2
The Current Policy Environment
In general, the U.S. government encourages and promotes the dissemination of basic research. The government also recognizes that some research may offer both benefits and risks and has, accordingly, developed policies to manage the dissemination of information in circumstances where it has authority. While acknowledging that its role has limits, the government recognizes that, given the nature of federal funding streams and the international scope of the life sciences research enterprise (see Box 2-1), there is significant value in frameworks and guiding principles that may be adopted by the larger community of researchers.1
The current U.S. government approach to the oversight of dual use research in general and dual use research of concern (DURC) in particular fits within the larger set of overlapping laws and regulations, policies, and guidelines that constitute the U.S. strategy for countering biological threats, including biological weapons and bioterrorism (see Figure 2-1).2
It is important to recognize that there are significant limitations to the reach of most regulations. In the particular case of DURC, the policies target research conducted with federal funding or at institutions that receive federal funding. They apply only to research that involves certain agents or pathogens and types of experiments. Moreover, the policies are aimed at seeking a level of oversight
___________________
1 While their focus is research conducted in the United States, two National Academies’ reports, Institute of Medicine and National Research Council, Guidelines for Human Embryonic Stem Cell Research (Washington, DC: The National Academies Press, 2005), doi:https://doi.org/10.17226/11278) and National Academies of Sciences, Engineering, and Medicine, Human Genome Editing: Science, Ethics, and Governance (Washington, DC: The National Academies Press, 2017), doi:https://doi.org/10.17226/24623, have provided guidelines that have wide applicability for the broader community of researchers. The guidelines have exerted particular influence internationally.
2 National Security Council, National Strategy to Counter Biological Threats (Washington, DC, 2009). Available at https://obamawhitehouse.archives.gov/sites/default/files/National_Strategy_for_Countering_BioThreats.pdf.
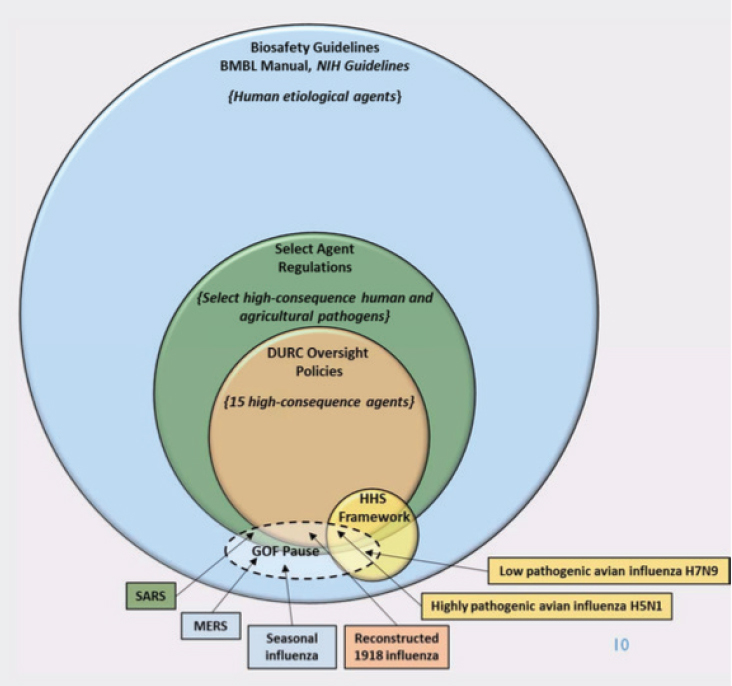
NOTE: BMBL = Biosafety in Microbiological and Biomedical Laboratories; DURC = Dual Use Research of Concern; GOF = gain-of-function; HHS = U.S. Department of Health and Human Services; MERS = Middle East respiratory syndrome; SARS = Severe acute respiratory syndrome; NIH = National Institutes of Health.
SOURCE: National Science Advisory Board for Biosecurity, Recommendations for the Evaluation and Oversight of Proposed Gain-of-Function Research (Washington, DC, 2016), p. 28. This image is a work of the National Science Advisory Board for Biosecurity, taken or made during the course of an employee’s official duties. As the work of the U.S. federal government, the image is in the public domain.
over research that could be used for dangerous purposes, not to prohibit such research or publication of research findings. These policies are complementary to other mechanisms that impose criminal penalties for misuse.
Policies governing the management of the dissemination of potentially sensitive scientific information focus on three categories of actors—research funders, research institutions, and researchers.3 In addition, while not the subject of federal policies, scientific journals are critical players in the management of dual use research as they must make determinations about whether to publish potentially harmful research findings.
THE DEVELOPMENT OF U.S. GOVERNMENT OVERSIGHT OF INFORMATION WITH NATIONAL SECURITY IMPLICATIONS
By the early 1980s, concerns had grown that U.S. adversaries, in particular the Soviet Union, were using the openness of academic research in the United States to obtain militarily useful information. The National Research Council (NRC) undertook a study of security issues related to university research that resulted in the publication of Scientific Communication and National Security in 1982.4 Known as the Corson Report, after study committee chair Dale Corson, the report concluded that for “the largest share [of university research], the benefits of total openness overshadow their possible near-term military benefits to the Soviet Union.” The report noted that there are areas of research for which classification is clearly indicated and observed that there is a small “gray area” between openly disseminated research and classified research for which some controls might be appropriate.5 The report does not explicitly consider the biological sciences.
In 1985, President Ronald Reagan issued National Security Decision Directive 189 (NSDD-189), which declared that “to the maximum extent possible, the products of fundamental research remain unrestricted . . . [and] where national security requires control, the mechanism for control of information
___________________
3 See, e.g., U.S. Government, United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern, 2012 (available at http://www.phe.gov/s3/dualuse/Documents/uspolicy-durc-032812.pdf) and U.S. Government, United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern, 2014 (available at http://www.phe.gov/s3/dualuse/Documents/durc-policy.pdf).
4 Institute of Medicine, National Academy of Sciences, and National Academy of Engineering, Scientific Communication and National Security (Washington, DC: The National Academies Press, 1982), doi:https://doi.org/10.17226/253.
5 Ibid, p. 4. The report (see p. 4) suggested that, for research in this area, “restrictions short of classification are appropriate.” The report set forth criteria to be met before the communication of research could be restricted: the research was dual use or had direct military application; the technology was rapidly developing with a short time frame from the basic science to the development of an application; the dissemination of the research could give short-term military benefit to adversaries; and information about the technology was held only by the U.S. or friendly nations (see p. 5).
generated during federally funded fundamental research in science, technology and engineering at colleges, universities and laboratories is classification.”6 The Reagan Administration did not seek to introduce controls on “gray areas.” NSDD-189 remains the foundation for U.S. policy related to potential restrictions on the publication of scientific research, and the directive has been reaffirmed by subsequent administrations, including by National Security Advisor Condoleeza Rice following the attacks of September 11, 2001, and most recently by Undersecretary of Defense Ashton Carter in 2010.
General U.S. government policy on classification is governed by federal statutes and executive orders. The most recent executive order (Executive Order 13526), issued in 2009, specifies that information may be classified if it is “owned by, produced by or for, or is under the control of the United States Government. . . . Basic scientific research information not clearly related to the national security shall not be classified.”7 There are only two limited exceptions. The government may classify even information that it does not own, does not control, or was not produced for it if: (1) the information is related to nuclear weapons (Atomic Energy Act of 1954), or (2) the information is a patent application whose disclosure “might . . . be detrimental to national security” (Invention Secrecy Act of 1951).8 Controls on certain types of unclassified information are also laid out in specific statutes. These include sensitive security information,9 unclassified controlled nuclear information,10 and protected critical infrastructure information.11
___________________
6 National Security Decision Directive 189 (NSDD-189): National Policy on the Transfer of Scientific, Technical and Engineering Information (September 21, 1985).
7 White House Office of the Press Secretary, Executive Order 13526—Classified National Security Information (Washington, DC, 2009). Available at https://www.whitehouse.gov/the-press-office/executive-order-classified-national-security-information.
8 The Invention Secrecy Act of 1951 (see https://www.govinfo.gov/content/pkg/STATUTE-66/pdf/STATUTE-66-Pg3.pdf) was enacted to prohibit the disclosure of inventions deemed by the Atomic Energy Commission, the Secretary of Defense, and the chief officers of defense agencies to be a detriment to national security. It allows for withholding of the granting of a patent if doing so is deemed to be in the national interest. Information on the number of patent secrecy order in effect at the end of fiscal year 2016 is available at https://fas.org/sgp/othergov/invention/.
9 “Sensitive Security Information (SSI) is a control designation used by the Department of Homeland Security, and particularly the Transportation Security Administration. It is applied to information about security programs, vulnerability and threat assessments, screening processes, technical specifications of certain screening equipment and objects used to test screening equipment, and equipment used for communicating security information relating to air, land, or maritime transportation.” See http://www.dhra.mil/perserec/osg/s2unclas/ssi.htm.
10 Unclassified Controlled Nuclear Information (UCNI) is “certain unclassified information about nuclear facilities and nuclear weapons that must be controlled because its unauthorized release could have a significant adverse effect on the national security or public health and safety.” See https://energy.gov/ehss/services/classification/unclassified-controlled-nuclear-information-ucni.
11 Protected Critical Infrastructure Information (PCII) is “information not customarily in the public domain and related to the security of critical infrastructure or protected systems.” See section 212(3) (6 U.S.C. 131(3)).
For information held by the government that is not classified or under specific statutory control, the Freedom of Information Act (FOIA) gives the public “the right to request access to records from any federal agency.” “Federal agencies are required to disclose any information requested under the FOIA unless it falls under one of nine exemptions which protect interests such as personal privacy, national security, and law enforcement.”12
Unless it is classified or subject to an exemption, data from research funded and held by the federal government are available under FOIA.13 In fact, information in the possession of the federal government that is not classified or covered by a FOIA exemption is subject to release even if it is not owned by the government. Investigative journalists and others have used FOIA as a means to obtain information about institutional research, animal research, and biosafety records residing with agencies.14 However, if the federal government is not in possession of federally funded information (e.g., DURC) held by researchers, it is not required to obtain such information from researchers in response to a FOIA request.15
Export controls are another mechanism by which dissemination of scientific information may be managed or restricted. As discussed in Chapter 3, these controls typically apply to the transfer of certain physical items and of non-public technical data associated with the items to particular destinations. Export controls may be brought to bear on information derived from research in two ways, but only if the transfer involves an export (the exchange of information among U.S. citizens in the United States is not limited or constrained by export controls). One involves the transfer of scientific information to foreign
___________________
12 See https://www.foia.gov/about.html. The nine exemptions are: Exemption 1: Information that is classified to protect national security; Exemption 2: Information related solely to the internal personnel rules and practices of an agency; Exemption 3: Information that is prohibited from disclosure by another federal law; Exemption 4: Trade secrets or commercial or financial information that is confidential or privileged; Exemption 5: Privileged communications within or between agencies, including deliberative process privilege, attorney-work product privilege, or attorney-client privilege; Exemption 6: Information that, if disclosed, would invade another individual’s personal privacy; Exemption 7: Information compiled for law enforcement purposes that: 7(A) could reasonably be expected to interfere with enforcement proceedings, 7(B) would deprive a person of a right to a fair trial or an impartial adjudication, 7(C) could reasonably be expected to constitute an unwarranted invasion of personal privacy, 7(D) could reasonably be expected to disclose the identity of a confidential source, 7(E) would disclose techniques and procedures for law enforcement investigations or prosecutions, or 7(F) could reasonably be expected to endanger the life or physical safety of any individual; Exemption 8: Information that concerns the supervision of financial institutions; Exemption 9: Geological information on wells.
13 Carrie Wolinetz, National Institutes of Health, Presentation to the committee, July 11, 2016, New York, NY.
14 See, e.g., A. Young and N. Penzenstadler, “Universities, Feds Fight to Keep Lab Failings Secret,” USA Today, May 28, 2015. Available at https://www.usatoday.com/story/news/2015/05/28/labs-fight-for-secrecy/26530719/.
15 State open access laws are also used to obtain information.
scientists, including even those working in the United States. The other relates to information redacted from a scientific publication. If a federal agency determines that aspects of a research study should not be exported, these aspects may be subject to export control regulations, such as requirements to obtain an export license before communicating the information to a foreigner.
In addition, the dissemination of scientific information can be controlled by the terms and conditions of a federal funder:
Most government grants for unclassified technical activity specify that if the grantee believes the results of that work warrant classification, the grantee has the responsibility to limit the dissemination of that work and to contact the appropriate U.S. government agency that would have the authority to classify it. In such extraordinary cases, the initiative to seek classification rests with the grantee, not the government.16
THE EVOLUTION OF U.S. GOVERNMENT POLICY ON DUAL USE RESEARCH IN THE LIFE SCIENCES
After the September 11 attacks and the anthrax mailings that followed, the federal government enacted regulations to provide additional oversight for research on select agents and toxins.17 The Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (PATRIOT Act) (P.L. 107-56) defined, in part, the reasons for which people may possess biological agents created through recombinant DNA technologies and specified those “restricted persons” who were not allowed to possess or transport select biological agents or toxins. In 2002, the Public Health Security and Bioterrorism Preparedness and Response Act (P.L. 107-188) added registration requirements for individuals working with biological select agents and toxins, added background checks for researchers, and provided additional guidance to the U.S. Department of Agriculture and the Department of Health and Human Services regarding listing select agents and safeguarding them during transfer. The Select Agent Program developed under the PATRIOT Act and the Public Health Security and Bioterrorism Preparedness and Response Act focuses on the handling of pathogens and toxins of concern; neither includes guidelines or requirements for the dissemination
___________________
16 Commission on Scientific Communication and National Security, Security Controls on Scientific Information and the Conduct of Scientific Research: A White Paper of the Commission on Scientific Communication and National Security (Washington, DC: Center for Strategic and International Studies, June 2005), p. 6.
17 Select agents and toxins are biological agents and toxins “which have the potential to pose a severe threat to public, animal or plant health or to animal or plant products.” See https://www.selectagents.gov/. The current list of select agents and toxins is available at https://www.selectagents.gov/SelectAgentsandToxinsList.html.
of research results regarding the pathogens and toxins, although certain types of experiments require review before they are carried out.18 The Select Agent Program requires reporting of “theft, loss, and release” of agents from laboratories registered with the program, and has made some of these data publicly available.
In 2005, based upon the recommendations of the Fink Report, the federal government established the National Science Advisory Board for Biosecurity (NSABB) to help assess the potential risks of life sciences research and offer advice to policymakers, research institutions, and researchers about the conduct, oversight, and communication of sensitive research.19
The NSABB was established to advise the Secretary of the Department of Health and Human Services, the Director of the National Institutes of Health (NIH), and heads of federal agencies that conduct, support, or have an interest in life sciences research. Originally, the board had a broad charge, but the current charge is much narrower (see Table 2-1). In particular, after the controversy over publication of work on influenza viruses with enhanced transmission properties, the April 2012 version of the charter removed the NSABB’s capacity to “Review and provide guidance on specific experiments insofar as they exemplify a significant or particularly complex permutation of an existing category of dual-use research, or represent a novel category of dual-use research that requires additional guidance from the NSABB.”20
NSABB activities during the board’s first 10 years included issuing recommendations on the oversight of life sciences research of dual use concern in general21 and on synthetic biology and gain-of-function (GOF) research involving pathogens with pandemic potential in particular. The NSABB also developed recommendations for codes of scientific conduct and encouraged a culture of
___________________
18 Further information about the Select Agent Program may be found at http://www.selectagents.gov/. The original Select Agent Program was created in 1996 under the Antiterrorism and Effective Death Penalty Act (P.L.104-132) in response to the efforts of a researcher with ties to white supremacist organizations to obtain Yersinia pestis samples from the American Type Culture Collection. The program’s purpose was to govern the transfer of an initial list of 42 pathogens and toxins. See J. E. Stern, “Larry Wayne Harris (1998)” in J. B. Tucker, ed., Toxic Terror. Assessing Terrorist Use of Chemical and Biological Weapons (Cambridge: MIT Press, 2000).
19 Information about the National Science Advisory Board for Biosecurity may be found at http://osp.od.nih.gov/office-biotechnology-activities/biosecurity/nsabb.
20 See G. D. Koblentz, “Is the NSABB Still Relevant to Today’s Biosecurity Challenges?,” July 16, 2014 (available at https://pandorareport.org/2014/07/16/is-the-nsabb-still-relevant-to-todays-biosecurity-challenges/) and Reuters, “US lawmakers question oversight of potentially dangerous experiments,” August 13, 2014 (available at http://www.foxnews.com/health/2014/08/13/us-lawmakers-question-oversight-potentially-dangerous-experiments.amp.html).
21 National Science Advisory Board for Biosecurity, Proposed Framework for the Oversight of Dual Use Life Sciences Research (Washington, DC, 2007).
| 2004 Charge | 2016 Charge |
|---|---|
|
|
| 2004 Charge | 2016 Charge |
|---|---|
|
a See Congressional Research Service, Oversight of Dual-Use Biological Research: The National Science Advisory Board for Biosecurity (Washington, DC, April 27, 2007). “The NSABB is chartered for two-year intervals and [. . . continues] its work pending biennial renewals of the charter by the Secretary of the Department of Health and Human Services (HHS).” See National Institutes of Health Office of Science Policy, “NSABB FAQs.” Available at: http://osp.od.nih.gov/office-biotechnology-activities/biosecurity/nsabb/faq.
responsibility, and educated scientists about DURC.22 The board also engaged the international community around issues in life sciences DURC.23
As noted in Chapter 1, the NSABB refined the Fink Report’s dual use concept in its 2007 Proposed Framework for the Oversight of Dual Use Life Sciences Research: Strategies for Minimizing the Potential Misuse of Research Information24 to underscore that only a small set of life sciences experiments should raise significant issues. This resulted in the creation of the special category of research termed “dual use research of concern.”25
The NSABB also addressed the communication and dissemination of research results, emphasizing the importance of monitoring research for dual use potential from experimental design through publication. In 2007, the board released a report titled Responsible Communication of Life Sciences Research with Dual-Use Potential.26 That report offered an approach to “facilitate consistent decision making about the responsible communication of research information with dual use potential” and provided a “tool set” that includes: (1) principles for the responsible communication of research with dual use potential; (2) points to consider for identifying and assessing the risks and benefits of communicating research information with dual use potential; and (3) considerations for the development of a communication plan for research with dual use potential.27 The NSABB also created two working groups on scientific journals’ review policies and conducted surveys of journals’ policies for reviewing DURC.28
The NSABB played a significant role in the 2011 H5N1 avian influenza (later described as the gain-of-function research) controversy and in the development of U.S. government policy for the oversight of DURC.29 The NSABB provided an initial review of two controversial influenza papers in December
___________________
22 C. Wolinetz, Presentation to the committee. See also “NSABB Reports and Recommendations” at http://osp.od.nih.gov/office-biotechnology-activities/biosecurity/nsabb/reports-andrecommendations.
23 See http://osp.od.nih.gov/office-biotechnology-activities/biosecurity/nsabb/nsabb-meetings-and-conferences/international-engagement for further information.
24 Available at http://osp.od.nih.gov/sites/default/files/resources/Framework%20for%20transmittal%20duplex%209-10-07.pdf.
25 “Research that, based on current understanding, can be reasonably anticipated to provide knowledge, products, or technologies that could be directly misapplied by others to pose a threat to public health and safety, agricultural crops and other plants, animals, the environment, or materiel.” See Proposed Framework for the Oversight of Dual Use Life Sciences Research, p. 17.
26 National Science Advisory Board for Biosecurity, Responsible Communication of Life Sciences Research with Dual Use Potential: A Set of Communication Tools Excerpted from the NSABB Proposed Framework for the Oversight of Dual Use Life Sciences Research” (Washington, DC, 2007).
27 Ibid, p. 3.
28 C. Wolinetz, Presentation to the committee.
29 See, e.g., G. Kwik Gronvall, H5N1: A Case Study for Dual-Use Research (New York: Council on Foreign Relations, July 2013). For a discussion of the GOF research controversy and the development of U.S. policy in this area, see Institute of Medicine and National Research Council, Potential
2011 and unanimously recommended against publication unless certain portions of the methods section were redacted. The board recommended that an arrangement be found to provide access to redacted information for researchers with a legitimate need.
The recommendations evoked a storm of controversy because they sought restrictions on publication.30 Following additional discussion and receipt of additional information about the research and in light of the inability of the U.S. government to require that the redacted material be withheld,31 the board voted in March 2012 to recommend publication of revised versions of both papers.32 Concurrently, the U.S. government announced a policy to govern federal oversight of DURC.
The GOF controversy resulted in a number of policies for the oversight of DURC. In 2012, the U.S. government released the United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern. The policy is intended to guide government agencies in the funding life sciences research. The policy applies to research involving one or more of 15 specific agents and toxins33 and using one of seven types of experiments that: increase an agent or toxin’s “harmful consequences;” disrupts immunity or effectiveness of immunizations to the agent or toxin; makes it resistant to prophylactic interventions or helps it evade detection; increases its stability, transmissibility,
___________________
Risks and Benefits of Gain-of-Function Research: Summary of a Workshop (Washington, DC: The National Academies Press., 2015), doi:https://doi.org/10.17226/21666.
30 See, e.g., the discussions described in K. Matchett, A. Mazza, and S. Kendall, Perspectives on Research with H5N1 Avian Influenza: Scientific Inquiry, Communication, Controversy: Summary of a Workshop (Washington, DC: National Academies Press, 2013) and G. Kwik Gronvall, H5N1: A Case Study for Dual-Use Research.
31 Francis Collins, at the time the director of the National Institutes of Health, informed the board that, upon the advice of counsel, the NSABB did not have the authority to redact manuscripts. See C. Wolinetz, Presentation to the committee; David A. Relman, Stanford University and VA Palo Alto Health Care System, Presentation to the committee, July 11, 2016, New York, NY; and Michael Imperiale, University of Michigan, Presentation to the committee, July 11, 2016, New York, NY.
32 See National Science Advisory Board for Biosecurity, National Science Advisory Board for Biosecurity Findings and Recommendations March 29-30, 2012 (Washington, DC, 2012) (available at http://osp.od.nih.gov/sites/default/files/resources/03302012_NSABB_Recommendations_1.pdf) and National Science Advisory Board for Biosecurity, March 29-30, 2012 Meeting of the National Science Advisory Board for Biosecurity to Review Revised Manuscripts on Transmissibility of A/H5N1 Influenza Virus: Statement of the NSABB (Washington, DC, 2012) (available at http://osp.od.nih.gov/sites/default/files/resources/NSABB_Statement_March_2012_Meeting.pdf). The board’s vote in favor of publication was unanimous for one of the papers. For the other, the vote on publication was 12 in favor and 6 against.
33 The agents and toxins are: 1) Avian influenza virus (highly pathogenic); 2) Bacillus anthracis; 3) Botulinum neurotoxin; 4) Burkholderia mallei; 5) Burkholderia pseudomallei; 6) Ebola virus; 7) Foot-and-mouth disease virus; 8) Francisella tularensis; 9) Marburg virus; 10) Reconstructed 1918 influenza virus; 11) Rinderpest virus; 12) Toxin-producing strains of Clostridium botulinum; 13) Variola major virus; 14) Variola minor virus; and 15) Yersinia pestis.
or ability to disseminate; alters its host range; makes a host population more susceptible to it; or “generates or reconstitutes an eradicated or extinct agent or toxin” on the list of 15 agents or toxins.34 A researcher proposing to undertake experiments covered by the policy must make an initial assessment of potential risk and, if needed, develop, in collaboration with the federal funder, a risk mitigation plan.35 The risk mitigation plan may limit the “venue and mode” of communication, or, if necessary, request voluntary redactions if risks cannot be adequately mitigated.36 In extreme cases, the funding agency could elect not to provide funding, apply classification rules as a term and condition of funding, or terminate federal funding. According to the White House Office of Science and Technology Policy, “no Department or Agency has reported use of voluntary redaction of publication as part of a risk mitigation plan for any dual-use research of concern research project that has been reported to the Assistant to the President for Homeland Security and Counterterrorism pursuant to the” 2012 policy.37
The 2012 policy was supplemented by a 2014 policy that outlined the responsibilities of research-performing institutions receiving federal funding for life sciences research.38 Researchers working with DURC were given a range of responsibilities including risk assessment and, in some cases, risk mitigation. Research institutions are required to fulfill their responsibility for the oversight of dual use research through an Institutional Review Entity that conducts reviews of institutional research of concern, develops risk mitigation plans, assesses and tracks compliance with the plans, and communicates information about activity of dual use concern to the funder of the research.
In addition, since February 2013, the Department of Health and Human Services (HHS) has conducted special reviews of requests for funding of GOF experiments involving highly pathogenic H5N1 avian influenza. The criteria used by HHS to evaluate proposed research are articulated in a 2013 HHS document titled A Framework for Guiding U.S. Department of Health and Human Services Funding Decisions About Research Proposals with the Poten-
___________________
34 United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern.
35 For DURC that is proposed and not yet funded, departments and agencies will assess whether to incorporate risk mitigation measures in the grant, contract, or agreement. See United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern.
36 For a further discussion, see National Institutes of Health, Tools for the Identification, Assessment, Management, and Responsible Communication of Dual Use Research of Concern: A Companion Guide to the United States Government Policies for Oversight of Life Sciences Dual Use Research of Concern (Washington, DC: The National Academies Press, 2014), pp. 49-53. Available at https://www.phe.gov/s3/dualuse/Documents/durc-companion-guide.pdf.
37 Gerald L. Epstein, Assistant Director for Biosecurity and Emerging Technologies, National Security and International Affairs Division, White House Office of Science and Technology Policy, communication with committee staff, June 19, 2017.
38 United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern.
tial for Generating Highly Pathogenic Avian Influenza H5N1 Viruses That Are Transmissible Among Mammals by Respiratory Droplets.39 Two of the criteria are relevant to dissemination. One criterion is that “the research information is anticipated to be broadly shared in order to realize its potential benefits to global health,” while the second is that the research “will be supported through funding mechanisms that facilitate appropriate oversight of the conduct and communication of the research.”40 The HHS document provides the only U.S. policy specific to GOF research.
Recent DURC policy and discussions have continued to focus on GOF research on pathogens with pandemic potential. Over time, the primary concerns about GOF research have shifted from biosecurity to biosafety. A series of serious biosafety lapses at federal laboratories during the summer of 2014, coupled with concerns about GOF research in light of the emergence of highly pathogenic avian influenza, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), led the U.S. government to institute a pause in federal funding for certain GOF research.41 The White House also instituted a deliberative process involving the NSABB. The board was tasked with developing recommendations for a system to provide oversight of GOF research. The National Academy of Sciences was asked to host two international symposia to collect broad stakeholder input for the NSABB’s deliberations. The meetings explored the scientific and technical considerations involved in assessing the risks and benefits of GOF research and discussed the NSABB’s draft recommendations.42 The final NSABB Recommendations for the Evaluation and Oversight of Proposed Gain-of-Function Research were
___________________
39 This document is available at http://www.phe.gov/s3/dualuse/Documents/funding-hpai-h5n1.pdf.
40 See U.S. Department of Health and Human Services, A Framework for Guiding U.S. Department of Health and Human Services Funding Decisions About Research Proposals with the Potential for Generating Highly Pathogenic Avian Influenza H5N1 Viruses That Are Transmissible Among Mammals by Respiratory Droplets (Washington, DC, 2013). Available at http://www.phe.gov/s3/dualuse/Documents/funding-hpai-h5n1.pdf.
41 White House, U.S. Government Gain-of-Function Deliberative Process and Research Funding Pause on Selected Gain-of-Function Research Involving Influenza, MERS, and SARS Viruses (Washington, DC, October 17, 2014). Available at http://www.phe.gov/s3/dualuse/Documents/gain-of-function.pdf.
The pause remains in place pending agency implementations of review mechanisms consistent with guidance issued in January 2017 (see Recommended Policy Guidance for Departmental Development of Review Mechanisms for Potential Pandemic Pathogen Care and Oversight, available at: https://www.phe.gov/s3/dualuse/Pages/GainOfFunction.aspx).
42 Institute of Medicine and National Research Council, Potential Risks and Benefits of Gain-of-Function Research: Summary of a Workshop (Washington, DC: The National Academies Press, 2015), doi:https://doi.org/10.17226/21666; National Academies of Sciences, Engineering, and Medicine, Gain-of-Function Research: Summary of the Second Symposium, March 10-11, 2016 (Washington, DC: The National Academies Press, 2016), doi:https://doi.org/10.17226/23484.
delivered to the White House in May 2016.43 In January 2017, in response to the NSABB recommendations and an interagency review, the White House Office of Science and Technology Policy (OSTP) issued policy guidance recommending pre-funding review mechanisms for federal agencies that conduct or support the creation, transfer, or use of enhanced pathogens of pandemic potential (PPPs).44 For federally sponsored research, the guidance recommends that agencies adopt the dissemination policies that currently apply to DURC research under the March 2012 DURC policy (i.e., “venue and mode” restrictions for risk mitigation, voluntary redactions, or classification).
A number of the NSABB’s findings about U.S. policy for GOF research are relevant to the broader discussion of DURC policy. The 2016 report highlighted the overlapping policy and regulatory frameworks that provide oversight for DURC and certain types of GOF research. While only some of the policies and regulations are directly relevant to specific publication issues, most have the potential to impact dissemination. (See Box 2-2.)
THE INTERNATIONAL CONTEXT
Efforts to develop oversight mechanisms to manage the dissemination of DURC information in the United States take place in a broader international context.45 In addition, the terms and conditions of U.S. government-funded research apply outside the United States, as the case of the NIH-funded researchers in the Netherlands embroiled in the 2011 GOF controversy illustrates. The United States may be global leader in biological research, however; absent a funding connection to the United States, U.S. DURC policies do not apply to activities in other countries.46
___________________
43 National Science Advisory Board for Biosecurity, Recommendations for the Evaluation and Oversight of Proposed Gain-of-Function Research (Washington, DC, 2016). Available at http://osp.od.nih.gov/sites/default/files/resources/NSABB_Final_Report_Recommendations_Evaluation_Oversight_Proposed_Gain_of_Function_Research.pdf.
44 Recommended Policy Guidance for Departmental Development of Review Mechanisms for Potential Pandemic Pathogen Care and Oversight. Available at: https://www.phe.gov/s3/dualuse/Pages/GainOfFunction.aspx.
45 This section of the report is intended to be descriptive. It does not speculate about how to manage research and publication that occurs outside the realm of U.S. influence or policy.
46 See, for example, C. Rhodes, International Governance of Biotechnology: Needs, Problems and Potential (London: Bloomsbury Academic, 2010) and the discussions during the two Academies symposia on GOF research; Institute of Medicine and National Research Council, Potential Risks and Benefits of Gain-of-Function Research: Summary of a Workshop (Washington, DC: The National Academies Press, 2015), doi:https://doi.org/10.17226/21666 and National Academies of Sciences, Engineering, and Medicine, Gain-of-Function Research: Summary of the Second Symposium, March 10-11, 2016 (Washington, DC: The National Academies Press, 2016), doi:https://doi.org/10.17226/23484.
As the Fink Report concluded in 2004:
Although the focus of this report is on the United States, this country is only one of many pursuing biotechnology research at the highest level. The techniques, reagents, and information that could be used for offensive applications are readily available and accessible. And the expertise and know-how to use or misuse them is distributed across the globe. Without international consensus and consistent guidelines for overseeing research in advanced biotechnology, limitations on certain types of research in the United States would only impede the progress of biomedical research here and undermine our own national interests. It is entirely appropriate for the United States to develop a system
to provide oversight of research activities domestically, but the effort will ultimately afford little protection if it is not adopted internationally. This is a challenge for governments, international organizations, and the entire international scientific community. Efforts to meet that challenge are under way, but they must be quickly expanded, strengthened, and harmonized.47
There is no single international institution with the mandate or capacity to provide oversight of DURC, nor is any institution currently giving these issues systematic attention. International treaty organizations, United Nations Security Council (UNSC) resolutions, less formal international structures such as supplier agreements, and various components of international law could play a role in the management of DURC.
The core of the international biological nonproliferation and disarmament regime is the Biological and Toxin Weapons Convention (BWC), which was signed in 1972 and entered into force in 1975. It built upon the Geneva Protocol banning use of chemical and biological agents in war and was the first international disarmament treaty to ban an entire class of weapons.48 The BWC prohibits development, production, stockpiling, and transfer of biological weapons, or the means of their delivery.49 The BWC has provided a forum for discussions of dual use issues in the context of oversight of research (see Chapter 3), but it does not ban research on defensive measures.50
___________________
47 National Research Council, Biotechnology Research in an Age of Terrorism (Washington, DC: The National Academies Press, 2004), p. 110.
48 The formal title of the Geneva Protocol, which was signed in 1925 and entered into force in 1928, is the “Protocol for the Prohibition of the Use in War of Asphyxiating, Poisonous or Other Gases, and of Bacteriological Methods of Warfare.” The Geneva Protocol prohibits first use of chemical and biological weapons. The BWC’s formal title is the “Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction.”
49 The BWC states that “Each State Party to this Convention undertakes never in any circumstances to develop, produce, stockpile or otherwise acquire or retain:
(1) Microbial or other biological agents, or toxins whatever their origin or method of production, of types and in quantities that have no justification for prophylactic, protective or other peaceful purposes;
(2) Weapons, equipment or means of delivery designed to use such agents or toxins for hostile purposes or in armed conflict.”
See U.S. Department of State, Text of the Biological Weapons Convention. Available at: https://www.state.gov/t/isn/bw/c48738.htm.
50 States Parties to the BWC, of which there were 178 as of January 2017, are obligated to enact national implementing legislation in support of the treaty. Countries may have an array of laws and regulations that address biological weapons (as well as bioterrorism) directly or that contribute indirectly by governing various aspects of research and commercial activities. The U.S. implementing legislation for the BWC, the Biological Weapons Anti-Terrorism Act of 1989 (P.L. 101-298) is the primary means for law enforcement to take action and is not confined to a specific list of agents or toxins.
In 2004, to eliminate any potential gaps in the ability of the international regimes against weapons of mass destruction to respond to terrorism, the United Nations Security Council adopted UNSC Resolution 1540 (UNSCR 1540). The resolution obligates all United Nations (UN) members not to provide “any form of support to non-State actors that attempt to develop, acquire, manufacture, possess, transport, transfer or use nuclear, chemical or biological weapons.”51 UNSCR 1540 carries an obligation for UN member states to pass implementing legislation. Implementation is overseen by the standing 1540 Committee, but the committee has given limited attention to dual use research as a security issue. In December 2016, the UN Security Council unanimously adopted Resolution 2325. This resolution “encourages States, as appropriate, to control access to intangible transfers of technology and to information that could be used for weapons of mass destruction and their means of delivery.”52 This oversight is potentially relevant to the dissemination of DURC.
From time to time, other international organizations have become engaged in dual use issues, particularly the World Health Organization (WHO).53 In 2005, for example, the WHO released a paper, Life Science Research: Opportunities and Risks for Public Health. This was followed by a workshop in 2006 on “Life Science Research and Global Health Security.”54 Additionally, a number of collaborative activities included regional workshops that addressed both biosafety and biosecurity issues. The final major WHO product prior to its involvement in the GOF controversy was a 2010 guidance document that provided a self-assessment tool for researchers and laboratories to evaluate their oversight of dual use research.55 The recommendations of such documents are not binding on member states.
The WHO also became embroiled in the GOF controversy because of its role in global planning for influenza research. In February 2012, the WHO held
___________________
In remarks to the committee at its first meeting, Ed You, Federal Bureau of Investigation, stated that the bureau relies on the Biological Weapons Anti-Terrorism Act in its law enforcement activities.
51 The text of the resolution may be found at http://www.un.org/en/ga/search/view_doc.asp?symbol=S/RES/1540(2004).
52 See http://www.un.org/en/ga/search/view_doc.asp?symbol=S/RES/2325(2016).
53 In May 1967, the WHO’s World Health Assembly had approved a statement that “scientific achievements, and particularly in the field of biology and medicine—the most humane science—should be used only for mankind’s benefit, but never to do it any harm.” World Health Organization, World Health Assembly Resolution WHA20.54 (1967).
54 World Health Organization, Life Science Research: Opportunities and Risks for Public Health (Geneva: World Health Organization, 2005) and World Health Organization, Scientific Working Group on Life Science Research and Global Health Security: Report of the First Meeting (Geneva: World Health Organization, 2007).
55 World Health Organization, Responsible Life Sciences Research for Global Health Security: A Guidance Document (Geneva: World Health Organization, 2010).
a technical consultation of public health and influenza experts to review the manuscripts. The meeting concluded:
On the question of limiting access to the results through publication of redacted versions, some participants observed that there was no current practical mechanism to limit access. Further, it would not be difficult for knowledgeable scientists to determine the information that had been removed, as novel methods had not been used. Limiting access to those with a need for the information would pose insurmountable practical problems. Chief among these problems are the development and implementation of a mechanism to disseminate the information to diverse and geographically distributed groups while maintaining the confidentiality of the detail. Therefore, such a mechanism would not realistically resolve concerns about dual-use research. There may be benefit in creating such a mechanism to deal with other dual-use research information in the future. However, this will require thorough consideration of and international agreement on practical issues such as security, access requirements, governance, and liability.56
The meeting was criticized by some who believed the meeting’s outcome was predetermined by the large number of influenza virologists who were selected to participate. The issue of restricting access to some information from these manuscripts also threatened to undermine the Pandemic Influenza Preparedness (PIP) Framework created in 2011. PIP was designed to ensure improving sharing of influenza strains and information in light of concerns by countries such as Indonesia that they were not reaping the full benefits of their cooperation on global pandemic influenza preparedness efforts.57
As discussed further in Chapter 3, the WHO also held a larger conference in March 2013 to address broader issues of DURC policy, although the organization was largely consumed by the global Ebola crisis during the renewed U.S. policy debates that began in 2014. In principle, the WHO could, in the future, take up the issue of research oversight as it affects global health security. The WHO could provide an important complement to the BWC.
In addition to formal international treaties and international law, groups of states may organize themselves to undertake tasks or coordinate policy. In the biosecurity realm, the Australia Group (AG) is the most relevant. The AG was created in 1985 to improve consultation among member states on export controls. Originally focused on chemical weapons, the AG added biological weapons in the 1990s. The AG now has 41 member countries including the member states of the European Union and the EU as an institutional member. “[T]hrough the harmonisation of export controls, [the AG] seeks to ensure that exports do not contribute to the development of chemical or biological weapons.” See http://australiagroup.net/en/.
___________________
56 See http://www.who.int/influenza/human_animal_interface/mtg_report_h5n1.pdf?ua=1.
57 See http://www.who.int/influenza/resources/pip_framework/en/.
The AG maintains common control lists for “dual use biological equipment and related technology and software, biological agents, and plant and animal pathogens” as the basis for promoting common standards and regulations. The second volume of the AG’s Common Control List Handbook notes that controls on technology do not apply to information “in the public domain” (i.e., “technology that has been made available without restrictions upon its further dissemination”); “Controls also do not apply to ‘basic scientific research,’” (i.e., “experimental or theoretical work undertaken principally to acquire new knowledge of the fundamental principles of phenomena or observable facts, not primarily directed towards a specific practical aim or objective”) or “the minimum information necessary to apply for patents.”58 The handbook also describes several types of knowledge that may be controlled.
The AG has also provided a forum for discussion of issues, including dissemination of information. For example, when the Netherlands, an AG member, chose to rely on export controls as the mechanism for its oversight of the dissemination of the results of the Dutch research on H5N1 avian influenza, the AG held discussions on the GOF controversy.59
Many investments have been made by major donors to assist foreign countries with enhancements to both biosafety capacity and biosecurity (e.g., physical security, access controls, pathogen accounting, etc.).60 Far fewer resources
___________________
58 See Australia Group, Common Control List Handbook, Volume II: Biological Weapons-Related Common Control Lists, June 2014, p. 255. Available at http://www.defence.gov.au/exportcontrols/_Master/docs/Australia_Group_Common_Control_List_Handbook_Volume_II.pdf.
59 For an account of the Dutch experience with the GOF controversy, see K. van der Bruggen, “Biosecurity Challenges in the 21st Century: The Case of Gain-of-function Experiments,” in S. Whitby, T. Novossiolova, G. Walther, and M. Dando, eds., Preventing Biological Threats: What You Can Do (Bradford: Bradford Disarmament Research Centre, 2015). Information about current Dutch policy, including export controls, is available from the Netherlands Biosecurity Office (see http://www.bureaubiosecurity.nl/en) and in the presentations in a side event during the 2015 BWC Meeting of Experts (the event may be found under “Side Events” at http://www.unog.ch/__80256ee600585943.nsf/(httpPages)/46cac219b57f8b49c1257db20030bce8?OpenDocument&ExpandSection=11#_Section11).
60 Comprehensive data on international or U.S. expenditures on biosafety and biosecurity assistance are not available. One can gain a sense of the priorities from Defense Threat Reduction Agency (DTRA), The Cooperative Biological Engagement Program Research Strategic Plan: Addressing Biological Threat Reduction Through Research, 2015. Available at: http://www.dtra.mil/Portals/61/Documents/Missions/CBEP%20Research%20Strategy_FINAL_July%202015.pdf.
The largest U.S. assistance program, the Cooperative Biological Engagement Program’s mission is to “establish and maintain international research collaborations with global partners to inform and enhance operational biosurveillance, enhance global health security, and foster safe, secure and sustainable bioscience capability with partner countries” (see p. 5).
The 5-year target for the Global Health Security Agenda’s Biosafety and Biosecurity action package is broader: “A whole-of-government national biosafety and biosecurity system is in place, ensuring that especially dangerous pathogens are identified, held, secured and monitored in a minimal number of facilities according to best practices; biological risk management training and educational outreach are conducted to promote a shared culture of responsibility, reduce dual
have been devoted to awareness-raising, education and training, and policy development.
The result is that the call in the Fink Report for the establishment of an international effort to confront the challenge presented by DURC has not yet been met.61
CONCLUSION
Over the past 20 years, the U.S. government has developed, on the basis of the principles articulated in NSDD-189, a set of mechanisms, regulations, and policies to guide institutions and researchers conducting dual use life sciences research. These policies build on the fundamental idea that basic research should be open, but should be subject to policies that provide for the assessment and mitigation of risks in certain cases. Nevertheless, despite years of effort, there are some who still do not believe that the current federal approach is adequate to address concerns raised by current and emerging dual use research in the life sciences.
___________________
use risks, mitigate biological proliferation and deliberate use threats, and ensure safe transfer of biological agents; and country-specific biosafety and biosecurity legislation, laboratory licensing, and pathogen control measures are in place as appropriate.” But its success is to be measured by the “number of countries who have completed/Completion of a national framework and comprehensive oversight system for pathogen biosafety and biosecurity, strain collections, containment laboratories and monitoring systems that includes identification and storage of national strain collections in a minimal number of facilities.” See Global Health Security Agenda Action Packages at https://www.ghsagenda.org/packages/p3-biosafety-biosecurity.
There are programs that do address policy issues, including DURC, but they are significantly smaller. See, e.g., another DTRA program, The Project on Advanced Systems and Concepts for Countering WMD (https://www.usafa.edu/df/inss/indexpascc.cfm).
61 Both the NSABB and the European Academies Scientific Advisory Council (EASAC) reports on GOF research, for example, call for greater efforts to engage the international community. The EASAC report explicitly references the Fink Report’s recommendation of an International Forum on Biosecurity. See European Academies Scientific Advisory Council (EASAC), Gain-of-Function: Experimental Applications Relating to Potentially Pandemic Pathogens. EASAC Policy Report 27, (Halle: EASAC, 2015). Available at http://www.easac.eu/fileadmin/PDF_s/reports_statements/Gain_of_Function/EASAC_GOF_Web_complete_centred.pdf.






















