers reporting to the medical clinic with upper respiratory tract infections (16 percent versus 12 percent for control and NNIF groups, respectively, p = 0.08) (Wood et al., in press). Thus, among the formulas tested, NNIF was the most effective in supporting the immune function of soldiers during stressful military training.
SIMILARITIES BETWEEN MILITARY STRESS-INDUCED IMMUNE CHANGES AND THOSE OCCURRING IN SENIOR POPULATIONS
Stress-induced changes observed in military training include a reduction in lymphocyte proliferation, DTH responsiveness, and vaccine responses (data not shown), an increased oxidative stress as well as several cellular changes that ultimately may increase the soldiers’ risk of infection. Similar results have been observed in senior populations. Factors that influence immune function, such as inadequate intake; GI changes that decrease nutrient absorption and nutrient metabolism; and the increased nutritional needs for optimal immune function, may be similar in both the military and aging populations. Therefore, the same multinutrient formula was tested in two studies of senior citizens (one study in a free- and assisted-living setting, and the other in an institutional setting). The first study evaluated whether senior citizens consuming NNIF for six months, before and during influenza season, experienced fewer days with symptoms of upper respiratory tract infections and improved response to influenza vaccine, compared with a control group consuming an isonitrogenous or isoenergetic formula. Significantly fewer days of upper respiratory tract infection symptoms (8.7 ± 3.8 versus 4.9 ± 3.2 [mean ± standard error of the mean for the control versus NNIF groups, respectively]) were observed for the NNIF group (see Figure B-48). Improved response to the influenza vaccine as measured by antibody responses; increased percentage of subjects with a four-fold increase in antibody production (an indication of response to the influenza vaccine); and increased lymphocyte proliferation to influenza antigens were also observed (Langkamp-Henken et al., 2004a) in the group consuming NNIF.
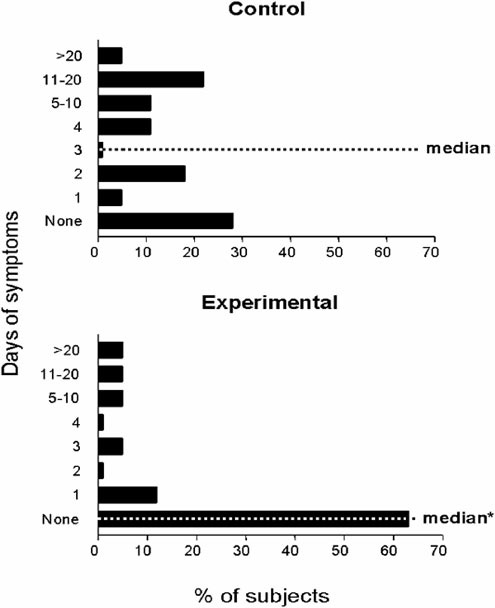
FIGURE B-48 Median days with symptoms of upper respiratory tract infections per subject were three (range 0–69) in the control group and zero (range 0–49, p < 0.05) in the experimental (NNIF) group. In the control group, 13 of 18 subjects recorded a total of 156 days of symptoms, while in the experimental group, 6 of 16 subjects recorded a total of 78 days of symptoms.
NOTE: NNIF = novel nutritional immune formulation.
SOURCE: Adapted from Langkamp-Henken et al. (2004a).
In the second study, institutionalized senior citizens consuming NNIF for three months experienced differences in immune function (response to influenza vaccines, lymphocyte subsets and activity, and cytokine production following stimulation with mitogens or influenza antigens) (Langkamp-Henken et al., 2004b).These findings were consistent with those noted in the previous studies of NNIF fed to soldiers undergoing training stress as well as to senior citizens in a free- and assisted-living setting.
SUMMARY AND CONCLUSIONS
Stress from military training and combat causes a reduction in immune function, placing soldiers at an increased risk of infection. The development of foods for people under stress, such as soldiers, requires the identification of stress-induced changes and specific nutrients to modulate these changes. The ability to identify gaps between healthy and stressed populations seems to be a critical point in developing such foods. Examples of nutritional manipulation of stress-induced changes were presented using immune function as a tool to evaluate susceptibility to infection. The similarities between stress-induced changes in soldiers in training courses and those observed in senior citizens in various settings provided an opportunity to evaluate the results of the same nutritional formula in two high-risk groups. A multinutrient formulation containing vitamins, antioxidants, minerals, structured lipids, fermentable carbohydrates, taurine, carnitine, and protein attenuated the stress-induced immune changes of soldiers in training. The same formula improved the immune system of senior citizens and led to increased responsiveness to influenza vaccine, fewer days of symptoms of upper respiratory tract infections, and less senescent immune cell profiles.
In summary, the multinutrient formulation attenuated lymphocyte proliferation decline and DTH anergy caused by stressful military training, and similarly, in senior citizens, reduced the number of days of symptoms of upper respiratory tract infections, improved responsiveness to influenza vaccine, and was associated with cellular changes indicating enhanced immunity. Thus, use of the NNIF could affect soldiers’ immune function, recovery, and readiness that ultimately could influence overall performance.
ACKNOWLEDGMENT
The author thanks COL Ron Shippee, PhD, Jeff Kennedy, MD, Joann Arsenault, MS, RD, E. Wayne Askew, PhD, Don Roberts, PhD, Scott Montain, PhD, Tim Kramer, PhD, Bobbi Langkamp-Henken, PhD, Brad Bender, MD, Kelli Herrlinger-Garcia, BS, Stephen DeMichele, PhD, John McEwen, BS, Mike Simpson, BS, John Cramblit, BS, Debra Thomas, MS, Joseph Schaller, PhD, Peter Ruey, PhD, Deb Ataya, PhD, and Geraldine Baggs, PhD, who contributed
significantly to the studies described. Furthermore, this work could not have been possible without the dedicated commitment of staff, command, soldiers, and personnel from US Army Research Institute of Environmental Medicine, Naval Health Research Center, US Army Special Operations, Marine Mountain Warfare Training Center, University of Utah, University of Florida, and Ross Products Division of Abbott Laboratories.
REFERENCES
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. 1967. Acute respiratory distress in adults. Lancet 2(7511):319–323.
Barrangou R, Altermann E, Hutkins R, Cano R, Klaenhammer TR. 2003. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc Natl Acad Sci U S A 100(15):8957–8962.
Beisel WR, Edelman R, Nauss K, Suskind RM. 1981. Single-nutrient effects on immunologic functions. Report of a workshop sponsored by the Department of Food and Nutrition and its nutrition advisory group of the American Medical Association. J Am Med Assoc 245(1):53–58.
Bernton E, Hoover D, Galloway R, Popp K. 1995. Adaptation to chronic stress in military trainees. Adrenal androgens, testosterone, glucocorticoids, IGF-1, and immune function. Ann N Y Acad Sci 774:217–231.
Bigatello LM, Zapol WM. 1996. New approaches to acute lung injury. Br J Anaesth 77(1):99–109.
Bonneau RH, Kiecolt-Glaser JK, Glaser R. 1990. Stress-induced modulation of the immune response. Ann N Y Acad Sci 594:253–269.
Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. 2002. Fatty acids and lymphocyte functions. Br J Nutr 87(Suppl 1):S31–S48.
Chao WH, Askew EW, Roberts DE, Wood SM, Perkins JB. 1999. Oxidative stress in humans during work at moderate altitude. J Nutr 129(11):2009–2012.
Christou NV, Meakins JL, Gordon J, Yee J, Hassan-Zahraee M, Nohr CW, Shizgal HM, MacLean LD. 1995. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg 222(4):534–546.
Corman LC. 1985. Effects of specific nutrients on the immune response. Selected clinical applications. Med Clin North Am 69(4):759–791.
Decombaz J, Arnaud MJ, Milon H, Moesch H, Philippossian G, Thelin AL, Howald H. 1983. Energy metabolism of medium-chain triglycerides versus carbohydrates during exercise. Eur J Appl Physiol Occup Physiol 52(1):9–14.
DeMichele SJ, Bistrian BR. 2001. Structured triacylglycerols in clinical nutrtion. In: Mansbach II CM, Tso P, Kuksis A, eds. Intestinal Lipid Metabolism. New York: Kluwer Acedemic/Plenum. Pp. 403–419.
DeMichele SJ, Karlstad MD, Babayan VK, Istfan N, Blackburn GL, Bistrian BR. 1988. Enhanced skeletal muscle and liver protein synthesis with structured lipid in enterally fed burned rats. Metabolism 37(8):787–795.
Fan YY, Chapkin RS. 1998. Importance of dietary g-linolenic acid in human health and nutrition. J Nutr 128(9):1411–1414.
Fraker PJ, King LE, Laakko T, Vollmer TL. 2000. The dynamic link between the integrity of the immune system and zinc status. J Nutr 130(5S Suppl):1399S–1406S.
Fulkerson WJ, MacIntyre N, Stamler J, Crapo JD. 1996. Pathogenesis and treatment of the adult respiratory distress syndrome. Arch Intern Med 156(1):29–38.
Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. 1999. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med 27(8):1409–1420.
Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. 1996. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 154(3 Pt 1):602–611.
Gray GC, Blankenship TL, Gackstetter G. 2001. History of respiratory illness at the US Naval Academy. Mil Med 166(7):581–586.
Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. 1999. Respiratory diseases among US military personnel: Countering emerging threats. Emerg Infect Dis 5(3):379–385.
Gray GC, Duffy LB, Paver RJ, Putnam SD, Reynolds RJ, Cassell GH. 1997. Mycoplasma pneumoniae: A frequent cause of pneumonia among US Marines in southern California. Mil Med 162(8):524–526.
Greenman E, Phillipich MJ, Meyer CJ, Charamella LJ, Dimitrov NV. 1988. The effect of selenium on phagocytosis in humans. Anticancer Res 8(4):825–828.
Herbert TB, Cohen S. 1993. Stress and immunity in humans: A meta-analytic review. Psychosom Med 55(4):364–379.
Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. 2000. Preexercise medium-chain triglyceride ingestion does not alter muscle glycogen use during exercise. J Appl Physiol 88(1):219–225.
Jeukendrup AE, Saris WH, Schrauwen P, Brouns F, Wagenmakers AJ. 1995. Metabolic availability of medium-chain triglycerides coingested with carbohydrates during prolonged exercise. J Appl Physiol 79(3):756–762.
Jeukendrup AE, Thielen JJ, Wagenmakers AJ, Brouns F, Saris WH. 1998. Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am J Clin Nutr 67(3):397–404.
Johnson RC, Young SK, Cotter R, Lin L, Rowe WB. 1990. Medium-chain-triglyceride lipid emulsion: Metabolism and tissue distribution. Am J Clin Nutr 52(3):502–508.
Kanter MM, Nolte LA, Holloszy JO. 1993. Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol 74(2):965–969.
Kasl SV, Evans AS, Niederman JC. 1979. Psychosocial risk factors in the developmental of infectious mononucleosis. Psychosom Med 41(6):445–466.
Kenler AS, Swails WS, Driscoll DF, DeMichele SJ, Daley B, Babineau TJ, Peterson MB, Bistrian BR. 1996. Early enteral feeding in postsurgical cancer patients. Fish oil structured lipid-based polymeric formula versus a standard polymeric formula. Ann Surg 223(3):316–333.
Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. 2003. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A 100(15):9090–9095.
Kramer TR, Moore RJ, Shippee RL, Friedl KE, Martinez-Lopez L, Chan MM, Askew EW. 1997. Effects of food restriction in military training on T-lymphocyte responses. Int J Sports Med 18(Suppl 1):S84–S90.
Langkamp-Henken B, Bender BS, Gardner EM, Herrlinger-Garcia KA, Kelley MJ, Murasko DM, Schaler JP, Stechmiller JK, Thomas DJ, Wood SM. 2004a. Nutritional formula enhanced immune function and reduced days of symptoms of upperrespiratory tract infection in seniors. J Am Geriatr Soc 52(1):3–12.
Langkamp-Henken B, Wood SM, Herrlinger-Garcia KA, Stechmiller JK, Thomas DJ, Bender BS, Schaller JP, Gardner EM, Murasko DM. 2004b. Nutritional formula improved immune profiles in a nursing home population. FASEB J 18(4):A9.
Lee DJ, Meehan RT, Robinson C, Mabry TR, Smith ML. 1992. Immune responsiveness and risk of illness in US Air Force Academy cadets during basic cadet training. Aviat Space Environ Med 63(6):517–523.
Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Claycombe KJ, Smith GT, Gregory TJ, Karlstad MD. 1997a. Effects of eicosapentaenoic and gamma-linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit Care Med 25(3):523–532.
Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Karlstad MD. 1997b. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med 25(7):1198–1206.
Marsland AL, Herbert TB, Muldoon MF, Bachen EA, Patterson S, Cohen S, Rabin B, Manuck SB. 1997. Lymphocyte subset redistribution during acute laboratory stress in young adults: Mediating effects of hemoconcentration. Health Psychol 16(4):341–348.
Martinez-Lopez LE, Friedl KE, Moore RJ, Kramer TR. 1993. A longitudinal study of infections and injuries of Ranger students. Mil Med 158(7):433–437.
Miyamura JB, McNutt SW, Lichton IJ, Wenkam NS. 1987. Altered zinc status of soldiers under field conditions. J Am Diet Assoc 87(5):595–597.
Mok KT, Maiz A, Yamazaki K, Sobrado J, Babayan VK, Moldawer LL, Bistrian BR, Blackburn GL. 1984. Structured medium-chain and long-chain triglyceride emulsions are superior to physical mixtures in sparing body protein in the burned rat. Metabolism 33(10):910–915.
Moser U. 1987. Uptake of ascorbic acid by leukocytes. Ann N Y Acad Sci 498:200–215.
Murray MJ, Kanazi G, Moukabary K, Tazelaar HD, DeMichele SJ. 2000. Effects of eicosapentaenoic and gamma-linolenic acids (dietary lipids) on pulmonary surfactant composition and function during porcine endotoxemia. Chest 117(6):1720–1727.
Murray MJ, Kumar M, Gregory TJ, Banks PL, Tazelaar HD, DeMichele SJ. 1995. Select dietary fatty acids attenuate cardiopulmonary dysfunction during acute lung injury in pigs. Am J Physiol 269(6 Pt 2):H2090–H2099.
Palombo JD, DeMichele SJ, Boyce PJ, Lydon EE, Liu JW, Huang YS, Forse RA, Mizgerd JP, Bistrian BR. 1999. Effect of short-term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit Care Med 27(9):1908–1915.
Palombo JD, DeMichele SJ, Lydon E, Bistrian BR. 1997. Cyclic vs continuous enteral feeding with omega-3 and gamma-linolenic fatty acids: Effects on modulation of phospholipid fatty acids in rat lung and liver immune cells. J Parenter Enteral Nutr 21(3):123–132.
Palombo JD, DeMichele SJ, Lydon EE, Gregory TJ, Banks PL, Forse RA, Bistrian BR. 1996. Rapid modulation of lung and liver macrophage phospholipid fatty acids in endotoxemic rats by continuous enteral feeding with n-3 and gamma-linolenic fatty acids. Am J Clin Nutr 63(2):208–219.
Pfeiffer JM, Askew EW, Roberts DE, Wood SM, Benson JE, Johnson SC, Freedman MS. 1999. Effect of antioxidant supplementation on urine and blood markers of oxidative stress during extended moderate-altitude training. Wilderness Environ Med 10(2):66–74.
Pierre F, Perrin P, Bassonga E, Bornet F, Meflah K, Menanteau J. 1999. T cell status influences colon tumor occurrence in min mice fed short chain fructo-oligosaccharides as a diet supplement. Carcinogenesis 20(10):1953–1956.
Prasad JS. 1980. Effect of vitamin E supplementation on leukocyte function. Am J Clin Nutr 33(3):606–608.
Sandstead HH, Henriksen LK, Greger JL, Prasad AS, Good RA. 1982. Zinc nutriture in the elderly in relation to taste acuity, immune response, and wound healing. Am J Clin Nutr 36(5 Suppl):1046–1059.
Santos JI, Arredondo JL, Vitale JJ. 1983. Nutrition, infection and immunity. Pediatr Ann 12(3):182–194.
Schley PD, Field CJ. 2002. The immune-enhancing effects of dietary fibres and prebiotics. Br J Nutr 87(Suppl 2):S221–S230.
Scrimshaw NS, SanGiovanni JP. 1997. Synergism of nutrition, infection, and immunity: An overview. Am J Clin Nutr 66(2):464S–477S.
Segerstrom SC, Miller GE. 2004. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull 130(4):601–630.
Selleck KJ, Wan JM, Gollaher CJ, Babayan VK, Bistrian BR. 1994. Effect of low and high amounts of a structured lipid containing fish oil on protein metabolism in enterally fed burned rats. Am J Clin Nutr 60(2):216–222.
Shippee RL, Wood S, Anderson P, Kramer TR, Neita M, Wolcott K. 1995. Effects of glutamine supplementation on immunological responses of soldiers during the Special Forces Assessment and Selection Course. FASEB J 9:A731.
Singh A, Day BA, DeBolt JE, Trostmann UH, Bernier LL, Deuster PA. 1989. Magnesium, zinc, and copper status of US Navy SEAL trainees. Am J Clin Nutr 49(4):695–700.
Tso P, Lee T, DeMichele SJ. 1999. Lymphatic absorption of structured triglycerides vs. physical mix in a rat model of fat malabsorption. Am J Physiol 277(40):G333–G340.
Tso P, Lee T, DeMichele SJ. 2001. Randomized structured triglycerides increase lymphatic absorption of tocopherol and retinol compared with the equivalent physical mixture in a rat model of fat malabsorption. J Nutr 131(8):2157–2163.
Van Zyl CG, Lambert EV, Hawley JA, Noakes TD, Dennis SC. 1996. Effects of medium-chain triglyceride ingestion on fuel metabolism and cycling performance. J Appl Physiol 80(6):2217–2225.
Wood SM, Kennedy JS, Arsenault J, Thomas DL, Buck RH, Shippee RL, DeMichele SJ, Winship TR, Schaller JP, Montain S, Cordle CT. In press. Novel nutritional immune formula maintains host defense mechanisms. Mil Med.
Food Intake Regulation: Liquid versus Solid
Richard D. Mattes, Purdue University
INTRODUCTION
Hunger is the term for sensations that prompt food seeking and ingestion. Satiation and satiety represent sensations that determine meal size and the intermeal interval, respectively. While hunger is on the opposite end of an ingestive behavior scale from satiation/satiety, the sensations do not stem from the presence or absence of a common set of cues. Different mechanisms regulate them, reflecting the fact that the sensations are multidimensional. For example, there is clearly a physiological basis for hunger, but there are cognitive and sensory components as well. One may eat to a high level of satiation, yet the availability of a palatable food can prompt sufficient hunger to eat more. Alternatively, stress may reduce interest in eating when sensations commonly associated with low satiation (e.g., weakness) are present. Thus, regulation of energy balance involves a complex interaction between these sensations and lifestyle.
In the context of soldiers in combat operations, where energy expenditure may surpass energy intake by 50 percent, there is particular concern about an energy deficit that may result in muscle loss and impaired performance. Such a deficit results from an inappropriate dominance of satiation/satiety cues relative
to hunger. It may also be attributable to a low priority for appetitive sensations and intake (i.e., the appetitive system may still be functional just ignored or minimized). The body of scientific literature comprises work addressing the predominant health concern in the general population, that is, energy surfeit. Whether the factors that promote positive energy balance in the general population can be applied in the combat environment in which intake is less than desired, is an empirical question that warrants further consideration and testing.
Features of a diet that may contribute to its low satiety value include a high fat content, a low fiber level, a low calcium concentration and an increased proportion of energy derived from beverages. The latter will be the focus of this presentation. Evidence will be presented that energy-yielding beverages have weak satiety properties and elicit incomplete dietary compensation. The properties of beverages that account for these effects will be outlined followed by a consideration of possible mechanisms. The following are a set of more specific questions that this paper attempts to address:
-
What are the rheological factors regulating food intake that could maximize the consumption of a food given to soldiers under physical, cognitive, and environmental stress?
-
What products may enhance appetite and be added to maximize the consumption of a food product for soldiers under stress?
-
What form (liquid versus solid and gels versus bars) could be given to a presumably healthy soldier to enhance appetite?
EFFECTS OF BEVERAGE CONSUMPTION ON FOOD INTAKE
Beverage Consumption and Appetite
Most studies of appetitive sensations (e.g., hunger, desire to eat, fullness, prospective consumption) support the view that fluids or semisolids are less satiating than solid foods (Haber et al., 1977; Hulshof et al., 1993; Raben et al., 1994; Tournier and Louis-Sylvestre, 1991). Indeed, an independent inverse influence of viscosity on hunger has been demonstrated (Mattes and Rothacker, 2001). There have been studies that failed to observe solid versus fluid differences in intake (Jordan and Spiegel, 1977; Kissileff et al., 1980; Levitz, 1975; Rolls et al., 1990), but their interpretation may be hampered by the use of foods that differed along many dimensions and testing under nonnormal eating conditions (e.g., delivery of load by pump or testing in a laboratory). For example, a number of these observations are based on studies conducted with soup (Hulshof et al., 1993; Jordan and Spiegel, 1977; Kissileff, 1985; Kissileff et al., 1980; Levitz, 1975; Raben et al., 1994; Rolls et al., 1990, 1999; Tournier and Louis-Sylvestre, 1991). Soup does appear to be an exception in the fluid–solid influ-
ence on appetite; although an explanation remains elusive, it likely involves a strong cognitive component (Mattes, 2005).
Beverage Consumption and Dietary Compensation
Dietary surveys indicate that the addition of a beverage to a meal leads to increased total energy intake at the meal by an amount roughly equal to the contribution of the beverage (de Castro, 1993). Similar findings have been reported over days for soda and alcohol (Colditz et al., 1991; Rose et al., 1995). The majority of work on this issue has been conducted with young adults, but the phenomenon also appears to hold with adolescents. Based on data from the 1994 Continuing Survey of Food Intake for Individuals (CSFII) survey, daily energy intake as well as carbohydrate energy tends to increase among adolescents with an increasing consumption of soft drinks (Harnack et al., 1999).
Intervention studies have yielded comparable results (DiMeglio and Mattes, 2000; Mattes, 1996; Tournier and Louis-Sylvestre, 1991). Interestingly, other studies noted that supplemental noncaloric fluid ingestion is not associated with increased intake or body weight, but the addition of an energy source to a fluid leads to increases in both (Poppitt et al., 1996; Raben et al., 2002; Tordoff and Alleva, 1990). Dietary compensation (energy intake adjustments made during the day to compensate for earlier intake) has been studied with solid, semisolid, and fluid foods. In a meta-analysis including more than 40 studies, the reported mean dietary compensation error for experimental loads of solid foods was 36 percent. This is in contrast to the mean dietary compensation for semisolid foods and for fluids (79 percent and 109 percent, respectively) (Mattes, 1996). This analysis suggests that dietary adjustments are made at other times of the day to offset all but 36 percent of the energy contributed by the experimental solid loads, but there is no compensation, indeed a slight reverse compensation (greater intake of the customary diet), for fluids.
Beverage Consumption and Body Weight
Survey data indicate caloric beverage consumption is associated with higher body weight or body mass index (BMI) (Gillis and Bar-Or, 2003; Ludwig et al., 2001). In a prospective observational study of schoolchildren, increments of soda servings were associated with quarter BMI unit increases (Ludwig et al., 2001). An intervention trial that involved ingestion of 450 kcal daily of sweetened fruit-flavored beverage revealed a significant increase in body weight that was not observed when 450 kcal were consumed in solid form by the same individuals (DiMeglio and Mattes, 2000). Although much of this work has focused on carbonated beverage intake because of its high level of consumption in the population, it is probably incorrect to assume that its effect on body weight is unique. In fact, noncarbonated beverages with energy derived from other ma-
cronutrients also elicit weak dietary compensation (de Castro, 1993; Shields et al., 2004), which suggests that they will also affect body weight.
Satiety Properties of Macronutrients
Important features of compensatory responses to beverages have not been adequately characterized. One concern is potential differences across macronutrients. Studies, primarily with solid foods, indicate that when macronutrients are presented as isoenergetic loads (preserving differences in energy density), there is a hierarchy of satiety values wherein protein > carbohydrate > fat (Blundell et al., 1996; Johnson and Vickers, 1993; Stubbs et al., 1997). Although there is some evidence that the hierarchy holds in fluids as well (e.g., Stubbs et al., 1997), the energy-yielding macronutrients have never been directly contrasted in a within-subject design study. Survey data indicate beverages with different macronutrient compositions (e.g., soda, alcohol, milk) are equally ineffective in prompting dietary compensation (de Castro, 1993; Mattes, 1996). A review of the literature reveals that most studies supporting a high satiety value of protein involved manipulations of solid foods (Booth et al., 1970; Hill and Blundell, 1986, 1990; Johnson and Vickers, 1993; Porrini et al., 1995; Stubbs et al., 1996; Vandewater and Vickers, 1996) whereas the high satiety value of protein is not as robust in studies using fluid preloads (de Graaf et al., 1992; Driver, 1988; Geliebter, 1979; Latner and Schwartz, 1999; Stockley et al., 1984; Sunkin and Garrow, 1982).
POSSIBLE MECHANISMS FOR THE WEAK APPETITIVE EFFECTS OF BEVERAGES
The mechanisms responsible for the weaker appetitive and compensatory dietary responses to fluids than to solids have not been identified. Candidates include cognitive effects, orosensory stimulation, gastrointestinal (GI) tract transit time, osmotic differences, gut hormone activity, absorptive processes, and postabsorptive endocrine or metabolic responses.
Beliefs about the energy content of a food or beverage can have a stronger influence on post-ingestive appetitive responses than the true energy content, at least acutely. When lean and obese individuals were presented with low- or high-energy meals, belief about their energy content was a better predictor of the hunger response than true energy content (Wooley et al., 1972). Beverages may be believed to hold weaker satiation value than isocaloric solid items (DiMeglio and Mattes, 2000). Orosensory cues also modulate the appetitive response to a food or beverage. For example, there is an indirect relationship between the self-reported viscosity of a beverage and hunger (Mattes and Rothacker, 2001). Food that passes through the GI tract more rapidly (a short transit time), such as fluids, may elicit weaker appetitive responses than those with a longer transit time, such
as soluble fiber–containing foods. This mechanism might partly explain the recognized satiating effects of fiber (Burton-Freeman, 2000; Delargy et al., 1997; Holt et al., 1992; Howarth et al., 2001). A beverage that holds comparable cognitive and orosensory properties to another beverage, but contains a compound that forms a viscous mass in the gut may be more satiating than the one lacking the compound because of its effects on GI function (Williams et al., 2004; Wolf et al., 2003). Cholecystokinin (CCK) is a gut peptide hormone that enhances satiation, especially in conjunction with gastric distention (Kissileff et al., 2003). A differential effect of ingested solid and fluid foods on CCK may be inferred by contrasting responses across studies. A fluid load (tomato soup) elicits only a modest increment in male adults (Nolan et al., 2003) whereas a more viscous homogenized vegetable load results in a marked CCK elevation in males (Santangelo et al., 1998). Similar, although quantitatively weaker, effects of fluids on CCK release have also been reported in females (Heini et al., 1998).
Several human studies have demonstrated that fluid versus solid forms of the same high-carbohydrate foods lead to comparable early rises of plasma glucose and greater insulin responses (Bolton et al., 1981; Haber et al., 1977). Numerous mechanisms for insulin’s satiety effects have been proposed. One is based on evidence that insulin is an independent determinant of the circulating leptin concentration (Mantzoros et al., 1998). Leptin may inhibit food intake (Karhunen et al., 1998; Larsson et al., 1998), in part though a reduction of hunger (Keim et al., 1998; Shimizu et al., 1998). Insulin stimulates both leptin gene expression and glucose oxidation within adipocytes. This leads to increased leptin secretion (Mueller et al., 1998) and higher plasma leptin concentrations in humans (Dirlewanger et al., 2000).
SUMMARY
There is compelling evidence that body weight is increasing in the population. Per capita consumption of energy-yielding beverages has increased in parallel with this trend and energy-yielding beverages evoke a weaker appetitive and compensatory dietary response than do solid foods. Thus, it is reasonable to postulate that the consumption of beverages contributes significantly to positive energy balance. There is also enough evidence suggesting that energy intake in the form of fluid beverages may have less satiating effects and induces more dietary compensation than do solid foods. None of these studies, however, have been conducted under the high-stress situation of combat, which might influence appetite and food intake in diverse ways. Nevertheless, these observations may provide an approach for enhancing the energy intake of individuals, such as soldiers, under emotional and environmental stress. To design diets providing a high proportion of energy in fluid form may be an effective strategy to increase total energy intake, if volume and size constraints are not problematic.
REFERENCES
Blundell JE, Lawton CL, Cotton JR, Macdiarmid JI. 1996. Control of human appetite: Implications for the intake of dietary fat. Ann Rev Nutr 16:285–319.
Bolton RP, Heaton KW, Burroughs LF. 1981. The role of dietary fiber in satiety, glucose, and insulin: Studies with fruit and fruit juice. Am J Clin Nutr 34(2):211–217.
Booth DA, Chase A, Campbell AT. 1970. Relative effectiveness of protein in the late stages of appetite suppression in man. Physiol Behav 5(11):1299–1302.
Burton-Freeman B. 2000. Dietary fiber and energy regulation. J Nutr 130(2S Suppl):272S–275S.
Colditz GA, Giovannucci E, Rimm EB, Stampfer MJ, Rosner B, Speizer FE, Gordis E, Willett WC. 1991. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr 54(1):49–55.
de Castro JM. 1993. The effects of the spontaneous ingestion of particular foods or beverages on the meal pattern and overall nutrient intake of humans. Physiol Behav 53(6):1133–1144.
de Graaf C, Hulshof T, Weststrate JA, Jas P. 1992. Short-term effects of different amounts of protein, fats, and carbohydrates on satiety. Am J Clin Nutr 55(1):33–38.
Delargy HJ, O’Sullivan KR, Fletcher RJ, Blundell JE. 1997. Effects of amount and type of dietary fibre (soluble and insoluble) on short-term control of appetite. Int J Food Sci Nutr 48(1):67–77.
DiMeglio DP, Mattes RD. 2000. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int J Obes Relat Metab Disord 24(6):794–800.
Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, Jequier E, Tappy L. 2000. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord 24(11):1413–1418.
Driver CJ. 1988. The effect of meal composition on the degree of satiation following a test meal and possible mechanisms involved. Br J Nutr 60(3):441–449.
Geliebter AA. 1979. Effects of equicaloric loads of protein, fat, and carbohydrate on food intake in the rat and man. Physiol Behav 22(2):267–273.
Gillis LJ, Bar-Or O. 2003. Food away from home, sugar-sweetened drink consumption and juvenile obesity. J Am Coll Nutr 22(6):539–545.
Haber GB, Heaton KW, Murphy D, Burroughs LF. 1977. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet 2(8040):679–682.
Harnack L, Stang J, Story M. 1999. Soft drink consumption among US children and adolescents: Nutritional consequences. J Am Diet Assoc 99(4):436–441.
Heini AF, Kirk KA, Lara-Castro C, Weinsier RL. 1998. Relationship between hunger-satiety feelings and various metabolic parameters in women with obesity during controlled weight loss. Obes Res 6(3):225–230.
Hill AJ, Blundell JE. 1986. Macronutrients and satiety: The effects of a high-protein or high-carbohydrate meal on subjective motivation to eat and food preferences. Nutr Behav 3(2):133–144.
Hill AJ, Blundell JE. 1990. Comparison of the action of macronutrients on the expression of appetite in lean and obese human subjects. Ann NY Acad Sci 575(575):529–531.
Holt S, Brand J, Soveny C, Hansky J. 1992. Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin responses. Appetite 18(2):129–141.
Howarth NC, Saltzman E, Roberts SB. 2001. Dietary fiber and weight regulation. Nutr Rev 59(5):129–139.
Hulshof T, De Graaf C, Weststrate JA. 1993. The effects of preloads varying in physical state and fat content on satiety and energy intake. Appetite 21(3):273–286.
Johnson J, Vickers Z. 1993. Effects of flavor and macronutrient composition of food servings on liking, hunger and subsequent intake. Appetite 21(1):25–39.
Jordan HA, Spiegel TA. 1977. Palatability and oral factors and their role in obesity. In: Kare MR, Maller O, eds. The Chemical Senses and Nutrition. New York: Academic Press. Pp. 393–410.
Karhunen LJ, Lappalainen RI, Haffner SM, Valve RH, Tuorila H, Miettinen H, Uusitupa MI. 1998. Serum leptin, food intake and preferences for sugar and fat in obese women. Int J Obes Relat Metab Disord 22(8):819–821.
Keim NL, Stern JS, Havel PJ. 1998. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr 68(4):794–801.
Kissileff HR. 1985. Effects of physical state (liquid-solid) of foods on food intake: Procedural and substantive contributions. Am J Clin Nutr 42(5 Suppl):956–965.
Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. 2003. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol 285(5):R992–R998.
Kissileff HR, Klingsberg G, Van Itallie TB. 1980. Universal eating monitor for continuous recording of solid or liquid consumption in man. Am J Physiol Regul Integr Comp Physiol 238(1):R14–R22.
Larsson H, Elmstahl S, Berglund G, Ahren B. 1998. Evidence for leptin regulation of food intake in humans. J Clin Endocrinol Metab 83(12):4382–4385.
Latner JD, Schwartz M. 1999. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 33(1):119–128.
Levitz LS. 1975. The susceptibility of human feeding behavior to external controls. In: Bray GA, ed. Obesity in Perspective. Proceedings of the Conference. October 1–3, 1973, Bethesda, MD. DHEW Publication No. 75-708. Washington, DC: US Government Printing Office. Pp. 53–60.
Ludwig DS, Peterson KE, Gortmaker SL. 2001. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 357(9255):505–508.
Mantzoros CS, Liolios AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, Moses AC, Flier JS. 1998. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res 6(3):179–186.
Mattes RD. 1996. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav 59(1):179–187.
Mattes RD. 2005. Soup and satiety. Physiol Behav 83:739–747.
Mattes RD, Rothacker D. 2001. Beverage viscosity is inversely related to postprandial hunger in humans. Physiol Behav 74(4–5):551–557.
Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ. 1998. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology 139(2):551–558.
Nolan LJ, Guss JL, Liddle RA, Pi-Sunyer FX, Kissileff HR. 2003. Elevated plasma cholecystokinin and appetitive ratings after consumption of a liquid meal in humans. Nutrition 19(6):553–557.
Poppitt SD, Eckhardt JW, McGonagle J, Murgatroyd PR, Prentice AM. 1996. Short-term effects of alcohol consumption on appetite and energy intake. Physiol Behav 60(4):1063–1070.
Porrini M, Crovetti R, Riso P, Santangelo A, Testolin G. 1995. Effects of physical and chemical characteristics of food on specific and general satiety. Physiol Behav 57(3):461–468.
Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A. 1994. Resistant starch: The effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr 60(4):544–551.
Raben A, Vasilaras TH, Moller AC, Astrup A. 2002. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 76(4):721–729.
Rolls BJ, Bell EA, Thorwart ML. 1999. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr 70(5):448–455.
Rolls BJ, Fedoroff IC, Guthrie JF, Laster LJ. 1990. Foods with different satiating effects in humans. Appetite 15(2):115–126.
Rose D, Murphy SP, Hudes M, Viteri FE. 1995. Food energy remains constant with increasing alcohol intake. J Am Diet Assoc 95(6):698–700.
Santangelo A, Peracchi M, Conte D, Fraquelli M, Porrini M. 1998. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br J Nutr 80(6):521–527.
Shields DH, Corrales KM, Metallinos-Katsaras E. 2004. Gourmet coffee beverage consumption among college women. J Am Diet Assoc 104(4):650–653.
Shimizu H, Tsuchiya T, Sato N, Shimomura Y, Kobayashi I, Mori M. 1998. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care 21(9):1470–1474.
Stockley L, Jones FA, Broadhurst AJ. 1984. The effects of moderate protein or energy supplements on subsequent nutrient intake in man. Appetite 5(3):209–219.
Stubbs RJ, Prentice AM, James WP. 1997. Carbohydrates and energy balance. Ann NY Acad Sci 819:44–69.
Stubbs RJ, van Wyk MC, Johnstone AM, Harbron CG. 1996. Breakfasts high in protein, fat or carbohydrate: Effect on within-day appetite and energy balance. Eur J Clin Nutr 50(7):409–417.
Sunkin S, Garrow JS. 1982. The satiety value of protein. Hum Nutr Appl Nutr 36(3):197–201.
Tordoff MG, Alleva AM. 1990. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr 51(6):963–969.
Tournier A, Louis-Sylvestre J. 1991. Effect of the physical state of a food on subsequent intake in human subjects. Appetite 169(1):17–24.
Vandewater K, Vickers Z. 1996. Higher-protein foods produce greater sensory-specific satiety. Physiol Behav 59(3):579–583.
Williams JA, Lai CS, Corwin H, Ma Y, Maki KC, Garleb KA, Wolf BW. 2004. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J Nutr 134(4):886–889.
Wolf BW, Wolever TMS, Lai CS, Bolognesi C, Radmard R, Maharry KS, Garleb KA, Hertzler SR, Firkins JL. 2003. Effects of a beverage containing an enzymatically induced-viscosity dietary fiber, with or without fructose, on the postprandial glycemic response to a high glycemic index food in humans. Eur J Clin Nutr 57(9):1120–1127.
Wooley OW, Wooley SC, Dunham RB. 1972. Can calories be perceived and do they affect hunger in obese and nonobese humans? J Comp Physiol Psychol 80(2):250–258.
A General Model of Intake Regulation: Diurnal and Dietary Composition Components
John M. de Castro, University of Texas at El Paso
INTRODUCTION
A considerable amount of evidence shows that nutrient intakes are affected by a wide range of factors, each of which accounts for only a small portion of the variance in intake. Models that attempt to explain intake control based on any single factor have failed to account for how intake can be controlled in the face of a complex array of influential variables. In addition, the levels and effects of many factors vary from individual to individual, and these individual differences are affected by heredity. These elements have been incorporated into a general model of intake regulation (de Castro and Plunkett, 2002) that is presented in Figure B-49. The model separates sets of uncompensated and compensated factors
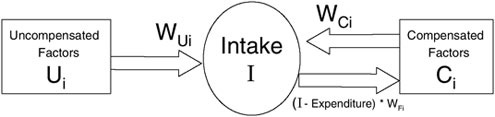
FIGURE B-49 The general intake regulation model wherein intake (I) is controlled by two sets of factors: compensated factors (Ci) that both affect and are affected by intake via negative feedback loops and uncompensated factors (Ui) that affect but are not affected by intake. Inheritance affects the system by (a) determining the preferred level for intake and compensated and uncompensated factors and (b) determining the level of impact of the factors on intake (W). Factors that are affected by heredity are indicated in bold.
SOURCE: de Castro and Plunkett (2002), used with permission from Elsevier.
with each factor having preferred levels that are influenced by heredity (e.g., genetic variation). Further, the model specifies that each factor in both sets has an individual impact factor (i.e., weight that specifies the magnitude of the factor’s effect on intake). The weights are assumed to be different for each individual, and their values are influenced by heredity. Simulations of the model indicate that although prolonged changes in the level of any factor can result in a prolonged change in intake and body weight, the size of the change depends on the inherited level of responsiveness to that factor. This implies that manipulation of a factor to alter intake will be effective in some individuals but not in others. For this reason, it is important to consider individual differences in responsiveness when developing an intervention designed to alter intake. The responsiveness of the individual to these influences can be measured and used to help construct an individualized intake recommendation.
The Committee on Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations was charged with designing the nutrient composition of a ration to be used for short-term, high-stress combat operations. It is a well-known fact that the energy intake of soldiers in these unique situations of high mental and physical stress is lower than the energy expenditure, and this energy deficit status might result in weight losses and other health and perfomance adverse effects. In addition to maintaining health and performance, the ration should be palatable and accepted by soldiers under such unique circumstances. Palatability is only one factor that drives consumption patterns; other factors that might be categorized as psychological factors are also important in defining eating behavior, including level of consumption.
In this paper, the role of diurnal rhythms and dietary energy density on dietary intake behavior is examined; the conclusions are derived with data from
reports from 669 free-living normal adult humans who adequately reported intake in a 7-day diet diary. The influence of the time of day and the dietary energy densities on intake were investigated. This model could be applied to soldiers under combat situations. Specifically, this paper attempts to answer the following questions:
-
What are the food factors, which regulate food intake, that could maximize the consumption of a food ration given to soldiers under physical, cognitive, and environmental stresses?
-
What are the issues to consider when developing a nutritionally enhanced food product in terms of energy density, satiability, behavior and food characteristics to maximize the consumption of the food product in specific relation to soldiers under stress?
DIURNAL FOOD INTAKE PATTERN
There are clear diurnal or circadian influences on intake. Studies using the diet diary technique (de Castro, 1987, 2004c) have demonstrated that substantial changes in intake occur over the course of the day. As the day progresses, the average meal size increases (Figure B-50, left) and the subsequent interval between meals decreases simultaneously (Figure B-50, center). We have found that this pattern is true for both North Americans and Europeans (de Castro et al., 2000) as determined by using the satiety ratio. The satiety ratio is the duration of the interval after the meal divided by the meal size (min/MJ), and it gauges the duration of satiety produced per unit of food energy ingested. This ratio markedly declines over the course of the day and becomes quite low during the late evening (Figure B-50, right) (de Castro, 1987, 2004c). This suggests that the satiating effect of food decreases over the course of the day and that a large proportion of intake in the evening could lead to increased overall intake.
Employing twin data, it was demonstrated that the time of day when people eat is significantly affected by heredity (de Castro, 2001). In particular, people differ in the proportion of daily intake ingested at different times of the day, with some eating a larger proportion of their intake in the morning, some in the afternoon, and some in the evening, and these proportions of intake are heritable. It was also demonstrated that the differences between the morning and afternoon, the morning and evening, and the afternoon and evening were significantly heritable (de Castro, 2001). This suggests that the time-of-day effects on intake vary among individuals and that this difference is, at least, partially caused by heredity. Hence genetic factors appear to affect not only when an individual will tend to eat, but also how big of an effect the selection of that time will have on intake.
Because meal sizes increase during the day, while the after-meal intervals and satiety ratios decrease over the day, it is reasonable to expect that the time of day of nutrient intake would be related to total intake. It was demonstrated that
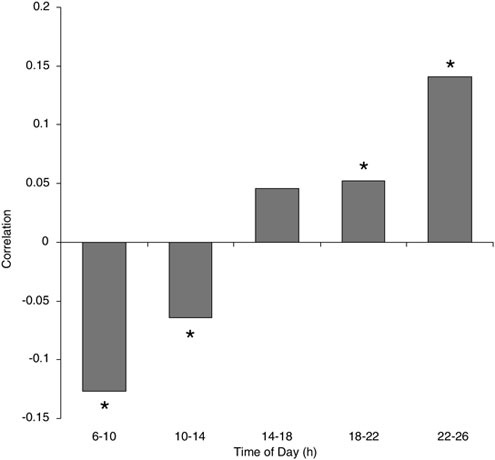
FIGURE B-51 Correlation between proportion of intake during four-hour periods and overall daily intake. The mean correlations between the proportions of food energy ingested and overall daily intake were self-reported in 7 day diet diaries during each of the five time periods and the total amount ingested.
NOTE: *Different from zero (p< 0.01).
SOURCE: Adapted from de Castro (2004c).
the proportion of intake ingested in the morning is negatively correlated with overall intake (Figure B-51), while the proportion ingested late in the evening is positively correlated with overall intake (de Castro, 2004c). The energy densities of intake during all periods of the day were positively related to overall intake. The results suggest that a high-density energy intake during any portion of the day could increase overall intake and that an intake in the late night lacks satiating value and can result in greater overall daily intake. Hence the best way to
promote higher levels of intake would be to increase the ingestion of high energy dense foods at night.
DENSITY
The energy density of a diet has been shown to markedly affect the total amount of food energy ingested. The greater the food energy content per gram of food consumed, the more total food energy ingested (Bell and Rolls, 2001; de Castro, 2004a; Yao and Roberts, 2001). We have shown that, for study participants consuming diets of varying energy densities during a seven-day period, the dietary energy density (calculated as the food energy content of the foods divided by their total weight) of meals is related to the total energy intakes from the meals; the dietary energy densities over the entire day are related to the total energy intakes over those same days; and the dietary energy densities of the participants’ diets are related to their total energy intakes. Dietary energy density is, to a large extent, positively correlated with intake, no matter whethermeals, daily intakes, or overall participant intakes were examined (Figure B-52). These findings resulted in the hypothesis that intake is not controlled on the basis of the energy content of the food, but rather on its volume, that is, the greater the food energy per unit volume of a meal, the more total food energy ingested.
Dietary energy density is calculated as the food energy content of the foods divided by the total weight of those foods. The food energy component comprises the macronutrients, carbohydrate, fat, protein, and alcohol; the weight component comprises the weight of the macronutrients, nonnutritive solids, water contained in the foods, and drinks (water) ingested with the foods. By calculating separately the energy density from carbohydrate, fat, and protein, we investigated whether the components of food energy play different roles in determining the effects of dietary energy density on intake. Each of these densities was positively correlated with intake regardless of whether meals, daily intakes, or weekly intakes were studied (Figure B-53, left). When the carbohydrate, fat, and protein dietary densities were used as independent variables in a multiple regression prediction of energy intake, a variable outcome was produced depending on whether meals, daily intakes, or overall participant intakes were employed (Figure B-53, center). The one consistent outcome was that the dietary energy density of fat always had the strongest positive relationship with energy intake. When the overall dietary energy density was added to the regression, only overall dietary energy density had a strong positive relationship with intake, but all of the macronutrient relationships became negative with respect to intake (Figure B-53, right). This occurred regardless of whether meals, daily intakes, or overall participant intakes were used. The negative relationship suggests that when the overall energy density of a meal is considered, high densities of any macronutrient have a negative influence on intake. Further, this suggested that the overall dietary energy density is more important than the dietary energy density
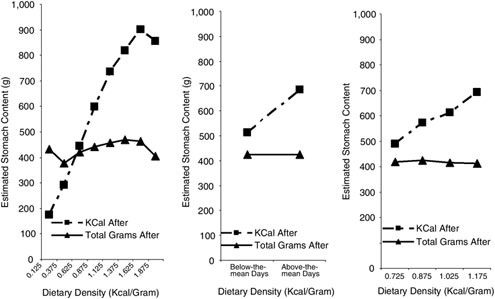
FIGURE B-52 The relationship of dietary density and after meal stomach contents. Participants with a physical activity level of > 1.1. Mean estimated contents of the stomach at the end of meals of food energy (kcal) or volume (total grams) as a function of dietary energy density for meal (left), daily (center), and participant (right) intakes.
SOURCE: Author’s unpublished data.
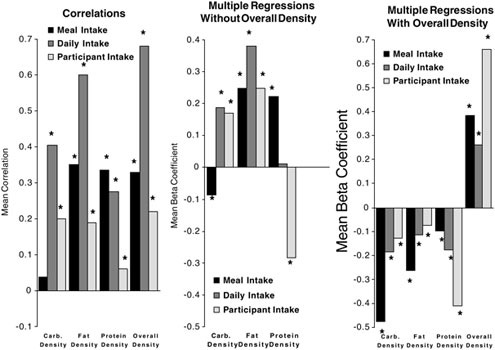
FIGURE B-53 Meal dietary density relationships with meal, daily, and participant intake. Mean univariate correlations (left), multiple regression β coefficients without (center) and with (right) inclusion of overall dietary energy density in the regression for the relationships of carbohydrate dietary energy density, fat dietary energy density, protein dietary energy density, and overall dietary energy density with meal (first bar of each set of three), daily (second bar), and participant (third bar) intakes.
SOURCE: Author’s unpublished results.
of any particular macronutrient, implying that it is the meal dietary energy density per se that is the important influence on intake, not the meal’s components.
The hypothesis that intake is controlled on the basis of the volume of food ingested infers that the volume of nutrients in the stomach at the end of the meal is the critical factor in determining intake. If the volume in the stomach at the end of the meal is relatively constant, then the food energy ingested would depend on the dietary energy density of the foods. The meal size is not an adequate estimate of the volume in the stomach because it does not include the nutrients from prior intake that still remain in the stomach. We attempted to test these predictions by estimating the contents of the stomach at the end of meals. Because the stomach empties in a very regular and predictable fashion (Hopkins, 1966; Hunt and Knox, 1968; Hunt and Stubbs, 1975), how much of the contents should have emptied over a given interval and how much should therefore be remaining at the time of a second meal is relatively simple to calculate (de Castro, 1987, 1999). Added to this model was an estimate of fluid movement into and out of the stomach according to the model of Toates (1978).
Employing the reported intakes from the seven-day diary records along with these stomach emptying models, both the food energy (kcal) contents and the total gram contents of the stomach at the end of each meal were estimated and expressed as a function of the dietary energy density of the meal (Figure B-52, left). Although the food energy content of the stomach increased significantly with the increase in dietary energy density, the total volume (grams) of contents estimated to be in the stomach was not significantly dissimilar over the nine different dietary energy density levels. An analysis of the mean after-meal stomach contents over the entire day (on days that an individual participant had below- or above-the-mean dietary energy density) revealed a similar picture (Figure B-52, center). Again, food energy content of the stomach was high on the above-the-mean dietary energy density days, but the estimated weight of the contents of the stomach remained unchanged. Finally, the average estimated after-meal stomach contents of participants whose dietary energy densities differed were compared (Figure B-52, right). Exactly as was seen in the meal and daily intake analyses, higher stomach contents of food energy resulted in higher dietary energy densities, but there was no difference in weight of the contents.
Using twin data, it was determined that the level of dietary energy density within the individual diets is affected by heredity (de Castro, 2004b). Additionally, the magnitude of the differences between the members of identical twin pairs in the density of their diet was positively related to the magnitude of the differences in the energy content of their daily intake (de Castro, 2004d). On the other hand, heredity did not seem to influence the relationship between density and intake. There were no significant heritable factors for either the correlation or the slope of the best fitting regression line between density and intake. Hence, it appears that heredity may influence the preferred dietary density that in turn
has a marked influence on intake. This infers that heredity does not cause individual differences in responsiveness to dietary density.
SUMMARY
The findings present a relatively clear picture of the roles of diurnal rhythms and dietary energy density in the control of short-term food intake. Intake in the morning appears to reduce overall intake, while intake at night appears to increase it. Dietary energy density, on the other hand, appears to be a major determinant of short-term food energy intake, with higher dietary energy density associated with substantially larger meals, daily intakes, and overall participant intakes. These results support the concept that short-term food intake is controlled on the basis of its weight and volume as opposed to its total food-energy content. This conclusion was supported by the observations that (a) overall dietary energy densities appear to be more important than the energy density of particular nutrients and (b) the estimated after-meal stomach contents appear to be constant regardless of the dietary energy density of the reported diet. These observations further suggest that short-term food regulation may occur on the basis of stomach filling.
Based on these findings, the overall dietary density of assault rations should be maximized, and intake at night should be emphasized. The fact that time-of-day effects on intake appear to be heritable suggests that there may be considerable individual differences in response to manipulating this factor. On the other hand, the effects of dietary energy density on intake do not appear to be influenced by heredity, and this suggests that individual differences in response to dietary energy density manipulation should be minimal. Hence, to maximize intake in the field, maximize energy density and encourage night-time eating.
REFERENCES
Bell EA, Rolls BJ. 2001. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr 73(6):1010–1018.
de Castro JM. 1987. Macronutrient relationships with meal patterns and mood in the spontaneous feeding behavior of humans. Physiol Behav 39(5):561–569.
de Castro JM. 1999. Inheritance of premeal stomach content influences on food intake in free living humans. Physiol Behav 66(2):223–232.
de Castro JM. 2001. Heritability of diurnal changes in food intake in free-living humans. Nutrition 17(9):713–720.
de Castro JM. 2004a. Dietary energy density is associated with increased intake in free-living humans. J Nutr 134(2):335–341.
de Castro JM. 2004b. The control of eating behavior in free-living humans. In: Stricker EM, Woods, eds. Handbook of Behavioral Neurobiology. 2nd ed. Vol 14. Neurobiology of Food and Fluid Intake. New York: Plenum Press. Pp. 469–504.
de Castro JM. 2004c. The time of day of food intake influences overall intake in humans. J Nutr 134(1):104–111.
de Castro JM. 2004d. When identical twins differ: An analysis of intrapair differences in the spontaneous eating behavior and attitudes of free-living monozygotic twins. Physiol Behav 82(4):733–739.
de Castro JM, Plunkett S. 2002. A general model of intake regulation. Neurosci Biobehav Rev 26(5):581–595.
de Castro JM, Bellisle F, Dalix AM. 2000. Palatability and intake relationships in free-living humans: Measurement and characterization in the French. Physiol Behav 68(3):271–277.
Hopkins A. 1966. The pattern of gastric emptying: A new view of old results. J Physiol 182(1):144–149.
Hunt JN, Knox MT. 1968. Regulation of gastric emptying. In: Coyle CF, Heidel W, eds. Handbook of Physiology. Vol 4. Alimentary Canal (Motility). Washington, DC: American Physiological Society. Pp. 1917–1935.
Hunt JN, Stubbs DF. 1975. The volume and energy content of meals as determinants of gastric emptying. J Physiol 245(1):209–225.
Toates FM. 1978. A physiological control theory of the hunger-thirst interaction. In: Booth DA, ed. Hunger Models: Computable Theory of Feeding Control. New York: Academic Press. Pp. 347–373.
Yao M, Roberts SB. 2001. Dietary energy density and weight regulation. Nutr Rev 59(8):247–258.