5
Review of the Evidence on Other ME/CFS Symptoms and Manifestations
This chapter reviews the evidence on symptoms and manifestations of ME/CFS other than the major ones addressed in Chapter 4. Discussed in turn are pain, immune impairment, neuroendocrine manifestations, and infection.
Description of Pain in ME/CFS
Pain is a defining characteristic of ME/CFS and is listed as either a required or additional symptom in all case definitions and diagnostic criteria evaluated in this report. The existing ME/CFS case definitions include muscle pain, joint pain, headaches, tender lymph nodes, and sore throat as pain symptoms (Carruthers et al., 2003, 2011; Fukuda et al., 1994; Jason et al., 2010; NICE, 2007). The Canadian Consensus Criteria (CCC), the Revised CCC, and the 2011 International Consensus Criteria for ME (ME-ICC) mention additional symptoms—including abdominal pain, chest pain, hyperalgesia, and stiffness—and such descriptors as myofascial, radiating, and migratory pain. Patients also described chronic pain behind the eyes, neck pain, neuropathic or nerve pain, “full-body ice-cream-headache-like pains,” and feeling like “my brain was going to explode” (FDA, 2013, p. 14).
The majority of ME/CFS patients experience some type of pain, although individual experiences with pain vary widely (FDA, 2013; Meeus et al., 2007; Unger, 2013). In one community-based study, 94 percent of respondents fulfilling the Fukuda definition reported muscle aches and pain,
and 84 percent reported joint pain (Jason et al., 1999). Recent preliminary data from the Centers for Disease Control and Prevention’s (CDC’s) Multi-Site Clinical Study of CFS indicate that 80 percent of patients enrolled had experienced pain in the past week (Unger, 2013). Muscle aches and pains were the most common pain complaint (reported by 72 to 79 percent of patients), followed by joint pain (reported by 58 to 60 percent of patients) and headaches (reported by 48 to 56 percent of patients). Less common pain complaints included tender lymph nodes (37 to 39 percent), abdominal pain (32 percent), sore throat (25 to 28 percent), eye pain (23 percent), and chest pain (15 percent).1,2
Pain interferes similarly in the life of someone with ME/CFS and someone with spinal cord injury, muscular dystrophy, or multiple sclerosis3 (Unger, 2013). More severely disabled ME/CFS patients may experience more pain (Marshall et al., 2010). Regardless of the definitions used, the presence of chronic regional and widespread pain in individuals with ME/CFS is associated with poor general health, physical functioning, and sleep quality independently of ME/CFS (Aaron et al., 2002). In a systematic review of chronic musculoskeletal pain in ME/CFS (which included studies using various ME/CFS diagnostic criteria), Meeus and colleagues (2007) concluded that there is no consensus on the definition of chronic widespread pain in ME/CFS, and while there is no strong proof of its exact cause or prevalence, this pain is strongly disabling and not necessarily related to depression.
Patients diagnosed with ME/CFS experience more pain than the general population (Ickmans et al., 2013; Jason et al., 2013b). Employing moderate thresholds for frequency and severity, Jason and colleagues (2013b) found that a greater percentage of ME/CFS patients experienced pain symptoms relative to healthy controls (see Figure 5-1).4
Pain in ME/CFS often is a component of post-exertional malaise (PEM), a symptom constellation triggered or worsened by physical and/or mental activity (see the discussion of PEM in Chapter 4). Exercise has
____________________
1 Personal communication from Elizabeth Unger, 2014. Preliminary analysis of CDC Multi-Site Clinical Study.
2 The percentages in the preceding two sentences reflect patients reporting that each symptom occurred with moderate severity at least half of the time.
3 The mean Patient-Reported Outcomes Measurement Information System (PROMIS) t scores for pain interference in the CDC Multi-Site Clinical Study were similar to or slightly higher than the scores published for spinal cord injury, muscular dystrophy, and multiple sclerosis (Unger, 2013).
4 Jason and colleagues (2013b) compared 236 ME/CFS patients with 86 healthy controls who completed the DePaul Symptom Questionnaire, rating the frequency and severity of 54 symptoms. Patient data were obtained from the SolveCFS BioBank, which includes patients diagnosed by a licensed physician using either the Fukuda definition or CCC.
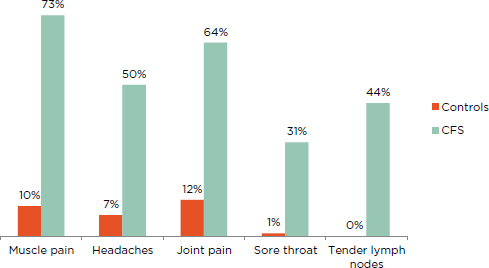
FIGURE 5-1 Percentage of ME/CFS patients and healthy controls reporting pain symptoms of at least moderate severity that occurred at least half of the time for the past 6 months.
NOTE: All patients fulfilled the Fukuda definition for CFS.
SOURCE: Jason et al., 2013b.
been shown to decrease pain threshold, increase pain severity, and worsen global symptoms in patients with ME/CFS compared with healthy controls (Van Oosterwijck et al., 2010; Whiteside et al., 2004) and in patients with both ME/CFS and fibromyalgia compared with patients with rheumatoid arthritis and healthy controls (Meeus et al., 2014).
Assessment of Pain in ME/CFS
Tools useful for evaluating pain clinically in ME/CFS include the 1990 and 2010 American College of Rheumatology (ACR) fibromyalgia criteria, numeric or visual analog scales (VASs), the Fibromyalgia Impact Questionnaire (FIQ), the Revised Fibromyalgia Impact Questionnaire (FIQR), and the Brief Pain Inventory (BPI) (Bennett et al., 2009; Boomershine, 2012). The VAS and the BPI are highly validated across many pain conditions, and the FIQ and FIQR are highly validated for fibromyalgia (Bennett et al., 2009; Herr and Garand, 2001; McCormack et al., 1988). Other tools used to assess pain include the Short Form 36-Item Questionnaire (SF-36) of the Medical Outcomes Study (MOS) and Patient-Reported Outcomes Measurement Information System (PROMIS) instruments for pain inter-
ference and pain behavior (Boomershine, 2012; Komaroff et al., 1996).5 Preliminary data from the CDC Multi-Site Clinical Study of CFS were used to compare pain interference scores as measured by PROMIS and the BPI, revealing a 0.75 correlation. PROMIS instruments are used primarily for research, while the BPI is an assessment tool more readily accessible to clinicians (Unger, 2013). Several instruments that were validated for diagnosing ME/CFS—the DePaul Symptom Questionnaire, CDC Symptom Inventory (SI), and CFS Questionnaire—include measures of pain (Jason et al., 2012).
Evidence for Pain in ME/CFS
The literature does not provide evidence on the cause, nature, and relevance of pain in ME/CFS as compared with normal controls, other subjectively defined conditions such as fibromyalgia, or other chronic pain conditions. Although some studies have investigated pain in ME/CFS and fibromyalgia, the ability to make comparisons across studies is limited by the use of varying case definitions. It is also challenging to elicit the characteristics and severity of pain attributable solely to ME/CFS in patients with comorbid fibromyalgia and ME/CFS. Nonetheless, the severity and frequency of pain have been studied in ME/CFS patients.
Pain Symptoms in Different Diagnostic Categories
Although pain symptoms are listed in all recent diagnostic criteria for ME/CFS, some of the diagnostic criteria identify patients with more frequent and more debilitating pain symptoms. In various studies, persons fulfilling the CCC,6 a revised definition of ME,7 and ME-ICC were found to have significantly greater disability due to bodily pain (as measured by the bodily pain subscale of the SF-36) than those fulfilling the Fukuda definition (Brown et al., 2013; Jason et al., 2012, 2013a, 2014b). People fulfilling the CCC and revised definition of ME also reported significantly worse (in terms of frequency and severity) headaches, chest pain, abdomen pain, eye
____________________
5 PROMIS “is a system of highly reliable, precise measures of patient-reported health status for physical, mental, and social well-being. PROMIS tools measure what patients are able to do and how they feel by asking questions. PROMIS’[s] measures can be used as primary or secondary endpoints in clinical studies of the effectiveness of treatment” (http://www.nihpromis.org/about/abouthome [accessed January 14, 2015]).
6 In one study, frequency and severity were specified according to the Revised CCC (Jason et al., 2013a). In another, for key symptoms, individuals had to have a frequency score of 2 or higher and symptoms had to be moderate or severe (rated at 50 or higher) as reported on the CFS Questionnaire (Jason et al., 2012).
7 Jason and colleagues (2012) created a revised case definition based on past case definitions, requiring PEM, neurological manifestation, and autonomic dysfunction.
pain, and tender/sore lymph nodes than those fulfilling the Fukuda definition (Jason et al., 2012). Those fulfilling the ME-ICC reported significantly worse headaches, chest pain, eye pain, muscle pain, pain in multiple joints, and tender/sore lymph nodes than those fulfilling the Fukuda definition (Brown et al., 2013; Jason et al., 2014b). In one study, those fulfilling the ME-ICC also experienced significantly worse abdomen/stomach pain and bloating than those fulfilling the Fukuda definition (Jason et al., 2014b).
Pain Symptoms in Other Fatigue Conditions
Chest pain and lymph node pain are more frequent in persons fulfilling the CCC than in those with chronic fatigue explained by psychiatric illness. Persons fulfilling the Fukuda definition experience more abdominal pain than those with chronic fatigue explained by psychiatric illness (Jason et al., 2004).
Pain Symptoms in Other Chronic Pain Conditions
Some evidence indicates that pain experienced by those with ME/CFS is different from chronic pain experienced in other conditions. For example, severity of pain has been associated with impaired cognitive performance in other chronic pain conditions such as fibromyalgia, chronic whiplash-associated disorders, and chronic low back pain (Antepohl et al., 2003; Park et al., 2001; Weiner et al., 2006). Pain levels in ME/CFS are similar to pain levels in other chronic pain conditions, however, pain severity is not correlated with cognitive impairment in ME/CFS, a finding suggesting that the pain in ME/CFS may be unique (Ickmans et al., 2013).
ME/CFS and Fibromyalgia
Population-based studies predict the prevalence of fibromyalgia to be 3-5 percent and the prevalence of ME/CFS to be 0.5-1 percent. The overlap of fibromyalgia and ME/CFS ranges from 20 to 70 percent across a number of studies using the 1990 ACR fibromyalgia criteria (Meeus et al., 2007). The revised 2010 ACR fibromyalgia criteria—which exclude the tender point exam; score pain distribution numerically; and include severity scores for fatigue, brain fog, unrefreshing sleep, and multisystem complaints—may greatly increase the overlap between ME/CFS and fibromyalgia (Wolfe et al., 2010).
The reason for this overlap of ME/CFS with the ACR fibromyalgia criteria has not been rigorously discussed. Some researchers suggested that the reason for the overlap has to do with both syndromes being a manifestation of somatic amplification (Clauw, 2014). There is a comparatively
large evidence base supporting fibromyalgia as a process involving heightened central sensitivity, hyperalgesia, and sensory amplification, causing widespread pain and contributing to other associated symptoms such as fatigue, brain fog, and unrefreshing sleep (Clauw, 2014). Other researchers used the existence of separate case definitions to ask the empirical question of whether the two syndromes are the same or different. Unfortunately, most studies on the pathophysiology of ME/CFS do not evaluate ME/CFS subjects with fibromyalgia separately from those unaffected by fibromyalgia, nor do they compare them with patients with fibromyalgia who do not meet ME/CFS criteria (Abbi and Natelson, 2013). This is primarily because ME/CFS studies do not assess the bodily distribution of pain and presence of hyperalgesia as defined by the tender point exam, and fibromyalgia studies do not use published case definitions to identify those fibromyalgia patients with comorbid ME/CFS (Reeves et al., 2007; Reyes et al., 2003; White et al., 1999; Wolfe et al., 1995).
Studies that do examine the differences between ME/CFS + fibromyalgia and ME/CFS alone suggest that the addition of widespread pain with tenderness to the usual diagnostic symptoms of ME/CFS leads to qualitative differences between the two groups. The first of these differences relates to spinal fluid Substance P, which has been shown to be elevated in fibromyalgia but not in ME/CFS (Evengård et al., 1998; Russell et al., 1994). While these studies were done on separate groups of ME/CFS or fibromyalgia patients, more recent studies carefully identified patients with ME/CFS alone, fibromyalgia alone, or ME/CFS with coexisting fibromyalgia. The results of these studies suggest that the pathophysiology of each group may present differently. Naschitz and colleagues (2008) used tilt table testing to develop a “hemodynamic instability score” based on blood pressure and heart rate changes, which enabled them to differentiate patients with ME/CFS alone from those with fibromyalgia alone. Natelson and colleagues conducted a number of studies comparing patients with ME/CFS alone and those with ME/CFS and fibromyalgia (Ciccone and Natelson, 2003; Cook et al., 2005, 2006; Natelson, 2010). Except for deficits in cognitive function, which were more prominent in the ME/CFS-only group (Cook et al., 2005), having comorbid fibromyalgia was an illness multiplier for patients with ME/CFS: self-ratings of severity of muscle and joint pain were higher and physical function on the SF-36 lower in the ME/CFS + fibromyalgia group; in addition, the rate of lifetime major depressive disorder was 52 percent in the ME/CFS + fibromyalgia group, approximately twice that seen in the ME/CFS-only group (Ciccone and Natelson, 2003). While no differences between ME/CFS patients and controls were found in any cardiopulmonary variable studied during a maximal stress test, patients with ME/CFS + fibromyalgia, but not those with ME/CFS only, perceived the exercise to be both more painful and more effortful compared with controls (Cook et al., 2006).
Summary
Sufficient evidence shows that pain is common in ME/CFS, and its presentation supports the diagnosis. However, while pain worsens ME/CFS when present, there is no conclusive evidence that the pain experienced by ME/CFS patients can be distinguished from that experienced by healthy people or those with other illnesses. Further, pain may be experienced in many areas, and while comprehensively assessing a patient’s pain symptoms is a challenging task, it is not specific to ME/CFS.
Conclusion: The committee elected not to include pain as a required element of its recommended diagnostic criteria for ME/CFS.
Description of Immune Impairment in ME/CFS
Symptoms related to inflammation are reported frequently by ME/CFS patients. When attempting to convey their illness experience to healthy persons, many patients describe it as similar to a perpetual flu-like state (Maupin, 2014). Patients also report persistent or recurrent sore throats, tender/swollen cervical and/or axillary lymph nodes, muscle pain, achy joints without swelling or redness, headaches, chills, “feverishness” (but not necessarily meeting objective criteria for fever), and new or worsened sensitivities to certain substances (e.g., foods, odors, medications) (FDA, 2013). Interestingly, “susceptibility to infections” was among the most common written-in symptoms submitted to the Food and Drug Administration during its April 2013 Drug Development Workshop for ME/CFS (FDA, 2013). These symptoms can fluctuate and may be unmasked or exacerbated with physical or cognitive activity as part of the constellation of symptoms associated with the individual patient’s PEM (see the section on PEM in Chapter 4).
All of the case definitions evaluated in this report include some inflammatory symptoms and/or signs; however, whether such symptoms or signs are mandatory and which are included varies among the definitions. The clinical case definition most commonly used in the United States, the Fukuda definition of 1994, includes five symptoms that are sometimes associated with systemic inflammation (tender cervical/axillary lymph nodes, joint pain, muscle pain, headache, and sore throat), but no specific symptom or group of symptoms is required (Fukuda et al., 1994). It is not clear, moreover, whether these symptoms have an infectious or inflammatory etiology in ME/CFS.
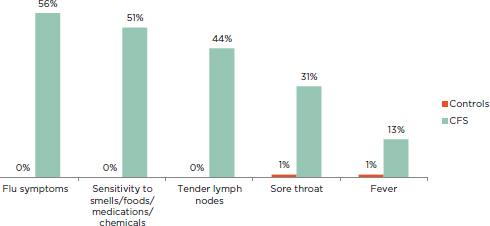
FIGURE 5-2 Percentage of ME/CFS patients and healthy controls reporting immune-related symptoms of at least moderate severity that occurred at least half of the time for the past 6 months.
NOTE: All patients fulfilled the Fukuda definition for CFS.
SOURCE: Jason et al., 2013b.
Although the prevalence of inflammatory symptoms reported in the literature ranges widely across studies that vary in time period, location, and case definition, it is evident that rates of this category of symptoms are elevated in ME/CFS patients: sore throat (19 to 84 percent), muscle pain (63 to 95 percent), joint pain (55 to 85 percent), tender/swollen axillary or cervical lymph nodes (23 to 76 percent), fever and/or chills (13 to 64 percent), flu-like feelings (56 to 81 percent), and new/worsened sensitivities (51 to 55 percent) (De Becker et al., 2001; Janal et al., 2006; Jason et al., 2013b; Kerr et al., 2010; Nacul et al., 2011; Naess et al., 2010; Solomon and Reeves, 2004). Jason and colleagues (2013b) showed that the presence of tender lymph nodes and sore throat was much greater in ME/CFS patients than in healthy controls (see Figure 5-2).
Assessment of Immune Impairment in ME/CFS
The evidence reviewed in this section pertains to the research setting because the usefulness of the tests employed in these studies has not yet been proved in the clinical setting. Alterations of natural killer (NK) cell count and function and perturbations in cytokine production have been the biomarkers studied most extensively because they have shown the most promising results.
NK cells are a part of the innate immune system and play an important role in preventing latent viruses from reactivating as well as in tumor surveillance. NK cell count is ascertained primarily by assay based on flow cytometry. Cytokine levels and function are most commonly measured through examination of plasma distribution via blood sample, but they may also be measured in cell supernatants following mitogenic stimulation of cells (Brenu et al., 2011, 2012b, 2014) or through various genomic methods (Carlo-Stella et al., 2006; Nakamura et al., 2013; Zhang et al., 2011). Most studies have not looked at cytokine function in the sense of measuring how well they function in their role as messengers (e.g., activation of the Janus kinase/signal transducer and activator of transcription [JAK-STAT] system). Other biomarkers with less consistent findings include humoral immunity and cellular cytotoxicity (Fischer et al., 2014). Overall, there is a large amount of variability across study protocols and laboratories in the methodology used for assessing biomarkers of immunology, which may contribute to inconsistency in the literature (Lyall et al., 2003).
Evidence for Immune Impairment in ME/CFS
The committee searched the literature for evidence with which to respond to one main question with respect to immunology: Is there a distinguishing feature of immune profile or function in ME/CFS? The methodology for the committee’s review of the literature is described in Chapter 1.
Impaired Immune Function
One of the most consistent findings in ME/CFS subjects is poor NK cell function. Using K562 cells as target cells, 16 of 17 studies reviewed found poor function in subjects compared with healthy controls. However, this finding should be interpreted with caution as even the strongest of these studies are subject to methodological limitations discussed at the beginning of Chapter 4. Furthermore, it is unclear from the description of the methodology of some of the studies whether multiple studies included the same subjects. The largest study compared 176 ME/CFS subjects with 230 healthy controls and found a significant group effect of poorer NK cell function in the ME/CFS cohort (Fletcher et al., 2010). Curriu and colleagues (2013) showed that there were differences in mean cytotoxicity between ME/CFS subjects and healthy controls, but the range was the same. Brenu and colleagues (2012b) studied 65 ME/CFS patients and 21 matched controls in a longitudinal study of three time points over 12 months and found significant deficits in NK cytotoxic activity in the patient group at each time point using peripheral blood mononuclear cells (PBMCs) and a
flow cytometric measure of killing. Caligiuri and colleagues (1987) demonstrated reduced cytotoxic activity of ME/CFS NK cells to K562 targets. On the other hand, one study with 26 ME/CFS patients and 50 controls failed to demonstrate impaired NK cell function in the ME/CFS patients using a K562 chromium (Cr) release assay of peripheral blood lymphocytes (PBLs) (Mawle et al., 1997). The authors of this study do not report NK cell counts or CD3-CD56+, but as described, NK numbers generally are not low in ME/CFS.
Low NK cytotoxicity is not specific to ME/CFS. It is also reported to be present in patients with rheumatoid arthritis, cancer, and endometriosis (Meeus et al., 2009; Oosterlynck et al., 1991; Richter et al., 2010). It is present as well in healthy individuals who are older, smokers, psychologically stressed, depressed, physically deconditioned, or sleep deprived (Fondell et al., 2011; Whiteside and Friberg, 1998; Zeidel et al., 2002).
A few studies found a correlation between the severity of NK cell functional impairment and the severity of disease in ME/CFS patients (Lutgendorf et al., 1995; Ojo-Amaize et al., 1994; Siegel et al., 2006). Others looked at mechanisms of cellular dysfunction in ME/CFS and identified abnormalities in early activation markers (Mihaylova et al., 2007) and perforin and granzyme concentration (Maher et al., 2005), as well as in the genes that regulate these cellular functions (Brenu et al., 2011, 2012a). However, no replication studies have been published.
There also are studies enumerating the numbers of NK cells in ME/CFS patients, sometimes employing different identifying markers. NK cell count shows substantial heterogeneity in these patients, and there are no consistent findings (Barker et al., 1994; Brenu et al., 2010, 2011, 2012b; Caligiuri et al., 1987; Curriu et al., 2013; Fletcher et al., 2010; Gupta and Vayuvegula, 1991; Henderson, 2014; Klimas et al., 1990; Levine et al., 1998; Maher et al., 2005; Mawle et al., 1997; Natelson et al., 1998; Peakman et al., 1997; Stewart et al., 2003; Straus et al., 1993; Tirelli et al., 1994).
Immune Activation
Immune activation has been studied using a variety of methods. The most consistent results are reported on pro-inflammatory cytokine production.
Cytokine abnormalities have been hypothesized to play a role in the pathogenesis of ME/CFS, although findings to support this idea are varied and inconsistent. Research to date has addressed levels of pro-inflammatory cytokines in ME/CFS patients compared with healthy controls.
The majority of studies yielded no significant findings (Jammes et al., 2009; Nakamura et al., 2010; Neu et al., 2014; White et al., 2010), including one study of monozygotic twins discordant for disease that showed no
difference in pro-inflammatory cytokine levels between the affected and unaffected twins (Vollmer-Conna et al., 2007). However, several studies found elevated pro-inflammatory cytokine levels, particularly for TNF-α, in ME/CFS patients (Brenu et al., 2011, 2012b, 2014; Broderick et al., 2012; Maes et al., 2013; Neu et al., 2014).
Sample sizes of individual studies generally have been small, and only a few types of pro-inflammatory cytokines have been measured in multiple studies. Two studies used subgrouping to identify differences in cytokine levels between those with low and high prevalence, duration, or severity of PEM (Maes et al., 2012) or after physical exertion (White et al., 2010). In one study, postexercise increases in both pro-inflammatory and anti-inflammatory cytokines were associated with more severe symptom flares after exertion (White et al., 2010).
Fewer peer-reviewed papers report on functional studies of other aspects of immune function, and these studies yielded less consistent findings. There are reports of Immunoglobulin G (IgG) subclass deficiencies, diminished T and B cell response to mitogens, and abnormalities of neutrophil and macrophage functions. However, the subjects studied were limited to small series, and there are no replication studies (Fletcher et al., 2009; Lattie et al., 2012; Nakamura et al., 2013; Neu et al., 2014).
Emerging Areas
Autoimmunity ME/CFS has been reported to be associated with autoimmune disorders such as hypothyroidism and Sjogren’s syndrome, raising the question of whether the disease may have an autoimmune component (Gaber and Oo, 2013; Nishikai et al., 1996; Sirois and Natelson, 2001). Antibodies frequently seen in other systemic autoimmune/rheumatic diseases are reported inconsistently in ME/CFS in the literature published to date. The prevalence of antinuclear antibodies in subjects varies widely (from 7 to 68 percent); antibody titers tend to be on the low side (less than 1:160); and no antigen (e.g., dsDNA, SS-A, Scl-70) has been identified as a unique marker (Buchwald and Komaroff, 1991; Konstantinov et al., 1996; Op De Beéck et al., 2012; Skowera et al., 2002; Tanaka et al., 2003; vonMikecz et al., 1997).
Three separate studies do point to one unusual antigen—the nuclear envelope protein lamin B, which has been associated with primary biliary cirrhosis. ME/CFS subjects with antibodies to this protein may be more likely to be affected by hypersomnia and cognitive difficulties (Konstantinov et al., 1996; Nishikai et al., 2001; vonMikecz et al., 1997); however, these observations have not been replicated in the ensuing years. Studies also found wide variation in autoantibodies to neural antigens (9 to 62 percent) (Klein and Berg, 1995; Ortega-Hernandez et al., 2009; Vernon and Reeves,
2005; vonMikecz et al., 1997) and cellular membranes (4 to 95 percent) (Hokama et al., 2009; Klein and Berg, 1995; Maes et al., 2006; Ortega-Hernandez et al., 2009). In other studies, ME/CFS or postviral fatigue syndrome patients showed antibodies to smooth muscle (36 percent) (Behan et al., 1985), heat shock protein 60 (24 percent) (Elfaitouri et al., 2013), and endothelial antigens (30 percent) (Ortega-Hernandez et al., 2009). The only clinical trial targeting antibodies found moderate to marked clinical improvements in 10 of 15 subjects treated with rituximab, a B cell depleting antibody, and 2 of 15 placebo arm subjects at a single time point (Fluge et al., 2011). This, however, was a post hoc analysis as the trial failed to meet its primary endpoint. Currently, researchers in the United Kingdom and Norway are conducting further studies addressing this question (Edwards, 2013; Mella and Fluge, 2014).
Systems biology Several groups are looking at the immune endocrine/neuropeptide homeostatic balance in innovative ways that could lead to a better understanding of ME/CFS. Using genomic and proteomic techniques together with studies of immune function, immune activation, and chemokine/cytokine expression represents a “big picture” approach to a complex illness, although no significant risk alleles or disease signatures have yet been identified (Presson et al., 2009; Smylie et al., 2013).
Summary
The committee’s literature review yielded data demonstrating poor NK cell cytotoxicity (NK cell function, not number) that correlates with illness severity in ME/CFS patients and could serve as a biomarker for the severity of the disease, although it is not specific to ME/CFS. More research is needed to address cytokine abnormalities and their potential use as biomarkers of possibly distinct subgroups of ME/CFS.
Conclusion: Sufficient evidence supports the finding of immune dysfunction in ME/CFS.
Description of Neuroendocrine Manifestations in ME/CFS
Linking a disease manifestation to a specific neuroendocrine abnormality is difficult. Neuroendocrine dysregulation or abnormalities may manifest with nonspecific signs and symptoms that present across multiple organ systems. Such manifestations as fatigue, achiness, weakness, sleep disturbances, and cognitive fog are nonspecific symptoms that may or may
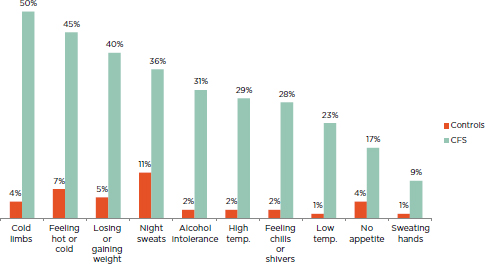
FIGURE 5-3 Percentage of ME/CFS patients and healthy controls reporting neuroendocrine manifestations of at least moderate severity that occurred at least half of the time for the past 6 months.
NOTE: All patients fulfilled the Fukuda definition for CFS.
SOURCE: Jason et al., 2013b.
not be caused by an underlying neuroendocrine abnormality. Further, if a neuroendocrine abnormality is contributing to these symptoms, it may be a secondary process.
The term and symptom category “neuroendocrine manifestations” appeared for the first time in the CCC. The Revised CCC retains the same group of symptoms, while the ME-ICC mentions only a few of these symptoms but classifies them under the “energy production/transportation impairments” category. According to Jason and colleagues (2013b), a greater percentage of ME/CFS patients compared to controls experience such symptoms as cold limbs, feeling hot or cold, losing or gaining weight, and night sweats compared with healthy controls (see Figure 5-3).
Assessment of Neuroendocrine Manifestations in ME/CFS
The complex multisystem nature of symptoms in ME/CFS patients has led researchers to explore central neural mechanisms such as dysregulation of the hypothalamic-pituitary-adrenal (HPA) and HP-growth hormone axes and the 5-hydroxytriptamine (5-HT) serotoninergic system (Fischer et al., 2014).
Numerous studies have focused on the HPA axis and measured cortisol concentrations in blood, saliva, and urine to assess the awakening, diurnal, and evening fluctuations in ME/CFS patients (Tomas et al., 2013). Researchers also have studied the role of the HP-growth hormone axis in ME/CFS and measured levels of growth hormone, insulin-like growth factor 1 (IGF-1), IGF-2, and IGF-binding protein 1 (IGFBP-1) (Allain et al., 1997). Various methods have been used to assess the HPA axis indirectly, including standardized stressors such as insulin-induced hypoglycemia; psychological duress or vigorous exercise; and administration of 5-HT (serotonin) precursors or receptor agonists, opioid antagonists, corticotropin-releasing hormone (CRH), or arginine vasopressin (AVP) (Tomas et al., 2013). For assessment of the 5-HT system, studies have measured plasma concentrations of 5-HT precursors (total and free tryptophan) in response to pharmacological challenge (i.e., d-fenfluramine) (Georgiades et al., 2003). A few other studies have examined both a serotonin transporter (5-HTT) gene promoter polymorphism and the density of serotonin transporters in the brain using positron emission tomography (PET) (Yamamoto et al., 2004).
Evidence for Neuroendocrine Manifestations in ME/CFS
Evaluation of the neuroendocrine literature was difficult for multiple reasons. First, there are myriad possible neuroendocrine abnormalities with different underlying pathophysiology. The physiology is sometimes complex, and knowledge about some of the physiology is evolving. For example, hormones of the neuroendocrine system generally are released in a pulsatile, cyclic, or feedback (negative and positive) responsive manner and in minutely detectable amounts. They may act on or be produced in several areas of the brain and be stored and released in others. Complex stimuli, including most of the “stressors” experienced by humans, are involved in their modulation and release, including peripheral and central signaling and sensory, autonomic, sleep, and emotional triggers, along with the positive and negative feedback of other endocrine signaling (Longo et al., 2012).
Second, studies addressing neuroendocrine manifestations have been heterogeneous and had multiple limitations, as described in Chapter 4. Most studies involved participants diagnosed with ME/CFS using the Fukuda definition, and they compared parameters in ME/CFS and healthy participants who were matched on age and gender. Few studies involved ME/CFS participants recruited from community samples or had comparison groups including patients with comorbidities. Studies evaluated multiple different stressors and used various measures to assess manifestations and outcomes. Some manifestations, such as fatigue severity and functional limitations, were rarely assessed. Cross-sectional comparisons that could not tease out cause-and-effect relationships were common. Some studies were small or
exploratory and involved multiple statistical comparisons. Others failed to control for such important factors as time of day of measurement or sleep quality on the night before neuroendocrine evaluation.
The committee conducted a literature search with emphasis on the HPA axis to explore the following questions: (1) Are particular neuroendocrine abnormalities or manifestations pathognomonic for ME/CFS? and (2) How do neuroendocrine manifestations experienced by adults diagnosed with ME/CFS differ from those experienced by adults diagnosed with other chronic illnesses?
Hypothalamic-Pituitary-Adrenal Axis
The question of the contribution of low cortisol to ME/CFS was galvanized by a 1991 study demonstrating reduced 24-hour urinary cortisol in ME/CFS patients (Demitrack et al., 1991). Numerous studies found reduced overnight cortisol or 24-hour cortisol in patients compared with healthy controls (Cevik et al., 2004; Cleare et al., 2001; Crofford et al., 2004; Gur et al., 2004; Jerjes et al., 2007; Nater et al., 2008; Roberts et al., 2004), including three metanalyses (Powell et al., 2013; Rosmalen et al., 2010; Tak et al., 2011); however, many studies yielded contradictory or normal results (Di Giorgio et al., 2005; Jerjes et al., 2006; Markopoulou et al., 2010). Several studies found that participants with lower cortisol levels prior to treatment did not respond well to cognitive-behavioral therapy (Jason et al., 2007; Roberts et al., 2010). Lattie and colleagues (2013) reported that patients with worse PEM had lower awakening cortisol and a flatter diurnal curve. The committee could discern no single explanation for these findings.
There is some evidence of low CRH and AVP and blunted adrenocorticotropin hormone (ACTH) response in ME/CFS (Altemus et al., 2001; Bakheit et al., 1993; Di Giorgio et al., 2005; Gaab et al., 2002, 2005; Ottenweller et al., 2001; Parker et al., 2001; Racciatti et al., 2001; Scott et al., 1998a,b,c); however, there have been negative studies on this question as well (Gaab et al., 2003; Papadopoulos et al., 2009; Van Den Eede et al., 2008). CDC studied ME/CFS subjects extensively during a 3-day inpatient stay in a population-based study and found only decreased heart rate variability during sleep and low aldosterone levels (Boneva et al., 2007).
The results of physiological studies suggesting reduced HPA function and reduced blood volume producing orthostatic intolerance have led to several therapeutic studies using corticosteroids and mineralocorticoids. The committee reviewed two placebo-controlled studies using hydrocortisone (Cleare et al., 1999; McKenzie et al., 1998). The first used hydrocortisone 20-30 mg in the morning and 5 mg at 2 PM for 12 weeks. Of numerous outcome measures, only one showed small but significant improvement in the treated group. In addition, the authors found demonstrable evidence of
adrenal cortical suppression in 12 of 33 hydrocortisone-treated participants and none in controls. They concluded that the risk of adrenal suppression negated the value of reported improvement (McKenzie et al., 1998). The second, much shorter study entailed administering hydrocortisone 5 or 10 mg daily for 1 month and placebo for 1 month in a crossover design. While on treatment, patients showed mild but statistically significant improvement in fatigue and disability compared with the placebo period without evidence of adrenal suppression (Cleare et al., 1999). Anecdotal discussion among clinicians during the open meetings held for this study indicated a lack of sustained effect using this treatment approach and concerns regarding the risks of long-term adrenal suppression. No further data on the effects of treating with hydrocortisone alone have appeared since 1999. A later study used both 5 mg of hydrocortisone and 50 mcg of fludrocortisone per day. These researchers found no effect on fatigue (Blockmans et al., 2003). Two studies that looked at treating with fludrocortisone alone also were negative (Peterson et al., 1998; Rowe et al., 2001).
Serotonin
Several studies found defective serotonergic signaling in the brain at or above the hypothalamus in ME/CFS patients (Bearn et al., 1995; Dinan et al., 1997; Sharpe et al., 1996). Higher prevalence of a 5-HTT gene promoter polymorphism (Narita et al., 2003) and reduced density of 5-HTT in the anterior cingulate (Yamamoto et al., 2004) were demonstrated in ME/CFS patients compared with healthy controls. Positive autoimmune activity was documented against 5-HT in a significant number of ME/CFS patients compared with chronic fatigue patients and healthy controls (Maes et al., 2013).
Growth Hormone
One study found normal levels of growth hormone or growth hormone responses in ME/CFS patients (Cleare et al., 2000), while others found reduced growth hormone (Allain et al., 1997; Bearn et al., 1995) and reduced nocturnal secretion of growth hormone (Berwaerts et al., 1998; Moorkens et al., 2000). Conflicting results were obtained in the growth hormone response to insulin-induced hypoglycemia, with responses being either reduced (Allain et al., 1997; Moorkens et al., 2000) or normal (Berwaerts et al., 1998). Various assessments of IGF-1 showed no consistent differences between ME/CFS patients and controls (Cleare et al., 2000; The et al., 2007).
Renin-aldosterone
Studies found low blood volume (orthostatic intolerance, small heart, and low cardiac index) and low aldosterone in ME/CFS patients (Miwa and Fujita, 2008, 2009, 2011, 2014). Renin is produced by the kidneys in response to low aldosterone and low blood volume. Abnormal function of the renin-angiotensin system, the autonomic nervous system, or the central nervous system could play a role (see the discussion of orthostatic intolerance and autonomic dysfunction in Chapter 4).
Summary
Patients with ME/CFS may have relatively reduced overnight cortisol, 24-hour urinary cortisol, CRH and/or AVP, and ACTH levels compared with healthy controls. The current preponderance of evidence points to normal adrenal function in such patients and suggests a secondary (central) rather than a primary (adrenal) cause of reduced but not absent cortisol production at the level of the pituitary, the hypothalamus, or higher. Patients with ME/CFS may have defective serotonergic signaling in the brain, localized to the level of the hypothalamus or higher, resulting in downstream dysregulation that may play a role in ME/CFS. The exact mechanism is not clear. Also, current evidence indicates that the growth hormone axis is intact in ME/CFS patients. If IGF-1 abnormalities are present, there may be many other causes (Brugts et al., 2009). ME/CFS patients may hyporeact to stressors, but that phenomenon may not be specific to a particular neurotransmitter or endocrine stimuli.
Conclusion: Evidence is insufficient to conclude that any specific neuroendocrine abnormalities cause ME/CFS, or that any such abnormalities either uniformly differentiate those with ME/CFS from individuals with other illnesses or distinguish a subset of ME/CSF patients.
Description of Infection in ME/CFS
Reports of several infectious disease outbreaks possibly leading to ME/CFS aroused early suspicion of an infectious etiology or an association of infection with the initiation of the illness (Acheson, 1955; Albrecht et al., 1964; Briggs and Levine, 1994; Buchwald et al., 1989; Clement et al., 1958; Daikos et al., 1959; Dillon et al., 1974; Klajman et al., 1960; Parish, 1978; Shelokov et al., 1957; Strickland et al., 2001). The observation that
ME/CFS cases commonly presented with an acute infection-like onset supported this belief (Komaroff, 1988), and an acute onset presentation was reported to be more common in ME/CFS patients than in those suffering from chronic fatigue only (Evengård et al., 2003). Yet while 25 to 80 percent of ME/CFS patients describe an infectious-like onset at the beginning of their illness (Ciccone and Natelson, 2003; Evengård et al., 2003; Naess et al., 2010), population-based studies using the Fukuda definition showed a predominance of gradual onset over an obvious acute infectious onset (Reyes et al., 2003). The variance in these rates likely is due to differences in recruitment of subjects, as well as varying interpretations of “acute,” “infectious,” and “gradual” onset.
While none of the case definitions discussed in Chapter 3 includes an infection-related onset as part of its main criteria, the CCC specifically uses this information to support the diagnosis of ME/CFS in patients who do not fulfill the sleep and pain criteria (Carruthers et al., 2003). Moreover, prospective studies of laboratory-documented acute Epstein-Barr virus (EBV)associated glandular fever, non-EBV-associated glandular fever, Ross River virus, Giardia duodenalis enteritis, parvovirus B19, and Q fever infections demonstrated that 1 to 22 percent of patients go on to develop ME/CFS (Hickie et al., 2006; Kerr et al., 2002; Naess et al., 2012; Seishima et al., 2008; White et al., 1998). One of the few pediatric studies on infection and ME/CFS found a rate within this range after EBV-associated infectious mononucleosis: 13 percent after 6 months, 7 percent after 12 months, and 4 percent after 24 months (see the section on infection in Chapter 6) (Katz et al., 2009). Additionally, postviral onset differs from slow-onset illness, showing higher chronic immune activation markers years after onset (Porter et al., 2010).
Assessment of Infection in ME/CFS
Researchers have made numerous attempts to determine whether an infectious agent, particularly a virus, plays a role in the ongoing pathogenesis of ME/CFS. However, detecting active, pathological infections or differentiating an active infection or reactivation from a latent infection is highly challenging. Several studies tested patients with ME/CFS for the presence of serum antibody levels in response to viruses such as EBV, human herpes virus-6 (HHV-6), and cytomegalovirus (CMV) and found increased levels in a subset of patients (Ablashi et al., 2000; Gascon et al., 2006; Kawai and Kawai, 1992; Krueger et al., 1988; Lerner et al., 2002, 2004). However, these viruses are highly prevalent and associated with other diseases, and antibodies to them may be present even in asymptomatic people (Sumaya, 1991). An increase in such antibodies also may reflect a reactivation of the virus in people with an altered immune system. Moreover, the find-
ings of increased serum antibody titers in ME/CFS patients have not been consistent, which may be due in part to the different serological tests used (Hellinger et al., 1988; Ilaria et al., 1995; Levine et al., 1992; Mawle et al., 1995; Reeves et al., 2000; Swanink et al., 1995; Whelton et al., 1992).
Prior studies also showed that peripheral blood samples may miss enterovirus, parvovirus, or herpes virus infections that continue to be present in, respectively, gut, brain, or heart tissues (Fotheringham et al., 2007; Halme et al., 2008; Kuhl et al., 2005; Yanai et al., 1997). Thus, ME/CFS researchers have looked for traces of infections elsewhere (Fremont et al., 2009; Ilaria et al., 1995). Enterovirus RNA assays have been performed on secretions, and immunohistochemistry has been used to look for enterovirus-specific antibodies or antigens in tissue (Chia and Chia, 2008; Chia et al., 2010). Newer techniques for detecting viruses, particularly when used in longitudinal therapeutic studies, may provide new insight into the etiology and treatment of some of these patients.
Because the immune system plays a vital role in the control of and response to pathogens, studies have been conducted to identify the presence of alterations to immune function in ME/CFS patients (Kerr and Tyrrell, 2003; Porter et al., 2010). The different markers tested to assess abnormalities in the immune function of ME/CFS patients were discussed earlier in the section of this chapter on immune impairment.
Evidence for Infection in ME/CFS
The committee searched the literature for evidence with which to address two main questions with respect to infection: (1) Is there an infectious agent that can precipitate ME/CFS? and (2) Is there evidence of an ongoing infection that plays a role in the disease? The description of the methodology used for the committee’s literature search can be found in Chapter 1.
Viral Infections
Numerous studies have assessed the possible association between viral infections and ME/CFS. However, EBV is the only viral infection showing some consistent findings as a possible trigger of ME/CFS in these studies.
Herpes virus The possibility that EBV infection can be a trigger for ME/CFS is suggested by results of some prospective studies (Fark, 1991; Hickie et al., 2006; Jason et al., 2014a; White et al., 1998). Several studies also found high titers of certain antibodies to EBV in ME/CFS patients, including viral capsid antigen (VCA) IgG, persistent titers of VCA Immunoglobulin M (IgM), or the persistence of early antigen IgG, whereas healthy individuals who were previously infected with EBV had only VCA IgG and nuclear
antigen IgG antibodies (Gascon et al., 2006; Kawai and Kawai, 1992; Lerner et al., 2004; Loebel et al., 2014; Manian, 1994; Natelson et al., 1990, 1994; Sairenji et al., 1995). Some studies, however, including a study of twins discordant for disease, were unable to find this difference (Buchwald et al., 1996; Fremont et al., 2009; Hellinger et al., 1988; Koelle et al., 2002; Levine et al., 1992; Mawle et al., 1995; Swanink et al., 1995). The severity of the acute illness may be a predictor of ME/CFS. Both a population-based study (Jason et al., 2014a) and a prospective study (Hickie et al., 2006) of ME/CFS patients after mononucleosis infection showed severity of illness, as measured by baseline autonomic symptoms and days spent in bed, to be a significant predictor of ME/CFS 6 months after the infection.
Evidence concerning the role of HHV-6 virus in ME/CFS is less consistent. Several studies found that ME/CFS patients have a significantly higher rate of HHV-6 antibodies (either IgG or IgM) compared with healthy controls (Ablashi et al., 2000; Patnaik et al., 1995; Sairenji et al., 1995; Yalcin et al., 1994). A few studies identified HHV-6 in human peripheral blood lymphocytes, recovered by culture and confirmed by immunofluorescence assay (IFA) and by polymerase chain reaction (PCR), more frequently in ME/CFS patients than in controls (Buchwald et al., 1992; Yalcin et al., 1994; Zorzenon, 1996). However, other research groups were unable to find any significant differences (Burbelo et al., 2012; Cameron et al., 2010; Enbom et al., 2000; Fremont et al., 2009).
A number of studies found release of early encoded viral proteins into the circulation of patients with ME/CFS, a phenomenon that some have suggested represents abortive lytic replication as whole virions are not present (Beqaj et al., 2008; Glaser et al., 2005, 2006; Jones et al., 1988; Lerner and Beqaj, 2011, 2012; Lerner et al., 2002, 2012; Loebel et al., 2014; Natelson et al., 1994; Patnaik et al., 1995). The clinical significance of unusual antibody profiles and incomplete viral replication in ME/CFS remains unclear.
Enterovirus A few studies focused on the role of persistent enterovirus infection in ME/CFS (Chia et al., 2010; Galbraith et al., 1995; Lane et al., 2003). Chia and Chia (2008) showed that in a subset of ME/CFS patients who reported significant gastrointestinal complaints, the prevalence of enterovirus infection as demonstrated in stomach biopsy samples was significantly higher compared with control subjects. Other investigators failed to reproduce an increased incidence of enterovirus infection in ME/CFS patients (Lindh et al., 1996).
Other viruses A few studies showed that ME/CFS may develop after an infection with parvovirus B19 (Fremont et al., 2009; Kerr et al., 2002; Seishima et al., 2008) and Ross River virus (Hickie et al., 2006). There is
insufficient evidence for an association between ME/CFS and various other viral infections, such as bornavirus (Evengård et al., 1999; Kitani et al., 1996; Li et al., 2003), retrovirus (Heneine et al., 1994; Honda et al., 1993; Khan et al., 1993), and HHV-7 (Fremont et al., 2009; Levine et al., 2001).
Response to treatment Some have argued that antiviral medication helps a subset of ME/CFS patients (Watt et al., 2012). One double-blind, placebo-controlled trial showed symptom improvement after 6 months in patients with elevated IgG antibody titers against EBV and HHV-6 following treatment with valganciclovir. There were also statistically significant changes in monocyte and cytokine levels, suggesting that immunomodulation may have been a factor in their improvement. However, the number of patients studied was small (N = 30), there were no differences in viral antibody titers between the two arms, and the patients were followed for only 9 months (Montoya et al., 2013). A prospective review of 106 ME/CFS patients with elevated serum antibody titers to EBV, CMV, or HHV-6 showed that 75 percent responded to long-term treatment with valacyclovir and/or valganciclovir (mean duration = 2.4 years). A patient was categorized as a responder if the Energy Index Point Score effect was greater than or equal to 1 (Lerner et al., 2010). A single-blind, placebo-controlled trial found a significant increase in NK cell activity in ME/CFS patients following treatment with isoprinosine (Diaz-Mitoma et al., 2003). However, another double-blind, placebo-controlled trial of ME/CFS patients with elevated antibodies to EBV failed to show a difference in clinical improvement between acyclovir-treated participants and placebo controls at 37 days follow-up (Straus et al., 1988). This study included a small number of patients (N = 27) and did not assess immune parameters.
Other Infections
The evidence on bacterial infection as a possible trigger of ME/CFS is limited mainly to Q-fever and Chlamydia pneumoniae (Nicolson et al., 2003; Wildman et al., 2002). A case-control study in an endemic area found a higher rate of ME/CFS in Q-fever cases (5 years postinfection) than in healthy controls (42 versus 26 percent), and ME/CFS occurred more often in individuals with more severe symptoms (Ayres et al., 1998). Further evidence of Q-fever as a trigger for ME/CFS is limited to case reports (Ledina et al., 2007). A cross-sectional study showed no increased prevalence of ME/CFS in Q-fever patients compared with healthy controls (Strauss et al., 2012). These findings are in contrast to the results of a prospective study that found that some patients would develop ME/CFS after a laboratory-confirmed Q-fever infection (Hickie et al., 2006). Evidence is unconvincing regarding Chlamydia pneumoniae infection as a possible trigger for ME/
CFS (Chia and Chia, 1999). Large interventional trials have not been performed; thus, data on response to antibacterial therapy are retrospective or limited to case reports (Bottero, 2000; Frykholm, 2009; Iwakami et al., 2005; Jackson et al., 2013). The committee’s literature review yielded no studies of posttreatment Lyme disease syndrome and ME/CFS; however, the two illnesses share some symptoms, and patients with Lyme disease often are identified as a subgroup among ME/CFS patients in specialty practices (Schutzer et al., 2011).
Literature on the association between ME/CFS and parasitic infection is limited to a few studies. Among a cohort of 1,262 laboratory-confirmed cases of Giardia duodenalis, 96 patients were diagnosed with long-lasting postinfectious fatigue; of those, 58 were diagnosed with ME/CFS (Naess et al., 2012). The data for a fungal etiology for ME/CFS are not convincing (Cater, 1995).
Summary
The literature indicates a possible relationship between EBV and ME/CFS. The evidence suggests that ME/CFS can be triggered by EBV infection, but there is insufficient evidence to conclude that all ME/CFS is caused by EBV or that ME/CFS is sustained by ongoing EBV infection. Improved diagnostic techniques may reveal as yet undetected associations. Further research in this area is warranted to determine whether patients in whom disease was triggered by EBV or patients with evidence of an ongoing abnormal response to EBV represent clinically significant subsets of ME/CFS.
There is insufficient evidence for an association between ME/CFS and bacterial, fungal, parasitic, and other viral infections. These infectious agents may, however, be comorbidities, and their presence may reflect the presence of problems with immune function in these patients. Future research may clarify the role of these infections in this illness.
Conclusion: There is sufficient evidence suggesting that ME/CFS follows infection with EBV and possibly other specific infections.
Aaron, L. A., L. M. Arguelles, S. Ashton, M. Belcourt, R. Herrell, J. Goldberg, W. R. Smith, and D. Buchwald. 2002. Health and functional status of twins with chronic regional and widespread pain. Journal of Rheumatology 29(11):2426-2434.
Abbi, B., and B. H. Natelson. 2013. Is chronic fatigue syndrome the same illness as fibromyalgia: Evaluating the “single syndrome” hypothesis. QJM: Monthly Journal of the Association of Physicians 106(1):3-9.
Ablashi, D. V., H. B. Eastman, C. B. Owen, M. M. Roman, J. Friedman, J. B. Zabriskie, D. L. Peterson, G. R. Pearson, and J. E. Whitman. 2000. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. Journal of Clinical Virology 16(3):179-191.
Acheson, E. D. 1955. Outbreak at the royal free. Lancet 266(6886):394-395.
Albrecht, R. M., V. L. Oliver, and D. C. Poskanzer. 1964. Epidemic neuromyasthenia. Outbreak in a convent in New York state. Journal of the American Medical Association 187:904-907.
Allain, T. J., J. A. Bearn, P. Coskeran, J. Jones, A. Checkley, J. Butler, S. Wessely, and J. P. Miell. 1997. Changes in growth hormone, insulin, insulinlike growth factors (IGFs), and IGF-binding protein-1 in chronic fatigue syndrome. Biological Psychiatry 41(5):567-573.
Altemus, M., J. K. Dale, D. Michelson, M. A. Demitrack, P. W. Gold, and S. E. Straus. 2001. Abnormalities in response to vasopressin infusion in chronic fatigue syndrome. Psychoneuroendocrinology 26(2):175-188.
Antepohl, W., L. Kiviloog, J. Andersson, and B. Gerdle. 2003. Cognitive impairment in patients with chronic whiplash-associated disorder—a matched control study. Neuro-Rehabilitation 18(4):307-315.
Ayres, J. G., N. Flint, E. G. Smith, W. S. Tunnicliffe, T. J. Fletcher, K. Hammond, D. Ward, and B. P. Marmion. 1998. Post-infection fatigue syndrome following Q fever. QJM: Monthly Journal of the Association of Physicians 91(2):105-123.
Bakheit, A. M., P. O. Behan, W. S. Watson, and J. J. Morton. 1993. Abnormal arginine-vasopressin secretion and water metabolism in patients with postviral fatigue syndrome. Acta Neurologica Scandinavica 87(3):234-238.
Barker, E., S. F. Fujimura, M. B. Fadem, A. L. Landay, and J. A. Levy. 1994. Immunologic abnormalities associated with chronic fatigue syndrome. Clinical Infectious Diseases 18(Suppl. 1):S136-S141.
Bearn, J., T. Allain, P. Coskeran, N. Munro, J. Butler, A. McGregor, and S. Wessely. 1995. Neuroendocrine responses to d-fenfluramine and insulin-induced hypoglycemia in chronic fatigue syndrome. Biological Psychiatry 37(4):245-252.
Behan, P. O., W. M. Behan, and E. J. Bell. 1985. The postviral fatigue syndrome—an analysis of the findings in 50 cases. The Journal of Infection 10(3):211-222.
Bennett, R. M., R. Friend, K. D. Jones, R. Ward, B. K. Han, and R. L. Ross. 2009. The Revised Fibromyalgia Impact Questionnaire (FIQR): Validation and psychometric properties. Arthritis Research & Therapy 11(4):R120.
Beqaj, S. H., A. M. Lerner, and J. T. Fitzgerald. 2008. Immunoassay with cytomegalovirus early antigens from gene products p52 and CM2 (UL44 and UL57) detects active infection in patients with chronic fatigue syndrome. Journal of Clinical Pathology 61(5):623-626.
Berwaerts, J., G. Moorkens, and R. Abs. 1998. Secretion of growth hormone in patients with chronic fatigue syndrome. Growth Hormone & IGF Research 8(Suppl. B):127-129.
Blockmans, D., P. Persoons, B. Van Houdenhove, M. Lejeune, and H. Bobbaers. 2003. Combination therapy with hydrocortisone and fludrocortisone does not improve symptoms in chronic fatigue syndrome: A randomized, placebo-controlled, double-blind, crossover study. American Journal of Medicine 114(9):736-741.
Boneva, R. S., M. J. Decker, E. M. Maloney, J. M. Lin, J. F. Jones, H. G. Helgason, C. M. Heim, D. B. Rye, and W. C. Reeves. 2007. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: A population-based study. Autonomic Neuroscience: Basic and Clinical 137(1-2):94-101.
Boomershine, C. S. 2012. A comprehensive evaluation of standardized assessment tools in the diagnosis of fibromyalgia and in the assessment of fibromyalgia severity. Pain Research and Treatment 2012:653714.
Bottero, P. 2000. Role of rickettsiae and chlamydiae in the psychopathology of chronic fatigue syndrome (CFS) patients: A diagnostic and therapeutic report. Journal of Chronic Fatigue Syndrome 6(3-4):147-161.
Brenu, E. W., D. R. Staines, O. K. Baskurt, K. J. Ashton, S. B. Ramos, R. M. Christy, and S. M. Marshall-Gradisnik. 2010. Immune and hemorheological changes in chronic fatigue syndrome. Journal of Translational Medicine 8.
Brenu, E. W., M. L. van Driel, D. R. Staines, K. J. Ashton, S. B. Ramos, J. Keane, N. G. Klimas, and S. M. Marshall-Gradisnik. 2011. Immunological abnormalities as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis. Journal of Translational Medicine 9:81.
Brenu, E. W., K. J. Ashton, M. van Driel, D. R. Staines, D. Peterson, G. M. Atkinson, and S. M. Marshall-Gradisnik. 2012a. Cytotoxic lymphocyte micrornas as prospective biomarkers for chronic fatigue syndrome/myalgic encephalomyelitis. Journal of Affective Disorders 141(2-3):261-269.
Brenu, E. W., M. L. van Driel, D. R. Staines, K. J. Ashton, S. L. Hardcastle, J. Keane, L. Tajouri, D. Peterson, S. B. Ramos, and S. M. Marshall-Gradisnik. 2012b. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. Journal of Translational Medicine 10:88.
Brenu, E. W., T. K. Huth, S. L. Hardcastle, K. Fuller, M. Kaur, S. Johnston, S. B. Ramos, D. R. Staines, and S. M. Marshall-Gradisnik. 2014. Role of adaptive and innate immune cells in chronic fatigue syndrome/myalgic encephalomyelitis. International Immunology 26(4):233-242.
Briggs, N. C., and P. H. Levine. 1994. A comparative review of systemic and neurological symptomatology in 12 outbreaks collectively described as chronic fatigue syndrome, epidemic neuromyasthenia, and myalgic encephalomyelitis. Clinical Infectious Diseases 18(Suppl. 1):S32-S42.
Broderick, G., B. Z. Katz, H. Fernandes, M. A. Fletcher, N. Klimas, F. A. Smith, M. R. O’Gorman, S. D. Vernon, and R. Taylor. 2012. Cytokine expression profiles of immune imbalance in post-mononucleosis chronic fatigue. Journal of Translational Medicine 10:191.
Brown, A. A., L. A. Jason, M. A. Evans, and S. Flores. 2013. Contrasting case definitions: The ME international consensus criteria vs. the Fukuda et al. CFS criteria. North American Journal of Psychology 15(1):103-120.
Brugts, M. P., J. G. Luermans, E. G. Lentjes, N. J. van Trooyen-van Vrouwerff, F. A. van der Horst, P. H. Slee, S. W. Lamberts, and J. A. Janssen. 2009. Heterophilic antibodies may be a cause of falsely low total IGF1 levels. European Journal of Endocrinology 161(4):561-565.
Buchwald, D., and A. L. Komaroff. 1991. Review of laboratory findings for patients with chronic fatigue syndrome. Reviews of Infectious Diseases 13(Suppl. 1):S12-S18.
Buchwald, D. S., R. Biddle, F. Jolesz, R. Kikinis, P. R. Cheney, D. L. Peterson, and A. L. Komaroff. 1989. Central nervous-system (CNS) abnormalities on magnetic-resonance imaging (MRI) in an outbreak of chronic fatigue syndrome (CFS). Clinical Research 37(2):A309.
Buchwald, D., P. R. Cheney, D. L. Peterson, B. Henry, S. B. Wormsley, A. Geiger, D. V. Ablashi, S. Z. Salahuddin, C. Saxinger, R. Biddle, R. Kikinis, F. A, Jolesz, T. Folks, N. Balachandran, J. B. Peter, R. Gallo, and A. L. Komaroff. 1992. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Annals of Internal Medicine 116(2):103-113.
Buchwald, D., R. L. Ashley, T. Pearlman, P. Kith, and A. L. Komaroff. 1996. Viral serologies in patients with chronic fatigue and chronic fatigue syndrome. Journal of Medical Virology 50(1):25-30.
Burbelo, P. D., A. Bayat, J. Wagner, T. B. Nutman, J. N. Baraniuk, and M. J. Iadarola. 2012. No serological evidence for a role of HHV-6 infection in chronic fatigue syndrome. American Journal of Translational Research 4(4):443-451.
Caligiuri, M., C. Murray, D. Buchwald, H. Levine, P. Cheney, D. Peterson, A. L. Komaroff, and J. Ritz. 1987. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. Journal of Immunology 139(10):3306-3313.
Cameron, B., L. Flamand, H. Juwana, J. Middeldorp, Z. Naing, W. Rawlinson, D. Ablashi, and A. Lloyd. 2010. Serological and virological investigation of the role of the herpesviruses EBV, CMV and HHV-6 in post-infective fatigue syndrome. Journal of Medical Virology 82(10):1684-1688.
Carlo-Stella, N., C. Badulli, A. De Silvestri, L. Bazzichi, M. Martinetti, L. Lorusso, S. Bombardieri, L Salvaneschi, and M. Cuccia. 2006. A first study of cytokine genomic polymorphisms in CFS: Positive association of TNF-857 and IFNgamma 874 rare alleles. Clinical and Experimental Rheumatology 24(2):179-182.
Carruthers, B. M., A. K. Jain, K. L. De Meirleir, D. L. Peterson, N. G. Klimas, A. M. Lemer, A. C. Bested, P. Flor-Henry, P. Joshi, A. C. P. Powles, J. A. Sherkey, and M. I. van de Sande. 2003. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols (Canadian case definition). Journal of Chronic Fatigue Syndrome 11(1):7-115.
Carruthers, B. M., M. I. van de Sande, K. L. De Meirleir, N. G. Klimas, G. Broderick, T. Mitchell, D. Staines, A. C. P. Powles, N. Speight, R. Vallings, L. Bateman, B. Baumgarten-Austrheim, D. S. Bell, N. Carlo-Stella, J. Chia, A. Darragh, D. Jo, D. Lewis, A. R. Light, S. Marshall-Gradisbik, I. Mena, J. A. Mikovits, K. Miwa, M. Murovska, M. L. Pall, and S. Stevens. 2011. Myalgic encephalomyelitis: International consensus criteria. Journal of Internal Medicine 270(4):327-338.
Cater II, R. E. 1995. Chronic intestinal candidiasis as a possible etiological factor in the chronic fatigue syndrome. Medical Hypotheses 44(6):507-515.
Cevik, R., A. Gur, S. Acar, K. Nas, and A. J. Sarac. 2004. Hypothalamic-pituitary-gonadal axis hormones and cortisol in both menstrual phases of women with chronic fatigue syndrome and effect of depressive mood on these hormones. BMC Musculoskeletal Disorders 5:47.
Chia, J. K. S., and A. Y. Chia. 2008. Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. Journal of Clinical Pathology 61(1):43-48.
Chia, J. K. S., and L. Y. Chia. 1999. Chronic chlamydia pneumoniae infection: A treatable cause of chronic fatigue syndrome. Clinical Infectious Diseases 29(2):452-453.
Chia, J., A. Chia, M. Voeller, T. Lee, and R. Chang. 2010. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. Journal of Clinical Pathology 63(2):165-168.
Ciccone, D. S., and B. H. Natelson. 2003. Comorbid illness in women with chronic fatigue syndrome: A test of the single syndrome hypothesis. Psychosomatic Medicine 65(2):268-275.
Clauw, D. J. 2014. Fibromyalgia: A clinical review. Journal of the American Medical Association 311(15):1547-1555.
Cleare, A. J., E. Heap, G. S. Malhi, S. Wessely, V. O’Keane, and J. Miell. 1999. Low-dose hydrocortisone in chronic fatigue syndrome: A randomised crossover trial. Lancet 353(9151):455-458.
Cleare, A. J., S. S. Sookdeo, J. Jones, V. O’Keane, and J. P. Miell. 2000. Integrity of the growth hormone/insulin-like growth factor system is maintained in patients with chronic fatigue syndrome. Journal of Clinical Endocrinology & Metabolism 85(4):1433-1439.
Cleare, A. J., D. Blair, S. Chambers, and S. Wessely. 2001. Urinary free cortisol in chronic fatigue syndrome. American Journal of Psychiatry 158(4):641-643.
Clement, W. B., P. Gorda, D. A. Henderson, J. W. Lawrence, and J. O. Bond. 1958. Epidemic neuromyasthenia, an outbreak in Punta Gorda, Florida; an illness resembling Iceland disease. The Journal of the Florida Medical Association 45(4):422-426.
Cook, D. B., P. R. Nagelkirk, A. Peckerman, A. Poluri, J. Mores, and B. H. Natelson. 2005. Exercise and cognitive performance in chronic fatigue syndrome. Medicine & Science in Sports & Exercise 37(9):1460-1467.
Cook, D. B., P. R. Nagelkirk, A. Poluri, J. Mores, and B. H. Natelson. 2006. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis & Rheumatism 54(10):3351-3362.
Crofford, L. J., E. A. Young, N. C. Engleberg, A. Korszun, C. B. Brucksch, L. A. McClure, M. B. Brown, and M. A. Demitrack. 2004. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain, Behavior & Immunity 18(4):314-325.
Curriu, M., J. Carrillo, M. Massanella, J. Rigau, J. Alegre, J. Puig, A. M. Garcia-Quintana, J. Castro-Marrero, E. Negredo, B. Clotet, C. Cabrera, and J. Blanco. 2013. Screening NK-, B- and T-cell phenotype and function in patients suffering from chronic fatigue syndrome. Journal of Translational Medicine 11:68.
Daikos, G. K., S. Garzonis, A. Paleologue, G. A. Bousvaros, and N. Papadoyannakis. 1959. Benign myalgic encephalomyelitis. An outbreak in a nurses’ school in Athens. Lancet 1(7075):693-696.
De Becker, P., N. McGregor, and K. De Meirleir. 2001. A definition-based analysis of symptoms in a large cohort of patients with chronic fatigue syndrome. Journal of Internal Medicine 250(3):234-240.
Demitrack, M. A., J. K. Dale, S. E. Straus, L. Laue, S. J. Listwak, M. J. Kruesi, G. P. Chrousos, and P. W. Gold. 1991. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. Journal of Clinical Endocrinology and Metabolism 73(6):1224-1234.
Di Giorgio, A., M. Hudson, W. Jerjes, and A. J. Cleare. 2005. 24-hour pituitary and adrenal hormone profiles in chronic fatigue syndrome. Psychosomatic Medicine 67(3):433-440.
Diaz-Mitoma, F., E. Turgonyi, A. Kumar, W. Lim, L. Larocque, and B. M. Hyde. 2003. Clinical improvement in chronic fatigue syndrome is associated with enhanced natural killer cell-mediated cytotoxicity: The results of a pilot study with isoprinosine®. Journal of Chronic Fatigue Syndrome 11(2):71-93.
Dillon, M. J., W. C. Marshall, J. A. Dudgeon, and A. J. Steigman. 1974. Epidemic neuromyasthenia: Outbreak among nurses at a children’s hospital. British Medical Journal 1(5903):301-305.
Dinan, T. G., T. Majeed, E. Lavelle, L. V. Scott, C. Berti, and P. Behan. 1997. Blunted serotonin-mediated activation of the hypothalamic-pituitary-adrenal axis in chronic fatigue syndrome. Psychoneuroendocrinology 22(4):261-267.
Edwards, J. 2013. UK rituximab trial—statements by professor Jonathan Edwards and invest in ME. http://www.ukrituximabtrial.org/Rituximab%20news-July13%2001.htm (accessed May 14, 2014).
Elfaitouri, A., B. Herrmann, A. Bölin-Wiener, Y. Wang, C. G. Gottfries, O. Zachrisson, R. Pipkorn, L. Rönnblom, and J. Blomberg. 2013. Epitopes of microbial and human heat shock protein 60 and their recognition in myalgic encephalomyelitis. PLoS ONE 8(11):e81155.
Enbom, M., A. Linde, and B. Evengård. 2000. No evidence of active infection with human herpesvirus 6 (HHV-6) or HHV-8 in chronic fatigue syndrome. Journal of Clinical Microbiology 38(6):2457.
Evengård, B., C. G. Nilsson, G. Lindh, L. Lindquist, P. Eneroth, S. Fredrikson, L. Terenius, and K. G. Henriksson. 1998. Chronic fatigue syndrome differs from fibromyalgia. No evidence for elevated substance P levels in cerebrospinal fluid of patients with chronic fatigue syndrome. Pain 78(2):153-155.
Evengård, B., T. Briese, G. Lindh, S. Lee, and W. I. Lipkin. 1999. Absence of evidence of Borna disease virus infection in Swedish patients with chronic fatigue syndrome. Journal of NeuroVirology 5(5):495-499.
Evengård, B., E. Jonzon, A. Sandberg, T. Theorell, and G. Lindh. 2003. Differences between patients with chronic fatigue syndrome and with chronic fatigue at an infectious disease clinic in Stockholm, Sweden. Psychiatry & Clinical Neurosciences 57(4):361-368.
Fark, A. R. 1991. Infectious mononucleosis, Epstein-Barr virus, and chronic fatigue syndrome: A prospective case series. Journal of Family Practice 32(2):202, 205-206, 209.
FDA (Food and Drug Administration). 2013. The voice of the patient: Chronic fatigue syndrome and myalgic encephalomyelitis. Bethesda, MD: Center for Drug Evaluation and Research (CDER), FDA.
Fischer, D. B., A. H. William, A. C. Strauss, E. R. Unger, L. A. Jason, G. D. Marshall, Jr., and J. D. Dimitrakoff. 2014. Chronic fatigue syndrome: The current status and future potentials of emerging biomarkers. Fatigue: Biomedicine, Health & Behavior 2(2):93-109.
Fletcher, M. A., X. R. Zeng, Z. Barnes, S. Levis, and N. G. Klimas. 2009. Plasma cytokines in women with chronic fatigue syndrome. Journal of Translational Medicine 7:96.
Fletcher, M. A., X. R. Zeng, K. Maher, S. Levis, B. Hurwitz, M. Antoni, G. Broderick, and N. G. Klimas. 2010. Biomarkers in chronic fatigue syndrome: Evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. PLoS ONE 5(5):e10817.
Fluge, O., O. Bruland, K. Risa, A. Storstein, E. K. Kristoffersen, D. Sapkota, H. Naess, O. Dahl, H. Nyland, and O. Mella. 2011. Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLoS ONE 6(10):e26358.
Fondell, E., J. Axelsson, K. Franck, A. Ploner, M. Lekander, K. Bälter, and H. Gaines. 2011. Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behavior and Immunity 25(7):1367-1375.
Fotheringham, J., N. Akhyani, A. Vortmeyer, D. Donati, E. Williams, U. Oh, M. Bishop, J. Barrett, J. Gea-Banacloche, and S. Jacobson. 2007. Detection of active human herpesvirus-6 infection in the brain: Correlation with polymerase chain reaction detection in cerebrospinal fluid. The Journal of Infectious Diseases 195(3):450-454.
Fremont, M., K. Metzger, H. Rady, J. Hulstaert, and K. De Meirleir. 2009. Detection of herpesviruses and parvovirus B19 in gastric and intestinal mucosa of chronic fatigue syndrome patients. In Vivo 23(2):209-213.
Frykholm, B. O. 2009. On the question of infectious aetiologies for multiple sclerosis, schizophrenia and the chronic fatigue syndrome and their treatment with antibiotics. Medical Hypotheses 72(6):736-739.
Fukuda, K., S. E. Straus, I. Hickie, M. C. Sharpe, J. G. Dobbins, A. Komaroff, A. Schluederberg, J. F. Jones, A. R. Lloyd, S. Wessely, N. M. Gantz, G. P. Holmes, D. Buchwald, S. Abbey, J. Rest, J. A. Levy, H. Jolson, D. L. Peterson, J. Vercoulen, U. Tirelli, B. Evengård, B. H. Natelson, L. Steele, M. Reyes, and W. C. Reeves. 1994. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine 121(12):953-959.
Gaab, J., D. Hüster, R. Peisen, V. Engert, V. Heitz, T. Schad, T. H. Schürmeyer, and U. Ehlert. 2002. Hypothalalmic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological and pharmacological stimulation. Psychosomatic Medicine 64(6):951-962.
Gaab, J., D. Huster, R. Peisen, V. Engert, V. Heitz, T. Schad, T. Schurmeyer, and U. Ehlert. 2003. Assessment of cortisol response with low-dose and high-dose ACTH in patients with chronic fatigue syndrome and healthy comparison subjects. Psychosomatics 44(2): 113-119.
Gaab, J., N. Rohleder, V. Heitz, V. Engert, T. Schad, T. H. Schlümeyer, and U. Ehlert. 2005. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology 30(2):188-198.
Gaber, T., and W. W. Oo. 2013. Prevalence of hypothyroidism in chronic fatigue syndrome patients (CFS/ME). Journal of Neurology 260:S98-S99.
Galbraith, D. N., C. Nairn, and G. B. Clements. 1995. Phylogenetic analysis of short enteroviral sequences from patients with chronic fatigue syndrome. Journal of General Virology 76(Pt. 7):1701-1707.
Gascon, J., T. Marcos, J. Vidal, A. G. Forcada, and M. Corachan. 2006. Cytomegalovirus and Epstein-Barr virus infection as a cause of chronic fatigue syndrome in travellers to tropical countries. Journal of Travel Medicine 2(1):41-44.
Georgiades, E., W. M. Behan, L. P. Kilduff, M. Hadjicharalambous, E. E. Mackie, J. Wilson, S. A. Ward, and Y. P. Pitsiladis. 2003. Chronic fatigue syndrome: New evidence for a central fatigue disorder. Clinical Science 105(2):213-218.
Glaser, R., D. A. Padgett, M. L. Litsky, R. A. Baiocchi, E. V. Yang, M. Chen, P. E. Yeh, N. G. Klimas, G. D. Marshall, T. Whiteside, R. Herberman, J. Kiecolt-Glaser, and M. V. Williams. 2005. Stress-associated changes in the steady-state expression of latent Epstein-Barr virus: Implications for chronic fatigue syndrome and cancer. Brain, Behavior & Immunity 19(2):91-103.
Glaser, R., M. L. Litsky, D. A. Padgett, R. A. Baiocchi, E. V. Yang, M. Chen, P. E. Yeh, K. B. Green-Church, M. A. Caligiuri, and M. V. Williams. 2006. EBV-encoded dutpase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology 346(1):205-218.
Gupta, S., and B. Vayuvegula. 1991. A comprehensive immunological analysis in chronic fatigue syndrome. Scandinavian Journal of Immunology 33(3):319-327.
Gur, A., R. Cevik, K. Nas, L. Colpan, and S. Sarac. 2004. Cortisol and hypothalamic-pituitarygonadal axis hormones in follicular-phase women with fibromyalgia and chronic fatigue syndrome and effect of depressive symptoms on these hormones. Arthritis Research & Therapy 6(3):R232-R238.
Halme, L., J. Arola, K. Hockerstedt, and I. Lautenschlager. 2008. Human herpesvirus 6 infection of the gastroduodenal mucosa. Clinical Infectious Diseases 46(3):434-439.
Hellinger, W. C., T. F. Smith, R. E. Van Scoy, P. G. Spitzer, P. Forgacs, and R. S. Edson. 1988. Chronic fatigue syndrome and the diagnostic utility of antibody to Epstein-Barr virus early antigen. Journal of the American Medical Association 260(7):971-973.
Henderson, T. A. 2014. Valacyclovir treatment of chronic fatigue in adolescents. Advances in Mind-Body Medicine 28(1):4-14.
Heneine, W., T. C. Woods, S. D. Sinha, A. S. Khan, L. E. Chapman, L. B. Schonberger, and T. M. Folks. 1994. Lack of evidence for infection with known human and animal retroviruses in patients with chronic fatigue syndrome. Clinical Infectious Diseases 18(Suppl. 1):S121-S125.
Herr, K. A., and L. Garand. 2001. Assessment and measurement of pain in older adults. Clinics in Geriatric Medicine 17(3):vi, 457-478.
Hickie, I., T. Davenport, D. Wakefield, U. Vollmer-Conna, B. Cameron, S. D. Vernon, W. C. Reeves, A. Lloyd, and G. Dubbo Infection Outcomes Study. 2006. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. British Medical Journal 333(7568):575.
Hokama, Y., C. E. Campora, C. Hara, T. Kuribayashi, D. Le Huynh, and K. Yabusaki. 2009. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. Journal of Clinical Laboratory Analysis 23(4):210-212.
Honda, M., K. Kitamura, T. Nakasone, Y. Fukushima, S. Matsuda, K. Nishioka, J. Matsuda, N. Hashimoto, and S. Yamazaki. 1993. Japanese patients with chronic fatigue syndrome are negative for known retrovirus infections. Microbiology and Immunology 37(10):779-784.
Ickmans, K., M. Meeus, D. Kos, P. Clarys, G. Meersdom, L. Lambrecht, N. Pattyn, and J. Nijs. 2013. Cognitive performance is of clinical importance, but is unrelated to pain severity in women with chronic fatigue syndrome. Clinical Rheumatology 32(10):1475-1485.
Ilaria, Jr., R. L., A. L. Komaroff, L. R. Fagioli, W. C. Moloney, C. A. True, and S. J. Naides. 1995. Absence of parvovirus B19 infection in chronic fatigue syndrome. Arthritis and Rheumatism 38(5):638-641.
Iwakami, E., Y. Arashima, K. Kato, T. Komiya, Y. Matsukawa, T. Ikeda, Y. Arakawa, and S. Oshida. 2005. Treatment of chronic fatigue syndrome with antibiotics: Pilot study assessing the involvement of Coxiella burnetii infection. Internal Medicine (Tokyo, Japan) 44(12):1258-1263.
Jackson, M., H. Butt, M. Ball, D. Lewis, and D. Bruck. 2013. An association between changes in the intestinal microbial flora and the alteration of sleep in chronic fatigue syndrome: A pilot open label trial with use of the antibiotic erythromycin. Sleep and Biological Rhythms 11:55.
Jammes, Y., J. G. Steinberg, S. Delliaux, and F. Bregeon. 2009. Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and hsp responses. Journal of Internal Medicine 266(2):196-206.
Janal, M. N., D. S. Ciccone, and B. H. Natelson. 2006. Sub-typing CFS patients on the basis of “minor” symptoms. Biological Psychology 73(2):124-131.
Jason, L. A., J. A. Richman, A. W. Rademaker, K. M. Jordan, A. V. Plioplys, R. R. Taylor, W. McCready, C. F. Huang, and S. Plioplys. 1999. A community-based study of chronic fatigue syndrome. Archives of Internal Medicine 159(18):2129-2137.
Jason, L. A., S. R. Torres-Harding, A. Jurgens, and J. Helgerson. 2004. Comparing the Fukuda et al. criteria and the Canadian case definition for chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome 12(1):37-52.
Jason, L. A., S. Torres-Harding, K. Maher, N. Reynolds, M. Brown, M. Sorenson, J. Donalek, K. Corradi, M. A. Fletcher, and T. Lu. 2007. Baseline cortisol levels predict treatment outcomes in chronic fatigue syndrome nonpharmacologic clinical trial. Journal of Chronic Fatigue Syndrome 14(4):39-59.
Jason, L. A., M. Evans, N. Porter, M. Brown, A. Brown, J. Hunnell, V. Anderson, A. Lerch, K. De Meirleir, and F. Friedberg. 2010. The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. American Journal of Biochemistry and Biotechnology 6(2):120-135.
Jason, L. A., A. Brown, E. Clyne, L. Bartgis, M. Evans, and M. Brown. 2012. Contrasting case definitions for chronic fatigue syndrome, myalgic encephalomyelitis/chronic fatigue syndrome and myalgic encephalomyelitis. Evaluation and the Health Professions 35(3):280-304.
Jason, L. A., A. Brown, M. Evans, M. Sunnquist, and J. L. Newton. 2013a. Contrasting chronic fatigue syndrome versus myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue 1(3):168-183.
Jason, L. A., M. Sunnquist, A. Brown, M. Evans, S. Vernon, J. Furst, and V. Simonis. 2013b. Examining case definition criteria for chronic fatigue syndrome and myalgic encephalomyelitis. Fatigue: Biomedicine, Health & Behavior 2(1).
Jason, L. A., B. Z. Katz, Y. Shiraishi, C. Mears, Y. Im, and R. R. Taylor. 2014a. Predictors of post-infectious chronic fatigue syndrome in adolescents. Health Psychology & Behavioural Medicine 2(1):41-51.
Jason, L. A., M. Sunnquist, A. Brown, M. Evans, and J. L. Newton. 2014b. Are myalgic encephalomyelitis and chronic fatigue syndrome different illnesses? A preliminary analysis. Journal of Health Psychology [Epub ahead of print].
Jerjes, W. K., N. F. Taylor, T. J. Peters, S. Wessely, and A. J. Cleare. 2006. Urinary cortisol and cortisol metabolite excretion in chronic fatigue syndrome. Psychosomatic Medicine 68(4):578-582.
Jerjes, W. K., N. F. Taylor, P. J. Wood, and A. J. Cleare. 2007. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology 32(2):192-198.
Jones, J. F., M. Williams, R. T. Schooley, C. Robinson, and R. Glaser. 1988. Antibodies to Epstein-Barr virus-specific DNase and DNA polymerase in the chronic fatigue syndrome. Archives of Internal Medicine 148(9):1957-1960.
Katz, B. Z., Y. Shiraishi, C. J. Mears, H. J. Binns, and R. Taylor. 2009. Chronic fatigue syndrome following infectious mononucleosis in adolescents. Pediatrics 124(1):189-193.
Kawai, K., and A. Kawai. 1992. Studies on the relationship between chronic fatigue syndrome and Epstein-Barr virus in Japan. Internal Medicine (Tokyo, Japan) 31(3):313-318.
Kerr, J. R., and D. A. J. Tyrrell. 2003. Cytokines in parvovirus B19 infection as an aid to understanding chronic fatigue syndrome. Current Pain and Headache Reports 7(5):333-341.
Kerr, J. R., J. Bracewell, I. Laing, D. L. Mattey, R. M. Bernstein, I. N. Bruce, and D. A. Tyrrell. 2002. Chronic fatigue syndrome and arthralgia following parvovirus B19 infection. Journal of Rheumatology 29(3):595-602.
Kerr, J. R., J. Gough, S. C. Richards, J. Main, D. Enlander, M. McCreary, A. L. Komaroff, and J. K. Chia. 2010. Antibody to parvovirus B19 nonstructural protein is associated with chronic arthralgia in patients with chronic fatigue syndrome/myalgic encephalomyelitis. Journal of General Virology 91(Pt. 4):893-897.
Khan, A. S., W. M. Heneine, L. E. Chapman, H. E. Gary, Jr., T. C. Woods, T. M. Folks, and L. B. Schonberger. 1993. Assessment of a retrovirus sequence and other possible risk factors for the chronic fatigue syndrome in adults. Annals of Internal Medicine 118(4):241-245.
Kitani, T., H. Kuratsune, I. Fuke, Y. Nakamura, T. Nakaya, S. Asahi, M. Tobiume, K. Yamaguti, T. Machii, R. Inagi, K. Yamanishi, and K. Ikuta. 1996. Possible correlation between Borna disease virus infection and Japanese patients with chronic fatigue syndrome. Microbiology and Immunology 40(6):459-462.
Klajman, A., B. Pinkhas, and L. Rannon. 1960. An outbreak of an epidemic of benign myalgic encephalomyelitis. Harefuah 58:314-315.
Klein, R., and P. A. Berg. 1995. High incidence of antibodies to 5-hydroxytryptamine, gangliosides and phospholipids in patients with chronic fatigue and fibromyalgia syndrome and their relatives: Evidence for a clinical entity of both disorders. European Journal of Medical Research 1(1):21-26.
Klimas, N. G., F. R. Salvato, R. Morgan, and M. A. Fletcher. 1990. Immunologic abnormalities in chronic fatigue syndrome. Journal of Clinical Microbiology 28(6):1403-1410.
Koelle, D. M., S. Barcy, M. L. Huang, R. L. Ashley, L. Corey, J. Zeh, S. Ashton, and D. Buchwald. 2002. Markers of viral infection in monozygotic twins discordant for chronic fatigue syndrome. Clinical Infectious Diseases 35(5):518-525.
Komaroff, A. L. 1988. Chronic fatigue syndromes: Relationship to chronic viral infections. Journal of Virological Methods 21(1-4):3-10.
Komaroff, A. L., L. R. Fagioli, T. H. Doolittle, B. Gandek, M. A. Gleit, R. T. Guerriero, I. R. J. Kornish, N. C. Ware, J. E. Ware, Jr., and D. W. Bates. 1996. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. American Journal of Medicine 101(3):281-290.
Konstantinov, K., A. von Mikecz, D. Buchwald, J. Jones, L. Gerace, and E. M. Tan. 1996. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. Journal of Clinical Investigation 98(8):1888-1896.
Krueger, G. R., B. Koch, A. Ramon, D. V. Ablashi, S. Z. Salahuddin, S. F. Josephs, H. Z. Streicher, R. C. Gallo, and U. Habermann. 1988. Antibody prevalence to HBLV (human herpesvirus-6, HHV-6) and suggestive pathogenicity in the general population and in patients with immune deficiency syndromes. Journal of Virological Methods 21(1-4):125-131.
Kuhl, U., M. Pauschinger, B. Seeberg, D. Lassner, M. Noutsias, W. Poller, and H. P. Schultheiss. 2005. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112(13):1965-1970.
Lane, R. J. M., B. A. Soteriou, H. Zhang, and L. C. Archard. 2003. Enterovirus related metabolic myopathy: A postviral fatigue syndrome. Journal of Neurology, Neurosurgery & Psychiatry 74(10):1382-1386.
Lattie, E. G., M. H. Antoni, M. A. Fletcher, F. Penedo, S. Czaja, C. Lopez, D. Perdomo, A. Sala, S. Nair, S. H. Fu, and N. Klimas. 2012. Stress management skills, neuroimmune processes and fatigue levels in persons with chronic fatigue syndrome. Brain, Behavior & Immunity 26(6):849-858.
Lattie, E. G., M. H. Antoni, S. Czaja, D. Perdomo, M. A. Fletcher, N. Klimas, and S. Nair. 2013. Post-exertional malaise symptoms, salivary cortisol, and coping self-efficacy in patients with ME/CFS. Psychosomatic Medicine 75(3):A66-A67.
Ledina, D., N. Bradaric, I. Milas, I. Ivic, N. Brncic, and N. Kuzmicic. 2007. Chronic fatigue syndrome after Q fever. Medical Science Monitor 13(7):CS88-CS92.
Lerner, A. M., and S. Beqaj. 2011. A paradigm linking herpesvirus immediate-early gene expression apoptosis and myalgic encephalomyelitis chronic fatigue syndrome. Virus Adaptation and Treatment 3(1):19-24.
Lerner, A. M., and S. Beqaj. 2012. Abortive lytic Epstein-Barr virus replication in tonsil-B lymphocytes in infectious mononucleosis and a subset of the chronic fatigue syndrome. Virus Adaptation and Treatment 4(1):85-91.
Lerner, A. M., S. H. Beqaj, R. G. Deeter, and J. T. Fitzgerald. 2002. IgM serum antibodies to human cytomegalovirus nonstructural gene products p52 and CM2(UL44 and UL57) are uniquely present in a subset of patients with chronic fatigue syndrome. In Vivo 16(3):153-159.
Lerner, A. M., S. H. Beqaj, R. G. Deeter, and J. T. Fitzgerald. 2004. IgM serum antibodies to Epstein-Barr virus are uniquely present in a subset of patients with the chronic fatigue syndrome. In Vivo 18(2):101-106.
Lerner, A. M., S. Beqaj, J. T. Fitzgerald, K. Gill, C. Gill, and J. Edington. 2010. Subset-directed antiviral treatment of 142 herpesvirus patients with chronic fatigue syndrome. Virus Adaptation and Treatment 2(1):47-57.
Lerner, A. M., M. E. Ariza, M. Williams, L. Jason, S. Beqaj, J. T. Fitzgerald, S. Lemeshow, and R. Glaser. 2012. Antibody to Epstein-Barr virus deoxyuridine triphosphate nucleotidohydrolase and deoxyribonucleotide polymerase in a chronic fatigue syndrome subset. PLoS ONE 7(11):e47891.
Levine, P. H., S. Jacobson, A. C. Pocinki, P. Cheney, D. Peterson, R. R. Connelly, R. Weil, S. M. Robinson, D. V. Ablashi, S. Z. Salahuddin, G. R. Pearson, and R. Hoover. 1992. Clinical, epidemiologic, and virologic studies in four clusters of the chronic fatigue syndrome. Archives of Internal Medicine 152(8):1611-1616.
Levine, P. H., T. L. Whiteside, D. Friberg, J. Bryant, G. Colclough, and R. B. Herberman. 1998. Dysfunction of natural killer activity in a family with chronic fatigue syndrome. Clinical Immunology and Immunopathology 88(1):96-104.
Levine, S., H. Eastman, and D. V. Ablashi. 2001. Prevalence of IgM and IgG antibody to HHV-6 and HHV-8 and results of plasma PCR to HHV-6 and HHV-7 in a group of CFS patients and healthy donors. Journal of Chronic Fatigue Syndrome 9(1-2):31-40.
Li, Y. J., D. X. Wang, F. M. Zhang, Z. D. Liu, A. Y. Yang, and K. Ykuta. 2003. Detection of antibody against Borna disease virus-p24 in the plasma of Chinese patients with chronic fatigue syndrome by western-blot analysis. Chinese Journal of Experimental and Clinical Virology 17(4):330-333.
Lindh, G., A. Samuelson, K. O. Hedlund, B. Evengård, L. Lindquist, and A. Ehrnst. 1996. No findings of enteroviruses in Swedish patients with chronic fatigue syndrome. Scandinavian Journal of Infectious Diseases 28(3):305-307.
Loebel, M., K. Strohschein, C. Giannini, U. Koelsch, S. Bauer, C. Doebis, S. Thomas, N. Unterwalder, V. von Baehr, P. Reinke, M. Knops, L. G. Hanitsch, C. Meisel, H. D. Volk, and C. Scheibenbogen. 2014. Deficient EBV-specific B- and T-cell response in patients with chronic fatigue syndrome. PLoS ONE 9(1):e85387.
Longo, D. L., A. S. Fauci, D. L. Kasper, S. L. Hauser, J. L. Jameson, and J. Loscalzo. 2012. Harrison’s principles of internal medicine: 18th edition. New York: McGraw-Hill.
Lutgendorf, S., N. G. Klimas, M. Antoni, A. Brickman, and M. A. Fletcher. 1995. Relationships of cognitive difficulties to immune measures, depression and illness burden in chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome 1(2):23-41.
Lyall, M., M. Peakman, and S. Wessely. 2003. A systematic review and critical evaluation of the immunology of chronic fatigue syndrome. Journal of Psychosomatic Research 55(2):79-90.
Maes, M., I. Mihaylova, and J.-C. Leunis. 2006. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuroendocrinology Letters 27(5):615-621.
Maes, M., F. N. M. Twisk, and C. Johnson. 2012. Myalgic encephalomyelitis (ME), chronic fatigue syndrome (CFS), and chronic fatigue (CF) are distinguished accurately: Results of supervised learning techniques applied on clinical and inflammatory data. Psychiatry Research 200(2-3):754-760.
Maes, M., K. Ringel, M. Kubera, G. Anderson, G. Morris, P. Galecki, and M. Geffard. 2013. In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. Journal of Affective Disorders 150(2):223-230.
Maher, K. J., N. G. Klimas, and M. A. Fletcher. 2005. Chronic fatigue syndrome is associated with diminished intracellular perforin. Clinical & Experimental Immunology 142(3):505-511.
Manian, F. A. 1994. Simultaneous measurement of antibodies to Epstein-Barr virus, human herpesvirus 6, herpes simplex virus types 1 and 2, and 14 enteroviruses in chronic fatigue syndrome: Is there evidence of activation of a nonspecific polyclonal immune response? Clinical Infectious Diseases 19(3):448-453.
Markopoulou, K., A. Roberts, A. Papadopoulos, S. Wessely, T. Chalder, and A. Cleare. 2010. The ratio of cortisol/DHEA in chronic fatigue syndrome. Journal of Affective Disorders 122:S71.
Marshall, R., L. Paul, A. K. McFadyen, D. Rafferty, and L. Wood. 2010. Pain characteristics of people with chronic fatigue syndrome. Journal of Musculoskeletal Pain 18(2):127-137.
Maupin, C. 2014. More than just “fatigue.” http://www.cfidsreport.com/Articles/CFS/Chronic_Fatigue_Syndrome-Fatigue.htm (accessed January 14, 2015).
Mawle, A. C., R. Nisenbaum, J. G. Dobbins, H. E. Gary, Jr., J. A. Stewart, M. Reyes, L. Steele, D. S. Schmid, and W. C. Reeves. 1995. Seroepidemiology of chronic fatigue syndrome: A case-control study. Clinical Infectious Diseases 21(6):1386-1389.
Mawle, A. C., R. Nisenbaum, J. G. Dobbins, H. E. Gary, Jr., J. A. Stewart, M. Reyes, L. Steele, D. S. Schmid, and W. C. Reeves. 1997. Immune responses associated with chronic fatigue syndrome: A case-control study. Journal of Infectious Diseases 175(1):136-141.
McCormack, H. M., D. J. Horne, and S. Sheather. 1988. Clinical applications of visual analogue scales: A critical review. Psychological Medicine 18(4):1007-1019.
McKenzie, R., A. O’Fallon, J. Dale, M. Demitrack, G. Sharma, M. Deloria, D. GarciaBorreguero, W. Blackwelder, and S. E. Straus. 1998. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: A randomized controlled trial. Journal of the American Medical Association 280(12):1061-1066.
Meeus, M., J. Nijs, and K. De Meirleir. 2007. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: A systematic review. European Journal of Pain 11(4):377-386.
Meeus, M., W. Mistiaen, L. Lambrecht, and J. Nijs. 2009. Immunological similarities between cancer and chronic fatigue syndrome: The common link to fatigue? Anticancer Research 29(11):4717-4726.
Meeus, M., L. Hermans, K. Ickmans, F. Struyf, D. Van Cauwenbergh, L. Bronckaerts, L. S. De Clerck, G. Moorken, G. Hans, S. Grosemans, and J. Nijs. 2014. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: A double-blind randomized controlled trial. Pain Practice [Epub ahead of print].
Mella, O., and O. Fluge. 2014. B-lymphocyte depletion using rituximab in chronic fatigue syndrome/myalgic encephalopathy (CFS/ME). A randomized phase-III study. (RituxME). https://clinicaltrials.gov/ct2/show/NCT02229942 (accessed September 17, 2014).
Mihaylova, I., M. DeRuyter, J. L. Rummens, E. Bosmans, and M. Maes. 2007. Decreased expression of CD69 in chronic fatigue syndrome in relation to inflammatory markers: Evidence for a severe disorder in the early activation of T lymphocytes and natural killer cells. Neuroendocrinology Letters 28(4):477-483.
Miwa, K., and M. Fujita. 2008. Small heart syndrome in patients with chronic fatigue syndrome. Clinical Cardiology 31(7):328-333.
Miwa, K., and M. Fujita. 2009. Cardiac function fluctuates during exacerbation and remission in young adults with chronic fatigue syndrome and “small heart.” Journal of Cardiology 54(1):29-35.
Miwa, K., and M. Fujita. 2011. Small heart with low cardiac output for orthostatic intolerance in patients with chronic fatigue syndrome. Clinical Cardiology 34(12):782-786.
Miwa, K., and M. Fujita. 2014. Renin-aldosterone paradox in patients with myalgic encephalomyelitis and orthostatic intolerance. International Journal of Cardiology 172(2):514-515.
Montoya, J. G., A. M. Kogelnik, M. Bhangoo, M. R. Lunn, L. Flamand, L. E. Merrihew, T. Watt, J. T. Kubo, J. Paik, and M. Desai. 2013. Randomized clinical trial to evaluate the efficacy and safety of valganciclovir in a subset of patients with chronic fatigue syndrome. Journal of Medical Virology 85(12):2101-2109.
Moorkens, G., J. Berwaerts, H. Wynants, and R. Abs. 2000. Characterization of pituitary function with emphasis on GH secretion in the chronic fatigue syndrome. Clinical Endocrinology 53(1):99-106.
Nacul, L. C., E. M. Lacerda, D. Pheby, P. Campion, M. Molokhia, S. Fayyaz, J. C. Leite, F. Poland, A. Howe, and M. L. Drachler. 2011. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: A repeated cross-sectional study in primary care. BMC Medicine 9:91.
Naess, H., E. Sundal, K. M. Myhr, and H. I. Nyland. 2010. Postinfectious and chronic fatigue syndromes: Clinical experience from a tertiary-referral centre in Norway. In Vivo 24(2):185-188.
Naess, H., M. Nyland, T. Hausken, I. Follestad, and H. I. Nyland. 2012. Chronic fatigue syndrome after giardia enteritis: Clinical characteristics, disability and long-term sickness absence. BMC Gastroenterology 12:13.
Nakamura, T., S. K. Schwander, R. Donnelly, F. Ortega, F. Togo, G. Broderick, Y. Yamamoto, N. S. Cherniack, D. Rapoport, and B. H. Natelson. 2010. Cytokines across the night in chronic fatigue syndrome with and without fibromyalgia. Clinical and Vaccine Immunology 17(4):582-587.
Nakamura, T., S. Schwander, R. Donnelly, D. B. Cook, F. Ortega, F. Togo, Y. Yamamoto, N. S. Cherniack, M. Klapholz, D. Rapoport, and B. H. Natelson. 2013. Exercise and sleep deprivation do not change cytokine expression levels in patients with chronic fatigue syndrome. Clinical and Vaccine Immunology 20(11):1736-1742.
Narita, M., N. Nishigami, N. Narita, K. Yamaguti, N. Okado, Y. Watanabe, and H. Kuratsune. 2003. Association between serotonin transporter gene polymorphism and chronic fatigue syndrome. Biochemical & Biophysical Research Communications 311(2):264-266.
Naschitz, J. E., G. Slobodin, D. Sharif, M. Fields, H. Isseroff, E. Sabo, and I. Rosner. 2008. Electrocardiographic QT interval and cardiovascular reactivity in fibromyalgia differ from chronic fatigue syndrome. European Journal of Internal Medicine 19(3):187-191.
Natelson, B. H. 2010. Chronic fatigue syndrome and fibromyalgia: A status report in 2010. MD Advisor 3(3):18-25.
Natelson, B. H., N. Ye, and Y. C. Cheng. 1990. Evidence of actively replacing Epstein-Barr-virus in patients with the chronic fatigue syndrome. Annals of Neurology 28(2):252.
Natelson, B. H., N. Ye, D. E. Moul, F. J. Jenkins, D. A. Oren, W. N. Tapp, and Y. C. Cheng. 1994. High titers of anti-Epstein-Barr virus DNA polymerase are found in patients with severe fatiguing illness. Journal of Medical Virology 42(1):42-46.
Natelson, B. H., J. J. LaManca, T. N. Denny, A. Vladutiu, J. Oleske, N. Hill, M. T. Bergen, L. Korn, and J. Hay. 1998. Immunologic parameters in chronic fatigue syndrome, major depression, and multiple sclerosis. American Journal of Medicine 105(3A):43S-49S.
Nater, U. M., L. S. Youngblood, J. F. Jones, E. R. Unger, A. H. Miller, W. C. Reeves, and C. Heim. 2008. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosomatic Medicine 70(3):298-305.
Neu, D., O. Mairesse, X. Montana, M. Gilson, F. Corazza, N. Lefevre, P. Linkowski, O. Le Bon, and P. Verbanck. 2014. Dimensions of pure chronic fatigue: Psychophysical, cognitive and biological correlates in the chronic fatigue syndrome. European Journal of Applied Physiology 114(9):1841-1851.
NICE (National Institute for Health and Clinical Excellence). 2007. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): Diagnosis and management of CFS/ME in adults and children. London, UK: NICE.
Nicolson, G. L., M. Y. Nasralla, K. De Meirleir, R. Gan, and J. Haier. 2003. Evidence for bacterial (mycoplasma, chlamydia) and viral (HHV-6) co-infections in chronic fatigue syndrome patients. Journal of Chronic Fatigue Syndrome 11(2):7-19.
Nishikai, M., K. Akiya, T. Tojo, N. Onoda, M. Tani, and K. Shimizu. 1996. “Seronegative” Sjögren’s syndrome manifested as a subset of chronic fatigue syndrome. British Journal of Rheumatology 35(5):471-474.
Nishikai, M., S. Tomomatsu, R. W. Hankins, S. Takagi, K. Miyachi, S. Kosaka, and K. Akiya. 2001. Autoantibodies to a 68/48 kDa protein in chronic fatigue syndrome and primary fibromyalgia: A possible marker for hypersomnia and cognitive disorders. Rheumatology 40(7):806-810.
Ojo-Amaize, E. A., E. J. Conley, and J. B. Peter. 1994. Decreased natural killer cell activity is associated with severity of chronic fatigue immune dysfunction syndrome. Clinical Infectious Diseases 18(Suppl. 1):S157-S159.
Oosterlynck, D. J., F. J. Cornillie, M. Waer, M. Vandeputte, and P. R. Koninckx. 1991. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertility and Sterility 56(1):45-51.
Op De Beéck, K., P. Vermeersch, P. Verschueren, R. Westhovens, G. Mariën, D. Blockmans, and X. Bossuyt. 2012. Antinuclear antibody detection by automated multiplex immunoassay in untreated patients at the time of diagnosis. Autoimmunity Reviews 12(2):137-143.
Ortega-Hernandez, O.-D., M. Cuccia, S. Bozzini, N. Bassi, S. Moscavitch, L.-M. Diaz-Gallo, M. Blank, N. Agmon-Levin, and Y. Shoenfeld. 2009. Autoantibodies, polymorphisms in the serotonin pathway, and human leukocyte antigen class II alleles in chronic fatigue syndrome: Are they associated with age at onset and specific symptoms? In Contemporary challenges in autoimmunity, edited by Y. Shoenfeld and M. E. Gershwin. Annals of the New York Academy of Sciences 1173:589-599.
Ottenweller, J. E., S. A. Sisto, R. C. McCarty, and B. H. Natelson. 2001. Hormonal responses to exercise in chronic fatigue syndrome. Neuropsychobiology 43(1):34-41.
Papadopoulos, A., M. Ebrecht, A. D. L. Roberts, L. Poon, N. Rohleder, and A. J. Cleare. 2009. Glucocorticoid receptor mediated negative feedback in chronic fatigue syndrome using the low dose (0.5 mg) dexamethasone suppression test. Journal of Affective Disorders 112(1-3):289-294.
Parish, J. G. 1978. Early outbreaks of “epidemic neuromyasthenia.” Postgraduate Medical Journal 54(637):711-717.
Park, D. C., J. M. Glass, M. Minear, and L. J. Crofford. 2001. Cognitive function in fibromyalgia patients. Arthritis & Rheumatology 44(9):2125-2133.
Parker, A. J. R., S. Wessely, and A. J. Cleare. 2001. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychological Medicine 31(8):1331-1345.
Patnaik, M., A. L. Komaroff, E. Conley, E. A. Ojo-Amaize, and J. B. Peter. 1995. Prevalence of IgM antibodies to human herpesvirus 6 early antigen (p41/38) in patients with chronic fatigue syndrome. Journal of Infectious Diseases 172(5):1364-1367.
Peakman, M., A. Deale, R. Field, M. Mahalingam, and S. Wessely. 1997. Clinical improvement in chronic fatigue syndrome is not associated with lymphocyte subsets of function or activation. Clinical Immunology and Immunopathology 82(1):83-91.
Peterson, P. K., A. Pheley, J. Schroeppel, C. Schenck, P. Marshall, A. Kind, J. M. Haugland, L. J. Lambrecht, S. Swan, and S. Goldsmith. 1998. A preliminary placebo-controlled crossover trial of fludrocortisone for chronic fatigue syndrome. Archives of Internal Medicine 158(8):908-914.
Porter, N., A. Lerch, L. A. Jason, M. Sorenson, M. A. Fletcher, and J. Herrington. 2010. A comparison of immune functionality in viral versus non-viral CFS subtypes. Journal of Behavioral and Neuroscience Research 8(2):1-8.
Powell, D. J. H., C. Liossi, R. Moss-Morris, and W. Schlotz. 2013. Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: A systematic review and subset meta-analysis. Psychoneuroendocrinology 38(11):2405-2422.
Presson, A. P., E. M. Sobel, J. C. Papp, C. J. Suarez, T. Whistler, M. S. Rajeevan, S. D. Vernon, and S. Horvath. 2009. Integrated weighted gene co-expression network analysis with an application to chronic fatigue syndrome. BMC Systems Biology 2.
Racciatti, D., M. T. Guagnano, J. Vecchiet, P. L. De Remigis, E. Pizzigallo, R. Della Vecchia, T. Di Sciascio, D. Merlitti, and S. Sensi. 2001. Chronic fatigue syndrome: Circadian rhythm and hypothalamic-pituitary-adrenal (HPA) axis impairment. International Journal of Immunopathology and Pharmacology 14(1):11-15.
Reeves, W. C., F. R. Stamey, J. B. Black, A. C. Mawle, J. A. Stewart, and P. E. Pellett. 2000. Human herpesviruses 6 and 7 in chronic fatigue syndrome: A case-control study. Clinical Infectious Diseases 31(1):48-52.
Reeves, W. C., J. F. Jones, E. Maloney, C. Heim, D. C. Hoaglin, R. S. Boneva, M. Morrissey, and R. Devlin. 2007. Prevalence of chronic fatigue syndrome in metropolitan, urban, and rural Georgia. Population Health Metrics 5.
Reyes, M., R. Nisenbaum, D. C. Hoaglin, E. R. Unger, C. Emmons, B. Randall, J. A. Stewart, S. Abbey, J. F. Jones, N. Gantz, S. Minden, and W. C. Reeves. 2003. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Archives of Internal Medicine 163(13):1530-1536.
Richter, J., V. Benson, V. Grobarova, J. Svoboda, J. Vencovsky, R. Svobodova, and A. Fiserova. 2010. CD161 receptor participates in both impairing NK cell cytotoxicity and the response to glycans and vimentin in patients with rheumatoid arthritis. Clinical Immunology 136(1):139-147.
Roberts, A. D. L., S. Wessely, T. Chalder, A. Papadopoulos, and A. J. Cleare. 2004. Salivary cortisol response to awakening in chronic fatigue syndrome. British Journal of Psychiatry 184:136-141.
Roberts, A. D. L., M. L. Charler, A. Papadopoulos, S. Wessely, T. Chalder, and A. J. Cleare. 2010. Does hypocortisolism predict a poor response to cognitive behavioural therapy in chronic fatigue syndrome? Psychological Medicine 40(3):515-522.
Rosmalen, J. G. M., L. M. Tak, J. Ormel, A. Manoharan, I. Kok, S. Wessely, and A. Cleare. 2010. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Journal of Psychosomatic Research 68(6):659-660.
Rowe, P. C., H. Calkins, K. DeBusk, R. McKenzie, R. Anand, G. Sharma, B. A. Cuccherini, N. Soto, P. Hohman, S. Snader, K. E. Lucas, M. Wolff, and S. E. Straus. 2001. Fludrocortisone acetate to treat neurally mediated hypotension in chronic fatigue syndrome: A randomized controlled trial. Journal of the American Medical Association 285(1):52-59.
Russell, I. J., M. D. Orr, B. Littman, G. A. Vipraio, D. Alboukrek, J. E. Michalek, Y. Lopez, and F. MacKillip. 1994. Elevated cerebrospinal fluid levels of substance p in patients with the fibromyalgia syndrome. Arthritis & Rheumatology 37(11):1593-1601.
Sairenji, T., K. Yamanishi, Y. Tachibana, G. Bertoni, and T. Kurata. 1995. Antibody responses to Epstein-Barr virus, human herpesvirus 6 and human herpesvirus 7 in patients with chronic fatigue syndrome. Intervirology 38(5):269-273.
Schutzer, S. E., T. E. Angel, T. Liu, A. A. Schepmoes, T. R. Clauss, J. N. Adkins, D. G. Camp, B. K. Holland, J. Bergquist, P. K. Coyle, R. D. Smith, B. A. Fallon, and B. H. Natelson. 2011. Distinct cerebrospinal fluid proteomes differentiate post-treatment Lyme disease from chronic fatigue syndrome. PLoS ONE 6(2):e17287.
Scott, L. V., F. Burnett, S. Medbak, and T. G. Dinan. 1998a. Naloxone-mediated activation of the hypothalamic-pituitary-adrenal axis in chronic fatigue syndrome. Psychological Medicine 28(2):285-293.
Scott, L. V., S. Medbak, and T. G. Dinan. 1998b. Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatrica Scandinavica 97(6):450-457.
Scott, L. V., S. Medbak, and T. G. Dinan. 1998c. The low dose ACTH test in chronic fatigue syndrome and in health. Clinical Endocrinology 48(6):733-737.
Seishima, M., Y. Mizutani, Y. Shibuya, and C. Arakawa. 2008. Chronic fatigue syndrome after human parvovirus B19 infection without persistent viremia. Dermatology 216(4): 341-346.
Sharpe, M., A. Clements, K. Hawton, A. H. Young, P. Sargent, and P. J. Cowen. 1996. Increased prolactin response to buspirone in chronic fatigue syndrome. Journal of Affective Disorders 41(1):71-76.
Shelokov, A., K. Habel, E. Verder, and W. Welsh. 1957. Epidemic neuromyasthenia; an outbreak of poliomyelitislike illness in student nurses. New England Journal of Medicine 257(8):345-355.
Siegel, S. D., M. H. Antoni, M. A. Fletcher, K. Maher, M. C. Segota, and N. Klimas. 2006. Impaired natural immunity, cognitive dysfunction, and physical symptoms in patients with chronic fatigue syndrome: Preliminary evidence for a subgroup? Journal of Psychosomatic Research 60(6):559-566.
Sirois, D. A., and B. Natelson. 2001. Clinicopathological findings consistent with primary Sjögren’s syndrome in a subset of patients diagnosed with chronic fatigue syndrome: Preliminary observations. Journal of Rheumatology 28(1):126-131.
Skowera, A., E. Stewart, E. T. Davis, A. J. Cleare, C. Unwin, L. Hull, K. Ismail, G. Hossain, S. C. Wessely, and M. Peakman. 2002. Antinuclear autoantibodies (ANA) in gulf war-related illness and chronic fatigue syndrome (CFS) patients. Clinical & Experimental Immunology 129(2):354-358.
Smylie, A. L., G. Broderick, H. Fernandes, S. Razdan, Z. Barnes, F. Collado, C. Sol, M. A. Fletcher, and N. Klimas. 2013. A comparison of sex-specific immune signatures in gulf war illness and chronic fatigue syndrome. BMC Immunology 14:29.
Solomon, L., and W. C. Reeves. 2004. Factors influencing the diagnosis of chronic fatigue syndrome. Archives of Internal Medicine 164(20):2241-2245.
Stewart, C. C., D. L. Cookfair, K. M. Hovey, K. E. Wende, D. S. Bell, and C. L. Warner. 2003. Predictive immunophenotypes: Disease-related profile in chronic fatigue syndrome. Cytometry Part B, Clinical Cytometry 53(1):26-33.
Straus, S. E., J. K. Dale, M. Tobi, T. Lawley, O. Preble, R. M. Blaese, C. Hallahan, and W. Henle. 1988. Acyclovir treatment of the chronic fatigue syndrome. Lack of efficacy in a placebo-controlled trial. New England Journal of Medicine 319(26):1692-1698.
Straus, S. E., S. Fritz, J. K. Dale, B. Gould, and W. Strober. 1993. Lymphocyte phenotype and function in the chronic fatigue syndrome. Journal of Clinical Immunology 13(1):30-40.
Strauss, B., M. Loschau, T. Seidel, A. Stallmach, and A. Thomas. 2012. Are fatigue symptoms and chronic fatigue syndrome following Q fever infection related to psychosocial variables? Journal of Psychosomatic Research 72(4):300-304.
Strickland, P. S., P. H. Levine, D. L. Peterson, K. O’Brien, and T. Fears. 2001. Neuromyasthenia and chronic fatigue syndrome (CFS) in northern Nevada/California: A ten-year follow-up of an outbreak. Journal of Chronic Fatigue Syndrome 9(3-4):3-14.
Sumaya, C. V. 1991. Serologic and virologic epidemiology of Epstein-Barr virus: Relevance to chronic fatigue syndrome. Reviews of Infectious Diseases 13(Suppl. 1):S19-S25.
Swanink, C. M., J. W. van der Meer, J. H. Vercoulen, G. Bleijenberg, J. F. Fennis, and J. M. Galama. 1995. Epstein-Barr virus (EBV) and the chronic fatigue syndrome: Normal virus load in blood and normal immunologic reactivity in the EBV regression assay. Clinical Infectious Diseases 20(5):1390-1392.
Tak, L. M., A. J. Cleare, J. Ormel, A. Manoharan, I. C. Kok, S. Wessely, and J. G. Rosmalen. 2011. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biological Psychology 87(2):183-194.
Tanaka, S., H. Kuratsune, Y. Hidaka, Y. Hakariya, K. I. Tatsumi, T. Takano, Y. Kanakura, and N. Amino. 2003. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. International Journal of Molecular Medicine 12(2):225-230.
The, G. K. H., G. Bleijenberg, and J. W. M. van der Meer. 2007. The effect of acclydine in chronic fatigue syndrome: A randomized controlled trial. PLOS Clinical Trials 2(5):e19.
Tirelli, U., G. Marotta, S. Improta, and A. Pinto. 1994. Immunological abnormalities in patients with chronic-fatigue-syndrome. Scandinavian Journal of Immunology 40(6):601-608.
Tomas, C., J. Newton, and S. Watson. 2013. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neuroscience 2013:1-8.
Unger, E. 2013. Measures of CFS in a multi-site clinical study. Paper read at FDA Scientific Drug Development Workshop, April 26, 2013, Washington, DC.
Van Den Eede, F., G. Moorkens, W. Hulstijn, B. Van Houdenhove, P. Cosyns, B. G. C. Sabbe, and S. J. Claes. 2008. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. Psychological Medicine 38(7):963-973.
Van Oosterwijck, J., J. Nijs, M. Meeus, I. Lefever, L. Huybrechts, L. Lambrecht, and L. Paul. 2010. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: An experimental study. Journal of Internal Medicine 268(3):265-278.
Vernon, S. D., and W. C. Reeves. 2005. Evaluation of autoantibodies to common and neuronal cell antigens in chronic fatigue syndrome. Journal of Autoimmune Diseases 2(5).
Vollmer-Conna, U., B. Cameron, D. Hadzi-Pavlovic, K. Singletary, T. Davenport, S. Vernon, W. C. Reeves, I. Hickie, D. Wakefield, and A. R. Lloyd. 2007. Postinfective fatigue syndrome is not associated with altered cytokine production. Clinical Infectious Diseases 45(6):732-735.
vonMikecz, A., K. Konstantinov, D. S. Buchwald, L. Gerace, and E. M. Tan. 1997. High frequency of autoantibodies to insoluble cellular antigens in patients with chronic fatigue syndrome. Arthritis and Rheumatism 40(2):295-305.
Watt, T., S. Oberfoell, R. Balise, M. R. Lunn, A. K. Kar, L. Merrihew, M. S. Bhangoo, and J. G. Montoya. 2012. Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein-Barr virus IgG antibody titers. Journal of Medical Virology 84(12):1967-1974.
Weiner, D. K., T. E. Rudy, L. Morrow, J. Slaboda, and S. Lieber. 2006. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Medicine 7(1):60-70.
Whelton, C. L., I. Salit, and H. Moldofsky. 1992. Sleep, Epstein-Barr virus infection, musculoskeletal pain, and depressive symptoms in chronic fatigue syndrome. Journal of Rheumatology 19(6):939-943.
White, A. T., A. R. Light, R. W. Hughen, L. Bateman, T. B. Martins, H. R. Hill, and K. C. Light. 2010. Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology 47(4):615-624.
White, K. P., M. Speechley, M. Harth, and T. Ostbye. 1999. The London fibromyalgia epidemiology study: The prevalence of fibromyalgia syndrome in London, Ontario. Journal of Rheumatology 26(7):1570-1576.
White, P. D., J. M. Thomas, J. Amess, D. H. Crawford, S. A. Grover, H. O. Kangro, and A. W. Clare. 1998. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. British Journal of Psychiatry 173:475-481.
Whiteside, A., S. Hansen, and A. Chaudhuri. 2004. Exercise lowers pain threshold in chronic fatigue syndrome. Pain 109(3):497-499.
Whiteside, T. L., and D. Friberg. 1998. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. American Journal of Medicine 105(3A):27S-34S.
Wildman, M. J., E. G. Smith, J. Groves, J. M. Beattie, E. O. Caul, and J. G. Ayres. 2002. Chronic fatigue following infection by Coxiella burnetii (Q fever): Ten-year follow-up of the 1989 UK outbreak cohort. QJM: Monthly Journal of the Association of Physicians 95(8):527-538.
Wolfe, F., K. Ross, J. Anderson, I. J. Russell, and L. Hebert. 1995. The prevalence and characteristics of fibromyalgia in the general population. Arthritis & Rheumatology 38(1):19-28.
Wolfe, F., D. J. Clauw, M. A. Fitzcharles, D. L. Goldenberg, R. S. Katz, P. Mease, A. S. Russell, I. J. Russell, J. B. Winfield, and M. B. Yunus. 2010. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research 62(5):600-610.
Yalcin, S., H. Kuratsune, K. Yamaguchi, T. Kitani, and K. Yamanishi. 1994. Prevalence of human herpesvirus-6 variant-A and variant-B in patients with chronic-fatigue-syndrome. Microbiology and Immunology 38(7):587-590.
Yamamoto, S., Y. Ouchi, H. Onoe, E. Yoshikawa, H. Tsukada, H. Takahashi, M. Iwase, K. Yamaguti, H. Kuratsune, and Y. Watanabe. 2004. Reduction of serotonin transporters of patients with chronic fatigue syndrome. NeuroReport: For Rapid Communication of Neuroscience Research 15(17):2571-2574.
Yanai, H., K. Takada, N. Shimizu, Y. Mizugaki, M. Tada, and K. Okita. 1997. Epstein-Barr virus infection in non-carcinomatous gastric epithelium. Journal of Pathology 183(3):293-298.
Zeidel, A., B. Beilin, I. Yardeni, E. Mayburd, G. Smirnov, and H. Bessler. 2002. Immune response in asymptomatic smokers. Acta Anaesthesiologica Scandinavica 46(8):959-964.
Zhang, H. Y., Z. D. Liu, C. J. Hu, D. X. Wang, Y. B. Zhang, and Y. Z. Li. 2011. Up-regulation of TGF-β1 mRNA expression in peripheral blood mononuclear cells of patients with chronic fatigue syndrome. Journal of the Formosan Medical Association 110(11):701-704.
Zorzenon, M. 1996. Active HHV-6 infection in chronic fatigue syndrome patients from Italy: New data. Journal of Chronic Fatigue Syndrome 2(1):3-12.
This page intentionally left blank.








































