9
Low Life Expectancy in the United States: Is the Health Care System at Fault?
Samuel H. Preston and Jessica Ho
The United States falls well behind the world’s leaders in life expectancy at birth. Some of the discrepancy is attributable to relatively high infant mortality and some to high mortality from violence among young adults. But the bulk of the discrepancy is attributable to mortality above age 50, an age to which 94 percent of newborns in the United States will survive according to the 2006 U.S. life table. Life expectancy at age 50 in the United States ranked 29th highest in the world in 2006 according to the World Health Organization (2009). It falls 3.3 years behind the leader, Japan, and more than 1.5 years behind Australia, Canada, France, Iceland, Italy, Spain, and Switzerland. About 4 million Americans reach age 50 each year, so an average loss of 1.5 years of life years per person means that some 6 million years of potential life are being lost annually. At the conventional value of $100,000 per additional year of life (Cutler, 2004), the relative loss of life in the United States above age 50 is valued at roughly $600 billion annually. Using Japan as a standard, the loss is $1.3 trillion.
The U.S. medical system is often blamed for this poor life-expectancy ranking. But measures of population health such as life expectancy do not depend solely on what transpires within the health care system—the array of hospitals, doctors, and other health care professionals, the techniques they employ, and the institutions that govern access to and utilization of them. Such measures also depend on a variety of personal behaviors that affect an individual’s health, such as diet, exercise, smoking, and compliance with medical protocols. The health care system could be performing exceptionally well in identifying and administering treatment for various diseases, but a country could still have poor measured health if personal health care practices were
unusually deleterious. This could be the case in the United States, which had the highest level of cigarette consumption per capita in the developed world over a 40-year period ending in the mid-1980s (Forey et al., 2002). Smoking in early life has left an imprint on mortality patterns that remains visible as cohorts age (Haldorsen and Grimsrud, 1999; Preston and Wang, 2006). One recent study estimated that, if deaths attributable to smoking were eliminated, the ranking of U.S. men and women in life expectancy at age 50 among 21 countries of the Organisation for Economic Co-operation and Development (OECD) would improve sharply (Preston, Glei, and Wilmoth, Chapter 4, in this volume). Recent trends in obesity are also more adverse in the United States than in other developed countries (Cutler, Glaeser, and Shapiro, 2003; Organisation for Economic Co-operation and Development, 2008).
This chapter begins with a review of previous international studies of the comparative performance of health care systems in disease identification and treatment. The review is focused on the major diseases of adulthood, cancer and cardiovascular disease, in the belief that disease-level analyses are more likely to reveal the forces at work than more highly aggregated studies (Garber, 2003). In 2005, cancer and major cardiovascular diseases were responsible for 61.0 percent of deaths in the United States at ages 45+ (National Center for Health Statistics, 2008). Because our concern is with mortality per se, the criterion we employ is effectiveness at preventing death, rather than cost-effectiveness or efficiency of resource deployment. These latter criteria have been used in several other recent comparative studies describing features of the U.S. health care system that appear inefficient by international standards (Garber and Skinner, 2008; McKinsey Global Institute, 2008). A comprehensive evaluation of the U.S. health care system would need to consider patient physical and emotional welfare, a much broader concept than survival, which is the sole focus of this chapter.
Health care systems can prevent death from a particular disease either by preventing it from developing or by effectively treating it once it has developed. A key element in effective treatment is accurate diagnosis. However, almost no internationally comparable data exist on the actual incidence of various diseases, which is the appropriate measure of the success of prevention. While cancer appears to be an exception because “incidence” data are published for various cancer registry sites (e.g., at the website of the International Agency for Research on Cancer), the data refer not to the origin of a disease but to its detection, a process that combines actual patterns of incidence with the mechanics of identification. And even if pure measures of it were available, actual disease incidence reflects not only features of a health care system but also many other factors of behavioral, social, and genetic origin.
Disease prevalence—the proportion of the population that has been diagnosed with a disease—is even more difficult to interpret. The United
States has a higher prevalence than Europe of the major adult diseases, including cancer, heart disease, and diabetes (Avendano et al., 2009; Thorpe, Howard and Galactionova, 2007a). But higher prevalence could reflect higher incidence, better detection, or longer survival resulting from more successful treatment. Because of these limitations of data and interpretation, our review focuses primarily on disease identification and treatment, elements that are customarily considered to be the provenance of health care systems.
A valuable but not unimpeachable indicator of the effectiveness of treatment is the comparative survival rate of individuals once a disease has been detected. Relatively high survival rates imply either that the disease has been detected unusually early or that treatment is unusually successful. Early detection is valuable to the extent that it permits better therapy. However, if early detection did not alter the clinical course of a disease but only increased the expected length of time from detection to death (socalled lead-time bias), then it would not be associated with reductions in mortality at the population level despite raising 5-year survival rates (e.g., Gatta et al., 2000).
Because they are not subject to this potential bias, we pay special attention to mortality rates. In particular, in the second half of the chapter we investigate comparative mortality trends for prostate cancer and breast cancer. We document that:
-
effective methods of screening for these diseases have been developed relatively recently;
-
these diagnostic methods have been deployed earlier and more widely in the United States than in most comparison countries;
-
effective methods are being used to treat these diseases; and
-
the United States has had a significantly faster decline in mortality from these diseases than comparison countries.
INTERNATIONAL STUDIES OF CANCER
The United States does well in international comparisons of the frequency of cancer screening. The OECD (2006, 2007) provides 2000-2005 data on the percentage of women ages 20-69 in 15 countries who had been screened for cervical cancer during the preceding 3 years. The United States has the highest percentage of women who have been screened in both tabulations.1 We present evidence below that the United States also
has exceptionally high screening rates for prostate cancer and breast cancer. Quinn (2003) reports U.S. colorectal screening rates that are “quite high” in comparison to Europe but does not provide comparative data. Gatta et al. (2000, p. 899) also suggest that access to and use of sigmoidoscopy, colonoscopy, and fecal occult blood tests are more common in the United States than in Europe. This difference is supported by the finding that colorectal cancer patients in the United States have less advanced disease at diagnosis than patients in Europe (Ciccolallo et al., 2005).
A higher rate of screening for cancer would produce a higher prevalence of ever-diagnosed cancer in the population, ceteris paribus. The elevated prevalence would occur simply because a higher fraction of the population would know about their disease. An additional boost to prevalence would be provided if early detection resulted in reduced mortality. Thus, in view of the higher frequency of screening in the United States, we would expect its reported prevalence of diagnosed cancer to be higher than in Europe.
That expectation is confirmed by data from the Health and Retirement Survey and its English and European counterparts. Thorpe et al. (2007a) found that 12.2 percent of Americans over age 50 reported having been diagnosed by physicians with cancer, compared with only 5.4 percent in a composite of 10 European countries. Avendano et al. (2009) reported similar figures for the age range 50-74, with England intermediate between the United States and Europe but closer to Europe. Some fraction of these very large differences in prevalence could, of course, be attributable to real differences in disease incidence or to reporting differences, which are discussed briefly below.
Thanks to a large number of cancer registries that record new cancer diagnoses and follow individuals forward from the point of diagnosis, 5-year survival rates for people initially diagnosed with cancer are widely available to provide evidence about the success of detection and treatment. Because of their relative comparability and pertinence to a major disease process, these data are among the best indicators of comparative health care system performance. In this summary, we use 5-year relative survival rates, which compare the survival of those diagnosed with cancer to that of an average person of the same age and sex as the person diagnosed.
International comparisons of cancer survival rates show a distinct advantage for the United States. Using cancer registry data, researchers from the Eurocare Working Group compare 5-year survival rates for cancers of 12 sites that were diagnosed between 1985 and 1989 (Gatta et al., 2000). The aggregate of 41 European registries, which were drawn from 17 countries, had lower survival rates than the United States from all cancer sites except the stomach, where differences were small and attributed to differences between the distributions of sites within the stomach. The U.S. data were drawn from the National Cancer Institute’s Surveillance, Epidemiology and
End Results (SEER) database, a population-based cancer registry covering approximately 14 percent of the U.S. population. For the major sites of lung, breast, prostate, colon, and rectum cancers, U.S. survival rates were the highest of any of the 18 countries investigated. Cancers first diagnosed on the death certificate (5 percent in Europe and 1 percent in the United States) were excluded from analysis; if they had been included, the U.S. survival advantage would have increased. The authors discount the possibility that the U.S. advantage was attributable to statistical or registration artifacts.
An updated analysis reached similar conclusions. Based on period survival data for 2000-2002 from 47 European cancer registries, 5-year survival rates were found to be higher in the United States than in a European composite for cancer at all major sites (Verdecchia et al., 2007). Table 9-1 presents the comparative data for all sites for which the U.S. 95 percent confidence interval was < 0.025. For men (all sites combined), 47.3 percent of Europeans survived 5 years, compared with 66.3 percent of Americans. For women, the contrast was 55.8 versus 62.9 percent. The male survival difference was much greater than the female primarily because of the very large difference in survival rates from prostate cancer.
Scattered data for cancer of various sites indicate that tumors are typically detected at an earlier stage in the United States (Ciccolallo et al., 2005; Gatta et al., 2000; Sant et al., 2004). Thus, the United States appears to screen more vigorously for cancer than Europe, and people in the United States who are diagnosed with cancer have higher 5-year survival probabilities. Of course, all of these phenomena could be the exclusive product of lead-time bias if early detection afforded no benefit for the clinical course
TABLE 9-1 5-Year Relative Survival Rates for Cancer of Different Sites, U.S. and European Cancer Registriesa
of the disease. Below, we present evidence that innovations in diagnosis and treatment of prostate and breast cancer were associated with faster declines in mortality in the United States than in OECD countries. Such a pattern would not be observed if lead-time bias were the only factor at work, that is, if early detection conferred no advantage.
INTERNATIONAL STUDIES OF CARDIOVASCULAR DISEASE
In contrast to cancer, nations do not have registries for heart disease and stroke. So information about the comparative performance of medical systems with respect to cardiovascular disease is not as systematic and orderly as it is for cancer. One useful source of comparative data is the Health and Retirement Survey (HRS) and its European counterpart, the Survey of Health, Ageing and Retirement in Europe (SHARE). Thorpe et al. (2007a) compared the United States with a composite of 10 European countries on the frequency with which people with a particular diagnosis reported using medication. Of people ages 50+ diagnosed with heart disease, 60.7 percent of Americans and 54.5 percent of Europeans reported being on medication. The proportions using medication after a stroke are comparable at 45.1 and 44.6 percent, respectively. Of those reporting high cholesterol levels, 88.1 percent of Americans report being medicated versus 62.4 percent of Europeans.2 Crimmins, Garcia, and Kim (Chapter 3, in this volume) show that a much higher fraction of Americans are using lipid-lowering drugs at a particular age than in Italy, Japan, or the Netherlands, even though the proportions with elevated cholesterol in these countries are similar to or higher than that in the United States.
Among those reporting high blood pressure in HRS and SHARE, the proportions reporting taking medication for the condition are similar in the United States (88.0 percent) and Europe (88.9 percent) (Thorpe et al., 2007a). However, when actual measures of blood pressure are used rather than self-reports, the U.S. position improves. Wolf-Maier et al. (2004) employed regional or national samples in the United States, Canada, and five European countries. Hypertension was defined as the population of persons who have systolic blood pressure of 160+ or diastolic blood pressure of 95+ or who are using antihypertensive medication. Of persons ages 35-64 with hypertension, 77.9 percent were being treated in the United States, compared with a range of 41.0 to 62.4 percent in the other six countries. Among those with hypertension, 65.5 percent were being successfully treated in the United States (i.e., their levels were reduced below the hypertension-defining threshold), compared with 24.8 to 49.1 percent in the other countries.
Survival data for cardiovascular disease start not from the point of diagnosis but from an acute event of heart attack or stroke. An OECD study, following up on a study by the Technological Change in Health Care Research Network, computed 1-year case fatality rates for people hospitalized for acute myocardial infarction in Australia, Canada, Denmark, Finland, Sweden, Great Britain, and the United States. The samples were sometimes regionally rather than nationally representative. Among the seven countries in 1996, the United States had the third-lowest case fatality rate for men ages 40-64 and the second-lowest rate for men ages 85-89. For women at these ages, the United States ranked fourth and first (Moise, 2003). Part of the explanation for why the U.S. performs better may be related to its unusually aggressive treatment regime. Of the seven countries, the United States had the highest proportion of male and female patients in both age intervals undergoing revascularization operations (percutaneous transluminal coronary angioplasty or coronary artery bypass graft) (Moise, 2003; see also Technological Change in Health Care (TECH) Research Network, 2001).3
One study has explicitly linked more aggressive surgical treatment in the United States to better outcomes. It compared Canadians and Americans who had just experienced an acute myocardial infarction and who enrolled in a drug trial (Kaul et al., 2004). Data are not nationally representative but rather reflect the patient base of hospitals participating in the trial. Americans had a small but statistically significant advantage in 5-year survival. Controlling many baseline characteristics, the hazard rate was 17 percent higher in Canada. When revascularization was added to the model, it was associated with a 28 percent reduction in the hazard rate and its addition reduced the international difference to an insignificant 7 percent. The authors conclude that “our findings are strongly suggestive of a survival advantage for the U.S. cohort based on more aggressive revascularization” (Kaul et al., 2004, p. 1758).
The OECD (2003) has conducted a large international study of ischemic stroke, which accounts for roughly 88 percent of stroke cases except in Japan, where it represents about 70 percent. They calculate in-hospital 7-day and 30-day survival rates for patients newly admitted with ischemic stroke. For both men and women ages 65-74, the U.S. ranking on 7-day survival rates was third out of nine; at ages 75+, it was second out of nine for both sexes. For 30-day hospital survival rates at ages 65-74, the United States was second for women and tied for second with two others among men. At ages 75+, the U.S. 30-day survival rate was first for men and second for women. Counting all deaths, not simply deaths in the hospital, and limiting comparison to six regions, including two in Canada, the U.S. survival
rate ranked first for men ages 65-74 and 75+ and second for women in these ages. However, the U.S. 1-year survival rate among this set of populations was considerably poorer, ranking fifth out of six for men ages 65-74 and fourth out of six for men ages 75+. For women at these two ages, the rankings were fourth and third. Consistently in these rankings, the U.S. position was better at ages 75+ than at ages 65-74.
Carotid endarterectomy (surgical removal of plaque from inside the carotid artery) is used to prevent stroke or the recurrence of stroke. Such surgery is much more common in the United States than in any of 11 comparison OECD countries (Organisation for Economic Co-operation and Development, 2003). We are unaware of any studies linking this surgery to international patterns of stroke mortality, but a randomized clinical trial reported a large survival advantage for persons undergoing the procedure (Halliday et al., 2004).
In summary, persons with high blood pressure or high serum cholesterol are more likely to be treated for these conditions in the United States than in other countries. Survival rates following a heart attack are somewhat above average in the United States, whereas survival rates following a stroke are comparable to those of comparison countries. The evidentiary basis for international comparisons of the treatment of cardiovascular diseases is much weaker than in the case of cancer.
CONTRARY EVIDENCE? “MORTALITY AMENABLE TO MEDICAL CARE”
The Commonwealth Fund (2008) has recently issued a “scorecard” on U.S. health care system performance that consists of 37 indicators. A prominent indicator is “mortality amenable to medical care,” on which the United States currently ranks last among 19 countries. This index was developed and applied in Nolte and McKee (2008), in which amenable deaths are described as “deaths from certain causes that should not occur in the presence of timely and effective health care” (p. 59). Only deaths below age 75 are included; these constitute 43.2 percent of deaths in the United States in 2005 (National Center for Health Statistics, 2008). For some causes of death, an earlier age cutoff is used.
The distribution of major causes of death included among the “amenable causes” is provided for the United States, the United Kingdom, and France (Nolte and McKee, 2008). A majority of amenable deaths in all three countries is attributed to ischemic heart disease and other circulatory diseases, even though only half of ischemic heart disease deaths are included because some are believed not to be amenable to health care. That rule of thumb is clearly a poor substitute for an effort to attribute international variation in mortality from ischemic heart disease to its various compo-
nents, including health care systems and behavioral and social factors.4 The authors note that a similar rule of thumb could have been introduced for cerebrovascular diseases, which constitute at least a quarter of the “amenable” deaths in the United States and the United Kingdom. But it would have been no more satisfactory for that cause of death.
In view of the studies that show that the United States does relatively well in treating cardiovascular disease, it seems inaccurate to attribute its high death rates from these causes to a poorly performing medical system. And these diseases contribute a majority of their set of amenable deaths, rendering the totality of amenable causes problematic. On one hand, a related objection could be raised to the inclusion of diabetes deaths in the set. On the other hand, prostate cancer is excluded from the list of amenable causes despite the fact that the 5-year survival rate from prostate cancer in the United States is above 99 percent and the disease can be readily identified (see below).
According to Nolte and McKee (2008), males in the United States had a faster fall in mortality from nonamenable causes of death (an 8 percent decline) than from amenable ones (4 percent) between the latest two readings, 1997-1998 and 2002-2003. This anomaly suggests either flaws in the index or the unimportance of medical care relative to other factors that are operating.
Causes of death whose inclusion in Nolte and McKee’s list of amenable causes at older ages is more defensible are influenza and pneumonia. Mortality from both causes is heavily influenced by smoking (Centers for Disease Control and Prevention, 2002), so the international distribution of mortality is a product of factors beyond the health care system. However, influenza is partially immunizable, and death from pneumonia can often be avoided through administration of vaccines or antibiotics or improvements in hospital sanitation.
The United States ranks ninth out of 23 OECD countries in the proportion of the population above age 65 offered an annual influenza vaccination (Organisation for Economic Co-operation and Development, 2007). Figure 9-1 demonstrates that the 2000-2004 age-standardized death rate from influenza at ages 50+ in the United States is among the lowest of the 16 countries investigated. The United States fares less well in mortality from pneumonia, having sixth highest rates among the 16 countries investigated (see Figure 9-2). However, the ranking is somewhat deceiving because its death rate is closer to all but one of the better ranked countries than to the five countries with higher rates. The U.S. death rate from pneumonia at
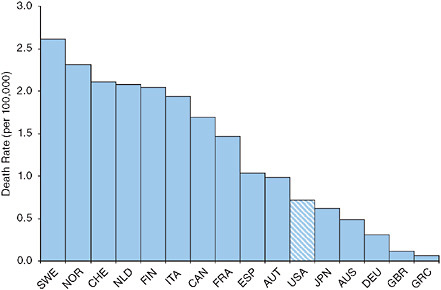
FIGURE 9-1 Age-standardized death rates at ages 50+ from influenza, 2000-2004.
NOTE: AUS = Australia, AUT = Austria, CAN = Canada, CHE = Switzerland, DEU = Germany, ESP = Spain, FIN = Finland, FRA = France, GBR = Great Britain, GRC = Greece, ITA = Italy, JPN = Japan, NLD = the Netherlands, NOR = Norway, SWE = Sweden, USA = United States.
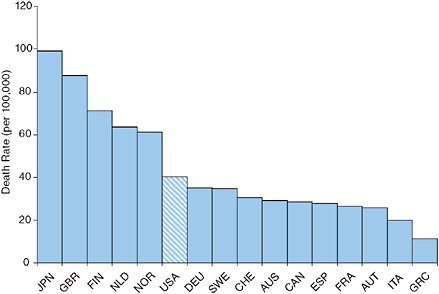
FIGURE 9-2 Age-standardized death rates at ages 50+ from pneumonia, 2000-2004.
NOTE: AUS = Australia, AUT = Austria, CAN = Canada, CHE = Switzerland, DEU = Germany, ESP = Spain, FIN = Finland, FRA = France, GBR = Great Britain, GRC = Greece, ITA = Italy, JPN = Japan, NLD = the Netherlands, NOR = Norway, SWE = Sweden, USA = United States.
ages 50+ is actually below the weighted or unweighted mean for the other 15 countries.
DISEASE PREVENTION
Medical procedures and survival rates are indicators of what happens to individuals whose health problems come to the attention of the health care system. But a health care system can also help prevent serious health problems from occurring in the first place. Of course, early identification of a disease is also preventive medicine in the sense that it may prevent death. But access to preventive medicine would appear to be an especially problematic area in the United States because 47 million people lack any form of health insurance (DeNavas-Walt, Proctor, and Smith, 2007).5 Such people are less likely to see a doctor and thus to receive routine testing that might detect the early stages of a disease and prevent its clinical manifestations (Institute of Medicine, 2001). They are also less likely to receive advice about health maintenance and disease prevention (Institute of Medicine, 2001). While this chapter focuses on ages above 50, the mortality levels in this age range reflect the conditions to which individuals have been exposed throughout their lives. Preventive medicine may have a large role to play at younger ages as well as older ones.
An additional factor that may inhibit disease prevention in the United States is the shortage of primary care physicians. The United States scores in the bottom group of 6 out of 18 OECD countries on a scale of the adequacy of primary care (Macinko, Starfield, and Shi, 2003). The scale is built from items relating to policy, finances, and personnel. In turn, the adequacy of primary care may be related to disease prevention (Macinko, Starfield, and Shi, 2003).
The best indication of the success of prevention is disease incidence—but international data on disease incidence are nil. As noted earlier, disease prevalence is higher in the United States than in a European composite for cancer, heart disease, stroke, chronic lung disease, and diabetes (Thorpe et al., 2007a). Such a difference could result from higher incidence, better detection, or longer survival after detection in the United States. It could also result from reporting differences, for example, a greater inclination to report disease in the United States. But a careful study by Banks et al. (2006) using biomarkers suggests that morbidity differences between England and the United States at ages 55-64 are real and not a result of differences in reportage. A related study found that, faced with the same set of health-
related vignettes, Americans were less likely to report themselves as disabled than the Dutch (Kapteyn, Smith, and van Soest, 2007).
Even if incidence data were available, analysts would have to disentangle the role of personal behavioral and social histories from that of health system performance. And these are not always readily distinguishable. Are the historically high rates of smoking in the United States attributable to the failure of the U.S. public health system to stem the smoking tide? The fact that Canada had for many years the second highest consumption of cigarettes per adult (Forey et al., 2002) makes it appear that geographic factors, perhaps related to conditions for growing or importing tobacco, had more to do with consumption patterns than did health care systems. And public health authorities were not passive in the United States. The U.S. surgeon general’s 1964 report on the health hazards of cigarette smoking was the first major indictment of the habit by a government authority (U.S. Department of Health, Education, and Welfare, 1964), and it was quickly followed up with a massive antismoking media campaign (Cutler and Glaeser, 2006). The United States had the largest reduction in manufactured cigarettes consumed per adult of any country between 1970 and 2000 (Forey et al., 2002). Some of that decline was likely attributable to public health efforts (Cutler and Glaeser, 2006).
However it is achieved, the high prevalence of disease in the United States adds considerably to health expenditure. Thorpe et al. (2007b) combine comparative prevalence data on 10 conditions in HRS (in the United States) and SHARE (in Europe) with U.S. Agency for Healthcare Research and Quality data on expenditure per medical condition for the population ages 50+. Their 95 percent confidence intervals on the per capita cost of higher disease prevalence in the United States are $1,195 to $1,750 per year, or 12.7 to 18.7 percent of total personal health care spending among those ages 50+. Inefficiencies in the U.S. health care system are not solely responsible for high per capita health expenditures; the high prevalence of major diseases is also substantially implicated (see also Michaud et al., 2009).
CASE STUDY I: PROSTATE CANCER
Accounting for 31,000 deaths in 2000, prostate cancer was, after lung cancer, the second leading cause of cancer deaths among U.S. men that year (National Center for Health Statistics, 2002). Unlike most chronic diseases, it is not associated with cigarette smoking (Lumey et al., 1997). A link with exercise has been suggested in several studies, but a review article found that “conclusions were quite variable … odds ratios [of developing prostate cancer] for men engaged in high levels of activity ranged from 0.2 to over 2.0” (Torti and Matheson, 2004). Dietary risk factors are suspected but not well established. The risk of prostate cancer is somewhat higher
for men with a high body mass index, but the risk is less than for other cancers (Crawford, 2003). Genetic factors, some of them associated with race, appear to be important in the risk of developing prostate cancer (Li et al., 2007). Its relatively flat landscape of behavioral risk factors, together with its medical preventability, make mortality from prostate cancer a purer indicator of health care system performance than mortality from many other chronic diseases of adulthood.
Prostate Cancer Screening
The Digital Rectal Examination (DRE) and Prostate Specific Antigen (PSA) test are the primary screening tools for prostate cancer. As a screening test, DRE is of limited value because it cannot investigate the entire prostate gland (Ilic et al., 2006). It is more difficult to detect cancer with DRE than with the PSA test (Harris and Lohr, 2002). The PSA test has the added benefits of being easy to perform, relatively inexpensive, and reproducible (Constantinou and Feneley, 2006).
The PSA blood test for the presence of prostate cancer was approved by the Food and Drug Administration in 1986 (Shampo, 2002). The test enables the detection of high and/or rapidly increasing levels of an antigen that often signal the presence of prostate cancer. High levels of the antigen can also be produced by other conditions; confirmation of cancer is made by transrectal ultrasound-guided biopsy (TRUS).
The PSA test is somewhat controversial. One reason is that, like many other medical screens, the PSA test can produce a false positive—a report of potential cancer when it is not present. According to a summary of studies of the sensitivity and specificity of PSA testing, an average of 75 percent of those with PSA readings above 4.0 ug/l have prostate cancer and 71 percent of men with prostate cancer have a PSA reading above 4.0 ug/l (Bunting, 2002). However, the main reservation about the use of the PSA test is that treatment for prostate cancer can produce impotence and/or incontinence. Because of these side effects, several organizations have recommended against PSA testing for men over 75 (U.S. Preventive Services Task Force, 2008). However, the American Cancer Society and the American Urological Association recommend that the PSA test should be offered annually to men over age 50 with at least a 10-year life expectancy.
By reputation the United States has been the world leader in PSA testing, especially in the early years after the test was developed (Bouchardy et al., 2008; De Koning et al., 2002; Hsing, Tsao, and Devesa, 2000; Vercelli et al., 2000). Table 9-2 compiles data on the frequency of PSA testing in various countries or regions. The age ranges used and the survey dates are not identical from country to country, preventing exact comparisons. The United States has the highest recorded percentage ever tested at older
TABLE 9-2 Indicators of Frequency of PSA Testing Among Men
|
A. Percentage of Men Ever Receiving a PSA Test |
||||
|
Country |
Percentage of Men Ever Receiving a PSA Test |
Year |
Age Group |
|
|
Australia |
49 |
2003 |
40+ |
|
|
Austria |
54.6 |
2006-2007 |
40+ |
|
|
Canada |
47.5a |
2000-2001 |
50+ |
|
|
France |
36 |
2005 |
40-74 |
|
|
Italy |
31.4 |
2003 |
50+ |
|
|
Netherlands (Rotterdam) |
12.7a |
1994 |
55-74 |
|
|
Switzerland (Vaud and Neuchâtel Cantons) |
10 |
Early 1990s |
65+ |
|
|
United States |
75 (BRFSS) |
2001 |
50+ |
|
|
|
62.7 (NHIS)b |
2005 |
50-79 |
|
|
B. Percentage of Men Recently Receiving a PSA Test |
||||
|
Country |
Percentage of Men Receiving a PSA Test in the Past x Years |
x |
Year |
Age Group |
|
Australia |
27 |
2 |
1995/1996 |
50+ |
|
Austria |
31.1 |
1 |
2006-2007 |
40+ |
|
Belgium (Limburg Province) |
23 |
1 |
1996-1998 |
40+ |
|
Canada |
26 |
1 |
2000-2001 |
40+ |
|
Italy |
15.9 |
1 |
2002 |
50+ |
|
Netherlands (Rotterdam) |
20.2 |
3 |
1997-2000 |
55-74 |
|
Norway (3 counties) |
7 |
1 |
1999 |
50-65 |
|
Spain (Getafe City) |
20.9 |
2 |
1997-1999 |
55+ |
|
Sweden |
25.3c |
1 |
2002 |
50+ |
|
United Kingdom |
7 |
1 |
1999-2001 |
45-84 |
|
United States |
57 (BRFSS) |
1 |
2001 |
50+ |
|
|
48.4 (NHIS)b |
2 |
2005 |
50-79 |
|
NOTE: BRFSS = Behavioral Risk Factor Surveillance System; NHIS = National Health Interview Survey. aOf the two sources of U.S. data presented in Table 9-2, the data from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System are less reliable because they are based on a telephone survey with a low response rate. bThis figure does not include men with a history of prostate cancer. cAccording to Sennfalt, Carlsson, and Varenhorst (2006), 430,000 PSA tests were performed in Sweden in 2002. We assume that all were performed on men ages 50+. The UN Population Division’s estimates for Sweden’s male population (ages 50+) for 2000 and 2005 were retrieved from the UN Statistics Division’s Common Database and interpolated to give a figure for 2002 of 1,699,442. SOURCES: Adapted from Beaulac, Fry, and Onysko (2006); Beemsterboer et al. (2000); D’Ambrosio et al. (2004); Eisinger et al. (2008); Gibbons and Waters (2003); Holden et al. (2006); Klimont, Ktir, and Leitner (2007); Levi et al. (1998); Lousbergh et al. (2002); Melia et al. (2003); Otto et al. (2003); Páez et al. (2002); Ross, Berkowitz, and Ekweume (2008); Sennfalt, Carlsson, and Varenhorst (2006); Sirovich, Schwartz, and Woloshin (2003); Smith and Armstrong (1998); Zappa et al. (2003). |
||||
ages (prevalence) as well as the highest percentage tested in a recent period (incidence).6 An analysis of survey data from HRS and SHARE at ages 50-64, 65-74, and 75+ shows that, among 16 OECD countries in 2004, the United States ranked either first or second in the proportion of the population having had a PSA test in the past year (Howard, Richardson, and Thorpe, 2009).
Evidence about the efficacy of PSA testing from randomized controlled trials has been mixed. The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial began in 1993 and involved 76,693 U.S. men ages 55-74. After 7 to 10 years of follow-up, the death rate from prostate cancer did not differ significantly between the study and the control groups. As noted by the authors, one possible explanation of the negative result is that PSA testing is already so frequent in the United States (see Table 9-2) that high levels of screening were already present among the control group. Furthermore, many cancers had already been identified in both the study and the control groups (Andriole et al., 2009). Results of the study are most reasonably interpreted as addressing the question of whether mortality advantages would pertain to extending PSA testing in a population in which half of men are already being tested every two years.
The second trial, the European Randomized Study of Screening for Prostate Cancer, was more than twice as large and was conducted in a region where prostate cancer screening is much less common. The trial began in the early 1990s in seven European countries and included a total of 162,243 men between the ages of 55 and 69. The study found that offering PSA screening to the treatment group reduced the death rate from prostate cancer by 20 percent (rate ratio of 0.73, 95% CI, 0.56 to 0.90). The absolute reduction was 0.71 prostate cancer deaths per 1,000 men. The median and average follow-up times were 9 and 8.8 years, respectively; death rates in the two study groups began diverging after 7 to 8 years and continued to diverge subsequently (Schröder et al., 2009).
The Goteborg, Sweden, component of the European trial followed 20,000 randomly selected men ages 50-66 for 10 years. Half were invited for biennial PSA testing, with 10,000 men serving as passive controls for whom diagnosis of metastatic prostate cancer was monitored by using the Swedish Cancer Registry. The risk of being diagnosed with metastatic (i.e., advanced) prostate cancer was reduced by 48.9 percent in the PSA treatment group relative to controls (p < .01) (Aus et al., 2007).
According to the SEER database, after the PSA test was introduced in the late 1980s, the recorded incidence of prostate cancer in the United States rose from 119 per 100,000 in 1986 to a peak of 237 per 100,000
|
6 |
Of the two sources of U.S. data presented in Table 9-2, the data from the Center for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System are less reliable because they are based on a telephone survey with a low response rate. |
in 1992 (National Cancer Institute, 2008).7 The proportion of tumors that are metastatic was 25 percent of newly diagnosed tumors in 1980 and only 4 percent in 2002 (Etzioni et al., 2008). Consistent with more extensive screening, the United States identifies prostate cancer at an earlier stage, on average, than Sweden (Stattin et al., 2005), Japan (Ogawa et al., 2008), or the United Kingdom (Collin et al., 2008). Stage at diagnosis is particularly important in prognosis—if detected at an early stage, prostate cancer can be treated by radical prostatectomy or radiotherapy.
Prostate Cancer Treatment
Once prostate cancer is detected, a variety of treatments can be employed, including radical prostatectomy, radiation by beam (external beam radiotherapy) or implanted seeds (brachytherapy), or hormone therapy. “Watchful waiting” is also an option. Since 1991, radical prostatectomy has been the most common treatment for localized prostate cancer in the United States. It serves as the initial treatment for over a third of newly diagnosed patients (Harris and Lohr, 2002). Observational studies have described apparent survival advantages from radical prostatectomy and radiation therapy (e.g., Trock et al., 2008; Wong et al., 2006) but not always from hormone therapy alone (Lu-Yao et al., 2008). The questions of possible selection bias that are always present in observational studies add uncertainty to these results.
Uncertainty has been reduced by several recent reports of randomized clinical trials. A key study of Scandinavian men examined survival after diagnosis of prostate cancer. Men were randomly assigned to radical prostatectomy or to watchful waiting (Bill-Axelson et al., 2005). Some of those assigned to prostatectomy did not have the operation, and some of those assigned to watchful waiting pursued radiation or hormonal therapy. Nevertheless, after a median follow-up period of 8.2 years, the group assigned to prostatectomy had cumulative proportions of death from prostate cancer that were lower by 44 percent, rates of disease progression that were lower by 67 percent, and rates of distant metastasis that were lower by 40 percent. All comparisons were statistically significant (Bill-Axelson et al., 2005). After a median follow-up period of 10.8 years, the group assigned to prostatectomy had relative reductions of 35 percent in risk of prostate cancer death and 35 percent in risk of distant metastases (Bill-Axelson et al., 2008). In summary, radical prostatectomy was found to reduce prostate cancer mortality and risk of metastases, although no further increase in benefit was observed 10+ years after surgery.
A randomized trial of variation in radiation dosage reported a highly significant beneficial effect on survival of heavier doses (Pollack et al., 2002). This study did not compare those radiated to those unradiated. Another randomized trial of adjuvant radiotherapy enrolled 425 men with pathologically advanced prostate cancer who had undergone radical prostatectomy between 1988 and 1997. Adjuvant radiotherapy significantly reduced the risk of PSA relapse and disease recurrence, although improvements in survival were not statistically significant (Thompson et al., 2006).
Several randomized clinical trials evaluate the use of hormone therapy as an adjunct to surgery or radiation in high-risk patients; the value of hormone therapy used alone or as primary therapy has been assessed only by observational studies. A population-based cohort study found that primary androgen deprivation therapy does not improve survival in elderly men compared with conservative management (no surgery, radiation, or hormone therapy) (Lu-Yao et al., 2008). However, three Phase III randomized trials have shown that a combination of radiotherapy and androgen suppression improve survival relative to radiotherapy alone (Bolla et al., 2002; Hanks et al., 2003; Pilepich et al., 2005).
Antonarakis and colleagues (2007) conducted a systematic review of studies published between 1986 and 2006 on hormone therapy for nonmetastatic prostate cancer. They extracted survival probabilities for men with localized or locally advanced prostate cancer receiving immediate hormone therapy as adjunct to radiation therapy, adjunct to radical prostatectomy, or stand-alone therapy. They found that survival in patients treated with hormone therapy for nonmetastatic prostate cancer may be longer than has been previously estimated. Men receiving hormone therapy alone had estimated 5-year disease-free survival (DFS) of 57 percent (median = 6.0 years) and overall survival (OS) of 70 percent (median = 7.0 years). Of the 10 studies used to estimate the DFS and OS for hormone therapy alone, 7 were Phase III randomized controlled trials and 3 were observational. The median follow-up was between 3.9 and 10.4 years. Comparative figures from two meta-analyses of primary hormone therapy in men with metastatic prostate cancer are a 5-year OS of 25 percent, a 10-year OS of 6 percent, and a median OS of 1.7-3.3 years (Antonarakis et al., 2007).
Thus far, few studies have compared all the standard treatment regimens in terms of overall survival and disease-specific survival. Zhou and colleagues (2009) used linked Ohio Cancer Incidence Surveillance System, Medicare, and death certificate files to examine overall and disease-specific survival for 10,179 men ages 65+ who were diagnosed with prostate cancer between 1999 and 2001 and received radical prostatectomy, brachytherapy, external beam radiotherapy, androgen deprivation therapy, or no treatment within 6 months after the initial diagnosis. At 7 years of follow-up and controlling for age, race, comorbidities, stage, and Gleason score, patients with
localized disease who received radical prostatectomy or brachytherapy had a significantly lower risk of dying from prostate cancer compared with patients who received no treatment (HR = 0.25, p < 0.0001 for radical prostatectomy, and HR = 0.45, p < 0.02 for brachytherapy). In this study, radical prostatectomy and brachytherapy significantly improved overall and disease-specific survival compared with no treatment, suggesting that earlier curative treatment is better than no treatment (Zhou et al., 2009).
Population-based information about the frequency of various treatments of prostate cancer is much skimpier than information about the use of the PSA test. Among U.S. men ages 65-80 in SEER who were diagnosed with low-grade tumors between 1991 and 1999, 25.5 percent received no treatment within 6 months of diagnosis, 9.6 percent received hormone therapy, and the remaining 64.8 percent received either radiation or prostatectomy (Wong et al., 2006).
Scandinavian countries rarely use radical therapies—radical prostatectomy or radiation—and rely primarily on watchful waiting or hormone therapy for palliation (Fleshner, Rakovitch, and Klotz, 2000; Sandblom et al., 2000). For example, the fraction of patients treated with curative intent in Norway was only 3 percent in 1985-1989 and rose to 6 percent in 1990-1994. In 1990-1994, radical prostatectomy was used to treat only 3.0 and 3.3 percent of all patients diagnosed with prostate cancer in Norway and Sweden, respectively (Kvåle et al., 2007). Low levels of surgery and radiation therapy are also reported in Japan (Ogawa et al., 2008).
Differences in treatment approach also exist between the United States and the United Kingdom, with U.S. approaches generally being more aggressive, particularly in the use of surgery (Collin et al., 2008). A survey of U.S. and Canadian urologists indicated that American urologists tended to have a more aggressive approach to case identification and surgical intervention. They were also more likely to perform radical prostatectomy on patients over the age of 70 (Fleshner, Rakovitch, and Klotz, 2000).
Prostate Cancer Survival
The combination of earlier detection and aggressive treatment in the United States has produced greatly improved survival chances for men diagnosed with prostate cancer. The 5-year relative survival rates in the United States increased from 71 to 83 percent between 1984-1986 and 1987-1989, whereas European rates improved from 55 to 59 percent during the same period (Post et al., 1998). According to the National Cancer Institute (2008), the U.S. 5-year relative survival rate had increased to 99.2 percent for those diagnosed in 2000.
Gatta et al. (2000) compared international survival rates for cancers diagnosed between 1985 and 1989. All of the European countries consid-
ered had lower prostate cancer survival rates than the United States. European patients had a 4.1 times greater risk of dying in the first year after diagnosis, suggesting that earlier diagnosis plays an important role in these survival differences (Gatta et al., 2000). The updated study whose results are presented in Table 9-1 found that 5-year survival rates for prostate cancer in 2000-2002 were 99.3 percent in the United States compared with 77.5 percent in Europe.
Prostate Cancer Mortality
Population-level data on mortality have one distinct advantage over data on survival rates among those newly diagnosed: they are not subject to lead-time bias. If one country is diagnosing cancer sooner than another but early diagnosis does not alter the clinical course of the disease and delay or prevent death, then that country will enjoy no advantage in mortality as a result of its earlier diagnoses. When early diagnosis improves prognosis, population-level mortality is responsive to the timeliness of diagnosis. It is also responsive to the efficacy of treatments employed, regardless of stage at diagnosis. Mortality data have a similar advantage relative to recorded incidence and prevalence data, both of which are subject to lead-time bias.
In order to investigate whether the relatively aggressive use of PSA testing and therapy in the United States has produced an unusually rapid decline in mortality from prostate cancer, we have used World Health Organization (WHO) data on deaths by cause and population by 5-year age groups. We have chosen a group of 15 economically developed OECD countries for purposes of comparison: Australia, Austria, Canada, Finland, France, Germany, Greece, Italy, Japan, the Netherlands, Norway, Spain, Sweden, Switzerland, and the United Kingdom.
Figure 9-3 compares levels of age-standardized death rates per 100,000 (all ages combined) in the United States to the unweighted mean death rate in these 15 comparison countries.8 With the exception of 1985, the United States had higher death rates each year from 1980 to 1995. Beginning in 1996, the United States had lower rates and the U.S. advantage grew every year thereafter. By 2003, the United States had death rates that were 20.4 percent lower than the mean of the comparison countries. Mortality rates among men ages 60-79 were lower in 1997 than in any year since 1950 (Tarone, Chu, and Brawley, 2000). Baade, Coory, and Aitken (2004) note that changes in risk factors and in the accuracy of procedures for recording cause-of-death information are unlikely to be responsible for the observed trends.
|
8 |
These rates are taken from the International Agency for Research on Cancer (http://www.dep.iarc.fr/ [accessed June 2010]), which extracts the World Health Organization mortality data and standardizes the rates to the world population in 1960 (Segi world standard). |
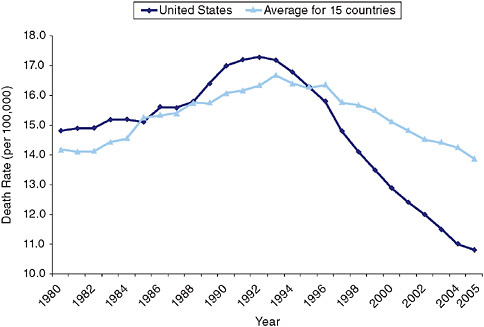
FIGURE 9-3 Age-standardized death rates from prostate cancer, 1980-2005.
Declines in prostate cancer mortality have been attributed to both PSA screening and improvements in treatment (Baade, Coory, and Aitken, 2004; Bouchardy et al., 2008; Collin et al., 2008; Kvåle et al., 2007; Potosky, Feuer, and Levin, 2001). An individual-level population model that used counterfactuals to simulate U.S. mortality and incidence of advanced-stage prostate cancer concluded that two-thirds of the decline in mortality between 1990 and 1999, and 80 percent of the decline in distant-stage incidence, was attributable to expanded PSA testing (Etzioni et al., 2008).
To test whether the faster mortality decline in the United States was statistically significant, we use a negative binomial regression in a fixed-effects model applied to data for these 15 countries for the period 1982-2005. The dependent variable is the log of the number of deaths from prostate cancer in a particular age, country and year cell, with population size in a particular cell used as the exposure. Independent variables are a set of age group identifiers, a set of period identifiers, a dummy variable for the United States, and a set of U.S./period interactions. Six 4-year-wide time periods are used, beginning with 1982-1985 and ending with 2002-2005. The period before PSA testing was begun, 1982-1985, was chosen as the reference period. Significance tests recognize the clustering of observations by country. Results are presented in Table 9-3.
The coefficient of the interactive variable for U.S. observations during
TABLE 9-3 Coefficients of Negative Binomial Regression Predicting the Log of the Number of Deaths from Prostate and Breast Cancer
|
|
|
Coefficient (standard error) |
|
|
|
Variable |
Prostate Cancer |
Breast Cancer |
|
Constant |
|
−10.37*** |
−7.657*** |
|
|
|
(0.079) |
(0.067) |
|
Age |
50-54 |
0.000 |
0.000 |
|
|
|
(−) |
(−) |
|
|
55-59 |
1.166*** |
0.247*** |
|
|
|
(0.026) |
(0.013) |
|
|
60-64 |
2.159*** |
0.413*** |
|
|
|
(0.026) |
(0.019) |
|
|
65-69 |
3.013*** |
0.550*** |
|
|
|
(0.032) |
(0.024) |
|
|
70-74 |
3.744*** |
0.721*** |
|
|
|
(0.034) |
(0.029) |
|
|
75-79 |
4.384*** |
0.925*** |
|
|
|
(0.038) |
(0.032) |
|
|
80-84 |
4.942*** |
1.157*** |
|
|
|
(0.041) |
(0.038) |
|
|
85+ |
5.455*** |
1.520*** |
|
|
|
(0.047) |
(0.046) |
|
Period |
1982-1985 |
0.000 |
0.000 |
|
|
|
(−) |
(−) |
|
|
1986-1989 |
0.0586*** |
0.0350*** |
|
|
|
(0.010) |
(0.011) |
|
|
1990-1993 |
0.103*** |
0.0276 |
|
|
|
(0.016) |
(0.015) |
|
|
1994-1997 |
0.0837*** |
−0.00241 |
|
|
|
(0.023) |
(0.028) |
|
|
1998-2001 |
0.0242 |
−0.0741* |
|
|
|
(0.029) |
(0.037) |
|
|
2002-2005 |
−0.0529 |
−0.114** |
|
|
|
(0.036) |
(0.042) |
|
Observation from United States |
0.125 |
0.108 |
|
|
|
|
(0.080) |
(0.082) |
|
Observation from United States in |
1982-1985 |
0.000 |
0.000 |
|
|
(−) |
(−) |
|
|
1986-1989 |
−0.0229* |
−0.0216* |
|
|
|
(0.010) |
(0.011) |
|
|
|
1990-1993 |
−0.00278 |
−0.0225 |
|
|
|
(0.015) |
(0.015) |
|
|
1994-1997 |
−0.0850*** |
−0.0585* |
|
|
|
(0.023) |
(0.028) |
|
|
1998-2001 |
−0.215*** |
−0.0892* |
|
|
|
(0.029) |
(0.036) |
|
|
2002-2005 |
−0.274*** |
−0.126** |
|
|
|
(0.036) |
(0.040) |
|
*p < 0.05, **p < 0.01, ***p < 0.001. |
|||
the period 2002-2005 is −0.274, which is significant at p < .001. Compared with expectations based on country and year, the United States had roughly 27 percent lower mortality in 2002-2005 than it did in 1982-1985. (The U.S./2002-2005 variable is always significant at p < 0.001 regardless of reference period used.) Likewise, the coefficient of the U.S./period interactive variable for the 1998-2001 period is −0.215 and is also significant at p < .001. So the United States had significantly faster declines in mortality from prostate cancer than did comparison countries between 1982-1985 and both 1998-2001 and 2002-2005.
Mortality trends from prostate cancer may be affected by “attribution bias”: people who have had prostate cancer detected may be more likely to have their death ascribed to it, even though some other morbid process was actually responsible (Feuer, Merrill, and Hankey, 1999). Such bias, combined with more aggressive screening, would produce a rise rather than a fall in prostate cancer mortality. This bias may account for the rise in prostate cancer mortality in the late 1980s and early 1990s (see Figure 9-3), but it obviously would minimize rather than accentuate the actual decline that is observed between 1982-1985 and 2002-2005.
African Americans have prostate cancer death rates that are among the highest in the world (Crawford, 2003). Perhaps the most prominent explanation of the racial disparity is that dark skin inhibits the absorption of Vitamin D, which is highly protective against prostate cancer (Li et al., 2007). A more tenuous connection to the health care system among African Americans is probably also a factor. Nevertheless, a sharp decline in prostate cancer mortality in the United States is evident among both whites and African Americans. Both whites and African Americans had rates that peaked in the early 1990s. Between 1992-1993 and 2004-2005, the death rate declined by 32.2 percent for African Americans and by 36.3 percent for whites (Li et al., 2007). The absolute decline in rates was much larger for African Americans: their 5-year survival rate increased from 68.4 percent for those diagnosed in 1986, the year PSA testing was approved, to 97.0 percent for those diagnosed in 2000. Among whites, the improvement was from 79.0 to 99.8 percent (National Cancer Institute, 2008).
CASE STUDY II: BREAST CANCER
Breast cancer is the most common cause of cancer death among women in a majority of high-income countries (Vainio and Bianchini, 2002). In contrast to prostate cancer, there are important behavioral risk factors for breast cancer. These include childlessness or low parity, late age at first birth, obesity, and use of hormone replacement therapy (Das, Feuer, and Mariotto, 2005; Levi et al., 2005). Thus, trends in mortality are more difficult to interpret as exclusively reflecting medical factors. But, like prostate
cancer, breast cancer is highly amenable to medical intervention through screening and therapy.
Breast Cancer Screening
Mammography, breast self-examination, clinical breast examination (CBE), and magnetic resonance imaging (MRI) are used to screen for breast cancer. No randomized trials of CBE alone have been completed, and casecontrol and ecological studies have provided only limited evidence for its efficacy in reducing mortality from breast cancer (Vainio and Bianchini, 2002). Breast self-examination is an appealing screening method because it is noninvasive, but it has weak ability to detect breast cancer (Elmore et al., 2005). Two randomized trials of breast self-examination have been conducted, and neither found evidence of mortality reduction. The International Agency for Research on Cancer (IARC) has concluded that there is inadequate evidence for the efficacy of CBE and breast self-examination in reducing breast cancer mortality (Vainio and Bianchini, 2002). The U.S. Preventive Services Task Force also found evidence from trials involving CBE and breast self-examination to be inconclusive (Humphrey et al., 2002). The third technique, MRI, is mainly employed in high-risk patients and after conventional diagnostic procedures have already been conducted (Veronesi et al., 2005). Because of its high cost (approximately 10 times that of mammography) and its relatively low specificity, MRI is not a feasible tool for routine screening in the general population (Elmore et al., 2005).
Thus, mammography is currently the most important diagnostic tool for breast cancer. It is the only screening test that has been shown to reduce mortality from breast cancer in randomized trials and population studies (Veronesi et al., 2005; Wells, 1998). The IARC concluded that there is sufficient evidence from randomized trials that offering of mammography to a treatment group reduces breast cancer mortality in women ages 50-69, by an average of 25 percent. After adjusting for the effect of nonacceptance of the screening invitation, this figure rises to 35 percent (Vainio and Bianchini, 2002). The U.S. Preventive Services Task Force reviewed eight randomized controlled trials of offering mammograms to treatment groups and concluded that, for studies that were designated as of fair quality or better, the relative mortality risk for women ages 40-74 was 0.84 (95% CI, 0.77 to 0.91) (Humphrey et al., 2002; see also Gøtzsche and Nielsen, 2009). While some concerns have been raised concerning flaws in the trials’ design and execution, in-depth independent reviews have concluded that they do not negate the trials’ results (Quinn, 2003).
The National Cancer Institute and the American Cancer Society issued the first formal guidelines for mammography in 1977, advocating screening for all women over the age of 50 (Wells, 1998). Currently, all major U.S.
medical organizations recommend screening mammography for women over the age of 40 (Ahern and Shen, 2009; Elmore et al., 2005). The United States is the only country that strongly endorses screening mammography for women under age 50 (Jatoi and Miller, 2003); recent evidence has supported the efficacy of screening in the age group 40-49 (Humphrey et al., 2002).
Use of mammographic screening in the United States increased very rapidly; the percentage of women ages 50-64 who reported having a mammogram in the past 2 years increased from 31.7 percent in 1987 to 73.7 percent in 1998 (Breen et al., 2001). Screening programs generally began later in Europe than in the United States (Møller et al., 2005). The start dates for organized screening programs in the countries under investigation range from 1986 to 1999 (Jatoi and Miller, 2003; Shapiro et al., 1998).
Table 9-4 presents international data on the frequency of screening for breast cancer in recent years. In the early to mid-1990s, the United States had the highest frequency of mammograms in the nine countries for which we are able to locate data. The OECD has collected more recent data showing that, while the frequency of mammograms has increased in the United States, it has grown faster in a number of other countries. The most recent tabulations, using data from HRS and SHARE, show that, among 11 OECD countries in 2004, the United States had the highest proportion of the population receiving a mammogram within the past 2 years at ages 50-64, 65-74, and 75+ (Howard, Richardson, and Thorpe, 2009).
Consistent with the relatively high frequency of mammograms in the United States, Sant et al. (2004) found that breast cancer is diagnosed at what is, on average, a later stage in Europe than in the United States.
Breast Cancer Treatment
In OECD countries, the large majority of cases of breast cancer are treated surgically. Surgery is often supplemented with some combination of radiotherapy, hormone therapy, and chemotherapy (i.e., adjuvant therapy). Descriptions of the Halsted mastectomy, which served as the treatment of choice for breast cancer for almost a century, were first published in 1894 (Veronesi et al., 2002). It was later replaced by the modified radical mastectomy, which was popular in the 1980s (Cotlar, Dubose, and Rose, 2003). Neither the original Halsted radical mastectomy nor the modified radical mastectomy was introduced on the basis of evidence from randomized clinical trials; however, observational studies confirm an enormous survival advantage for surgery relative to no surgery (e.g., Sant et al., 2004).
In most high-income countries, breast-conserving surgery (BCS, also known as lumpectomy) is currently the most common primary treatment for breast cancer (Veronesi et al., 2005). Relative to total mastectomy, its
TABLE 9-4 Percentage of Women Receiving a Mammogram in Previous 2 Years: 1994 and 2003a
|
Country |
Earlier Year |
Later Year |
||||
|
% Screened |
Year |
Age Group |
% Screened |
Year |
Age Group |
|
|
Australia |
51.4 |
1996-1997 |
50-69 |
55.6 |
2003-2004 |
50-69 |
|
Austria |
23.1 |
1995 |
40-79 |
|
|
|
|
|
35.7 |
|
50-54 |
|
|
|
|
Belgium |
49.2 |
1997 |
50-69 |
54.0 |
2003 |
50-69 |
|
Canada |
50.0 |
1994 |
50+ |
70.6 |
2003 |
50-69 |
|
Finland |
|
|
|
87.7 |
2003 |
50-59 |
|
France |
|
|
|
72.8 |
2003 |
50-69 |
|
Hungary |
|
|
|
60.2 |
2003 |
45-65 |
|
Iceland |
|
|
|
62.0 |
2003 |
40-69 |
|
Ireland |
|
|
|
79.5 |
2003 |
50-64 |
|
Italy |
|
|
|
29.0 |
2000 |
55-69 |
|
Japan |
|
|
|
2.6 |
2003 |
50-69 |
|
Luxembourg |
|
|
|
62.4 |
2003 |
50-69 |
|
Netherlands |
53.2 |
1994 |
50-69 |
79.0 |
2003 |
50-75 |
|
New Zealand |
|
|
|
62.3 |
2003 |
50-64 |
|
Norway |
|
|
|
98.0 |
2003 |
50-69 |
|
Portugal |
|
|
|
60.1 |
2003 |
50-69 |
|
Spain |
28.0 |
1994 |
40-70 |
|
|
|
|
Sweden |
|
|
|
83.6 |
2004 |
50-74 |
|
Switzerlandb |
20.0 |
1992-1993 |
50-64 |
27.0 |
2002 |
50-69 |
|
United Kingdomc |
63.9 |
1995 |
50-64 |
74.7 |
2003 |
50-64 |
|
United States |
66.5 |
1994 |
50-64 |
76.0 |
2003 |
50-69 |
|
aFor later years, when there are two observations for the same country, we use survey rather than program data in order to maximize comparability with the United States (this affected only Canada and the Netherlands). bFor 1992-1993, the data for Switzerland is for the canton of Vaud only, and the screening interval is 1 year. cFor the United Kingdom, the recall period is 3 years. SOURCES: Adapted from Australian Institute of Health and Welfare (2008); Bulliard, De Landtsheer, and Levi (2003); Capet, Arbyn, and Abarca (2003); Centraal Bureau voor de Statistiek (2009); Department of Health (1999); Luengo et al. (1996); National Center for Health Statistics (2000); Organisation for Economic Co-operation and Development (2006, 2008); Snider et al. (1997); Vutuc, Haidinger, and Waldhoer (1998). |
||||||
advantages are reduced disfigurement and morbidity rather than further reductions in mortality (Wood, 1994). After 20 years of follow-up in a randomized trial, Fisher et al. (2002) reported finding no differences in disease-free survival, distant-disease-free survival, or overall survival between women who underwent lumpectomy alone compared with those having a total mastectomy (see also Veronesi et al., 2002). In 1990, the
National Institutes of Health Consensus Development Conference recommended breast conservation therapy for the majority of women with Stage I or II breast carcinoma (Lazovich et al., 1999).
Since radiation treatment of breast cancer was first used in 1896, equipment and techniques have improved substantially, particularly since the 1960s (Ragaz et al., 1997). The Early Breast Cancer Trialists’ Collaborative Group conducted a meta-analysis of 36 trials of radiotherapy. They found that the local recurrence rate with radiotherapy and surgery was three times lower than with surgery alone, and that radiotherapy was associated with a 6 percent reduction in the relative risk of death due to breast cancer (odds ratio, 0.94) (Early Breast Cancer Trialists’ Collaborative Group, 1995). Ragaz et al. (1997) found that, after 15 years of follow-up, women assigned to chemotherapy plus radiotherapy had a 33 percent reduction in the recurrence rate and a 29 percent reduction in mortality from breast cancer compared with women treated with chemotherapy alone.
Adjuvant systemic multiagent chemotherapy and the drug tamoxifen have been estimated to reduce mortality (in terms of the relative reduction of the annual odds of death) by 27 percent and 47 percent, respectively (Early Breast Cancer Trialists’ Collaborative Group, 1998a, 1998b). These figures are derived from the meta-analyses of all randomized trials of any aspect of treatment for early breast cancer that began before 1990. There were 47 trials of adjuvant polychemotherapy involving 18,000 women (Early Breast Cancer Trialists’ Collaborative Group, 1998a). Greater benefits were reported in women under age 50, who experienced significant reductions in recurrence and mortality of 35 and 27 percent, respectively. For women between ages 50 and 69, these figures were 20 and 11 percent, respectively (Early Breast Cancer Trialists’ Collaborative Group, 1998a).
Cole et al. first reported the clinical efficacy of tamoxifen for disseminated breast cancer in 1971. The Early Breast Cancer Trialists’ Collaborative Group summarized the results of 55 randomized controlled trials involving more than 37,000 women. Compared with a placebo, adjuvant tamoxifen resulted in annual reductions of 26 percent in recurrence and 14 percent in death. Among women treated for 5 years, these figures rose to 50 and 28 percent, respectively (Early Breast Cancer Trialists’ Collaborative Group, 1998b; Osborne, 1998). Tamoxifen produces significant benefits in women of all age groups (Early Breast Cancer Trialists’ Collaborative Group, 1998b; Jaiyesimi et al., 1995). Following pharmacological and clinical evaluations, the U.S. Food and Drug Administration approved tamoxifen for the treatment of metastatic breast cancer in postmenopausal women in 1977. Tamoxifen was also approved as the initial endocrine therapy for disseminated breast cancer in premenopausal women.
Together, these studies constitute a substantial body of evidence supporting the effectiveness of treatment for breast cancer. A number of ran-
domized trials have demonstrated that surgical options, radiation therapy, chemotherapy, and tamoxifen reduce recurrence rates and breast cancer mortality.
Information on international differences in breast cancer treatment is limited. A comparison of the Eurocare and SEER registry data found that 97 percent of women in SEER were treated surgically compared with 90 percent in the Eurocare registries. Rates of lymphadenectomy (surgical removal of one or more groups of lymph nodes) were slightly more extensive in the United States, and more axillary lymph nodes were examined in the United States (Sant et al., 2004). Hughes (2003) compared patterns of breast cancer care in Belgium, Canada (Manitoba and Ontario), France, Italy, Norway, Sweden, the United Kingdom (England), and the United States. During the latest period investigated, 1990-1993, at least 90 percent of women diagnosed with breast cancer received a mastectomy or breast-conserving surgery in all areas except Ontario, where the figure was 82 percent, and England (71 percent). The use of radiotherapy with BCS has also risen over time and varied considerably among countries. Among women receiving BCS in 1995-1997, Belgium, Canada, France, and the United Kingdom had the highest proportions of women receiving radiation therapy. The United States ranked below these countries and above Sweden and Italy (Hughes, 2003).
Adjuvant chemotherapy became standard treatment for breast cancer patients in the United States in the late 1970s (Ragaz et al., 1997). Tamoxifen began to be widely used in the late 1970s and early 1980s, after the Nolvadex Adjuvant Trial Organization trials demonstrated its effectiveness (Mariotto et al., 2002). It has since become the most widely prescribed antineoplastic agent for treatment of breast cancer in the United States and Great Britain (Jaiyesimi et al., 1995). Between 1975 and 2000, the percentage of breast cancer patients receiving chemotherapy in the United States increased from essentially 0 to 80 percent, while tamoxifen use increased from 0 to 50 percent (Berry et al., 2006). Starting in the mid-1980s, tamoxifen use in the United Kingdom also increased rapidly. By 1990, 50 percent of women with breast cancer over the age of 50 in the Thames region were receiving tamoxifen (Blanks et al., 2000). We have not found comparable international data on the use of chemotherapy and tamoxifen. Variations in stage and type of tumor, age of patient, type of surgery, and other factors make it impossible to reliably compare the few national or regional data that exist.
Breast Cancer Survival
Several studies have compared international survival rates from breast cancer. As noted above, the survival advantage of U.S. breast cancer pa-
tients compared with their European counterparts is well documented. The U.S. survival advantage is particularly sharp among older women (Hughes, 2003). International differences in survival are challenging to interpret, but three studies using cancer registry data for European and American women have attributed the survival differences from breast cancer to earlier diagnosis and more aggressive care in the United States. These factors have also been introduced to explain better breast cancer survival rates in the United States than in Canada (Ugnat et al., 2005).
Gatta et al. (2002) found that European breast cancer patients diagnosed in 1985-1989 had significantly lower 5-year relative survival rates than American patients (73 versus 82 percent). None of the 17 European countries had higher 5-year relative survival than the United States. In the first year after diagnosis, the risk of death from breast cancer was much higher in European than American patients. Survival rates fell with increasing age at diagnosis in both the United States and Europe, but the fall was more marked in Europe. Gatta et al. suggest that the survival rate differences may be attributable to earlier diagnosis in the United States.
The most thorough study compared American and European women diagnosed with breast cancer between 1990 and 1992 (Sant et al., 2004). The 5-year survival rate was higher in the United States than in Europe (89 versus 79 percent), and survival for each stage-at-diagnosis category was also higher in the United States. Early-stage tumors were more frequent in the United States (41 percent of cases) than in Europe (29 percent). Treatment was more aggressive in the United States, where 97.1 percent of women underwent surgery compared with 90.2 percent in Europe. In the United States, 50.7 percent of women had 15+ lymph nodes evaluated for metastasis, compared with 27.8 percent in Europe. The overall relative risk of death was 37 percent higher among European women (95 percent confidence interval, 25-50 percent). The excess risk was reduced to 20 percent by adjustment for surgical intervention, which was associated with a 90 percent reduction in mortality. Adjustment for stage at diagnosis reduced the relative risk to 12 percent, and further adjustment for the number of lymph nodes evaluated to determine cancer progression reduced the excess risk of death among the European women to an insignificant 7 percent. Introducing information on the use of radiotherapy did not alter the relative risk of European women. Thus, the higher survival rate in the United States appears to be a result both of earlier diagnosis and more aggressive treatment.
The most recent study compared cancer survival differences between Europe and the United States in 2000-2002 based on period rather than cohort survival data. As shown in Table 9-1, the 5-year survival rate for breast cancer was 79.0 percent in Europe, compared with 90.1 percent in the United States. Verdecchia et al. (2007) hypothesize that these differences were most likely due to differences in timeliness of diagnosis.
Trends in screening and in survival in the United States are consistent with the idea that earlier screening improves survival. The percentage of American women ages 50-64 who had received a mammogram in the previous two years increased from 32 percent in 1987 to 74 percent in 1998 and was accompanied by an increase in 5-year survival rates from 79 percent for those diagnosed in 1985 to 91 percent for those diagnosed in 2000 (National Cancer Institute, 2008).
Breast Cancer Mortality
In many developed countries, breast cancer mortality rates began declining around 1990 (Botha et al., 2003; Veronesi et al., 2005). It is unlikely that the declines in mortality were caused by changes in the major risk factors for the disease. In fact, the risk factor profile of women in high-income countries has, if anything, become less favorable over the past few decades as a result of rising obesity and delayed and reduced childbearing (Levi et al., 2005). Reductions after 2002 in the use of hormone replacement therapy could work in the opposite direction, but the risk is sufficiently small (Chlebowski et al., 2003; Writing Group for the Women’s Health Initiative Investigators, 2002), and lags sufficiently long, that the decline should not be reflected in a data series that ends in 2005. Chu et al. (1996) rule out changes in coding or ascertainment as contributors to the mortality decline in the United States, noting that there had been no coding changes affecting breast cancer and that no systematic problems with ascertainment were identified after 1989.
Studies of trends in breast cancer mortality have attributed the declines mainly to earlier detection—in particular, rising rates of mammographic screening—and improved treatment (Chu et al., 1996; Levi et al., 2005; Veronesi et al., 2005). A careful, detailed simulation for the United States by Berry and colleagues (2006) concluded that “we can say with high probability that both screening and adjuvant therapy have contributed to the reductions in U.S. breast cancer mortality observed from 1975 (and especially from 1990) to 2000. Our best estimate is that about two-thirds of the reduction is due to therapy and one-third to screening” (p. 36). Using less precise methods, Blanks and colleagues (2000) reached a similar conclusion about the decline in breast cancer mortality in England and Wales from 1990 to 1998. Evidence that states with greater use of mammography had greater mortality declines between 1992 and 1999 supports the link between screening and mortality (Das et al., 2005).
We hypothesize that the United States has had a faster decline in breast cancer mortality than the comparison countries because it took better advantage of technological advances in screening and treatment. Mortality data alone do not permit us to distinguish between the effects of screening and treatment, but that distinction is not central to our argument.
Figure 9-4 shows the annual age-standardized death rate in the United States and the unweighted mean for our 15 OECD countries since 1980. Clearly, the United States has had a faster decline in breast cancer mortality than average among the comparison countries. Is the faster decline in the United States statistically significant? To answer this question, we repeat the approach used for prostate cancer, using WHO data files on deaths by cause and population by 5-year age groups. We employ negative binomial regression on data at ages 50+ (in 5-year-wide age groups until 85+). The dependent variable is the log of the number of deaths from breast cancer in a certain age group for a particular country and time period. Independent variables are a set of age group identifiers, a set of period identifiers, a dummy variable for the United States, and a set of U.S./period interactions. We designate six 4-year-wide time periods, beginning with 1982-1985 and ending with 2002-2005, and choose 1982-1985 as the reference period. Because of the rapid increase in the proportion of women receiving mammograms from less than a third in 1987 to 74 percent in 1998, a reference period in the early 1980s appears appropriate. Significance tests recognize the clustering of observations by country. Results are presented in Table 9-3.
Using 1982-1985 as the reference period, we find that the U.S./2002-2005 interaction term is significant at .01. With a coefficient of −.126, the coefficient implies that mortality in the United States has fallen 13 percent faster since 1982-1985 than in other countries. U.S. interactive coefficients
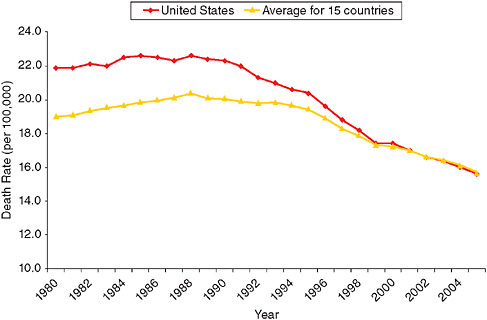
FIGURE 9-4 Age-standardized death rates from breast cancer, 1980-2005.
for 1994-1997 and 1998-2001 are also negative and significant at 5 percent. The interactive variable, U.S./2002-2005, is always significant at p < 0.01 regardless of which date is selected as the reference period (not shown). Thus, the United States has experienced a significantly faster decline in breast cancer mortality than comparison countries.
SUMMARY
We have demonstrated that mortality reductions from prostate cancer and breast cancer have been significantly more rapid in the United States than in a set of peer countries. We have argued that these unusually rapid declines are attributable to wider screening and more aggressive treatment of these diseases in the United States. It appears that the U.S. medical care system has worked effectively to reduce mortality from these important causes of death.
This conclusion is consistent with other evidence that we have reviewed on the performance of the U.S. health care system in enhancing survival: screening for other cancers also appears unusually extensive; 5-year survival rates from all of the major cancers are very favorable; survival rates following heart attack and stroke are also favorable (although 1-year survival rates following stroke are not above average); the proportion of people with elevated blood pressure or cholesterol levels who are receiving medication is well above European standards.
These performance indicators pertain primarily to what happens after a disease has developed. It is possible that the U.S. health care system performs poorly in preventing disease in the first place; however, there are no satisfactory international comparisons of disease incidence. Individuals report a higher prevalence of cancer and cardiovascular disease in the United States than in Europe, and biomarkers confirm the higher prevalence of many disease syndromes in the United States compared with England and Wales. Higher disease prevalence is prima facie evidence of higher disease incidence, although it could also be produced by better identification (e.g., through screening programs) or better survival. The history of exceptionally heavy smoking in the United States, and the more recent massive increase in obesity, suggest that a high disease incidence in the United States could not be laid entirely at the feet of the health care system, unless that system were held responsible for all health-related behaviors.
Evidence that the major diseases are effectively diagnosed and treated in the United States does not mean that there may not be great inefficiencies in the U.S. health care system. A list of prominent charges include fragmentation, duplication, inaccessibility of records, the practice of defensive medicine, misalignment of physician and patient incentives, limitations of access for a large fraction of the population, and excessively fast adoption
of unproven technologies (Garber and Skinner, 2008; Cebul et al., 2008; Commonwealth Fund, 2008). Some of these inefficiencies have been identified by comparing performance across regions of the United States. Of course, the fact that certain regions do poorly relative to others does not imply that the United States does poorly relative to other countries. And many of the documented inefficiencies of the U.S. health care system add to its costs rather than harm patients.
Just as we are not addressing issues of efficiency on the production side, we are not treating patient welfare as the main outcome. Practices that produce greater longevity do not necessarily enhance well-being. This potential disparity is central to the controversy involving PSA testing, which uncovers many cancers that would never kill patients but whose treatment often produces adverse side effects.
The question that we have posed is much simpler: Does a poor performance by the U.S. health care system account for the low international ranking of longevity in the United States? Our answer is “No.”
ACKNOWLEDGMENTS
This research was supported by the U.S. Social Security Administration (SSA) through grant no. 10-M-98363-1-01 to the National Bureau of Economic Research (NBER) as part of the SSA Retirement Research Consortium. The findings and conclusions expressed are solely those of the authors and do not represent the views of SSA, any agency of the federal government, or the NBER. We are grateful to Beth Soldo, Jason Schnittker, Eileen Crimmins, and anonymous reviewers for useful comments and suggestions.
REFERENCES
Ahern, C.H., and Shen, Y. (2009). Cost-effectiveness analysis of mammography and clinical breast examination strategies: A comparison with current guidelines. Cancer Epidemiology, Biomarkers & Prevention, 18(3), 718-725.
Andriole, G.L., Grubb, R.L., Buys, S.S., Chia, D., et al. (2009). Mortality results from a randomized prostate-cancer screening trial. New England Journal of Medicine, 360, 1310-1319.
Antonarakis, E.S., Blackford, A.L., Garrett-Mayer, E., and Eisenberger, M.A. (2007). Survival in men with nonmetastatic prostate cancer treated with hormone therapy: A quantitative systematic review. Journal of Clinical Oncology, 25(31), 4998-5008.
Aus, G., Bergdahl, S., Lodding, P., Lilja, H., et al. (2007). Prostate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer—Results from a prospective, population-based randomized controlled trial. European Urology, 51, 659-664.
Australian Institute of Health and Welfare. (2008). BreastScreen Australia Monitoring Report 2004-2005. Cancer series no. 42, cat. no. CAN 37. Canberra: Author.
Avendano, M., Glymour, M., Banks, J., and Mackenbach, J.P. (2009). Health disadvantage in U.S. adults ages 50 to 74 years: A comparison of the health of rich and poor Americans with that of Europeans. American Journal of Public Health, 99(3), 540-548.
Baade, P.D., M.D. Coory, and J.F. Aitken. (2004). International trends in prostate-cancer mortality: The decrease is continuing and spreading. Cancer Causes & Control, 15(3), 237-241.
Banks, J., Marmot, M., Oldfield, Z., and Smith, J.P. (2006). Disease and disadvantage in the United States and in England. Journal of the American Medical Association, 295(17), 2037-2045.
Beaulac, J.A., Fry, R.N., and Onysko, J. (2006). Lifetime and recent prostate specific antigen (PSA) screening of men for prostate cancer in Canada. Canadian Journal of Public Health, 97(3), 171-6.
Beemsterboer, P.M.M., de Koning, H.J., Kranse, R., Trienekens, T.H., van der Maas, P.J., and Schröder, F.H. (2000). Prostate specific antigen testing and digital rectal examination before and during a randomized trial of screening for prostate cancer: European Randomized Study of Screening for Prostate Cancer, Rotterdam. Journal of Urology, 164, 1216-1220.
Berry, D.A., Cronin, K.A., Plevritis, S.K., Fryback, D.G., et al. (2005). Effect of screening and adjuvant therapy on mortality from breast cancer. New England Journal of Medicine, 353, 1784-1792.
Berry, D.A., Inoue, L., Shen, Y., Venier, J., et al. (2006). Modeling the impact of treatment and screening on U.S. breast cancer mortality: A Bayesian approach. Journal of the National Cancer Institute Monograph, 36, 30-36.
Bill-Axelson, A., Holmberg, L., Ruutu, M., Haggman, M., Andersson, S., Bratell, S., Spangberg, A., Busch, C., Nordling, S., Garmo, H., Palmgren, J., Adami, H., Norlen, B., and Johansson, J. (2005). Radical prostatectomy versus watchful waiting in early prostate cancer. New England Journal of Medicine, 352(19), 1977-84.
Bill-Axelson, A., Holmberg, L., Filén, F., Ruutu, M., et al. (2008). Radical prostatectomy versus watchful waiting in localized prostate cancer: The Scandinavian prostate cancer group-4 randomized trial. Journal of the National Cancer Institute, 100, 1144-1154.
Blanks, R.G., Moss, S.M., McGahan, C.E., Quinn, M.J., et al. (2000). Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990-1998: Comparison of observed with predicted mortality. British Medical Journal, 321, 665-669.
Bolla, M., Collette, L., Blank, L., Warde, P., et al. (2002). Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A Phase III randomised trial. Lancet, 360(9327), 103-8.
Botha, J.L., Bray, F., Sankila, R., and Parkin, D.M. (2003). Breast cancer incidence and mortality trends in 16 European countries. European Journal of Cancer, 39, 1718-1729.
Bouchardy, C., Fioretta, G., Rapiti, E., Verkooijen, H.M., et al. (2008). Recent trends in prostate cancer mortality show a continuous decrease in several countries. International Journal of Cancer, 123, 421-429.
Breen, N., Wagener, D.K., Brown, M.L., Davis, W.W., et al. (2001). Progress in cancer screening over a decade: Results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. Journal of the National Cancer Institute, 93(22), 1704-1713.
Bulliard, J.-L., De Landtsheer, J.-P., and Levi, F. (2003). Results from the Swiss mammography screening pilot programme. European Journal of Cancer, 39, 1761-1769.
Bunting, P. (2002). Screening for prostate cancer with prostate-specific antigen: Beware the biases. Clinica Chimica Acta, 315, 71-97.
Capet, F., Arbyn, M., and Abarca, M. (2003). Mammografische opsporing van borstkanker in België: analyse van de gezondheidsenquêtes 1997 en 2001. IPH / EPI—REPORTS Nr. 2003-008. Brussels.
Cebul, R.D., Rebitzer, J.B., Taylor, L.J., and Votruba, M.E. (2008). Organizational fragmentation and care quality in the U.S. healthcare system. Journal of Economic Perspectives, 22(4), 93-113.
Centers for Disease Control and Prevention. (2002). Annual smoking-attributable mortality, years of potential life lost, and economic costs: United States, 1995-1999. Morbidity and Mortality Weekly Report, 51(14), 300-303.
Centraal Bureau voor de Statistiek. (2009). Statline. Available: http://statline.cbs.nl/StatWeb/default.aspx [accessed May 2010].
Chlebowski, R.T., Hendrix, S.L., Langer, R.D., et al. (2003). Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women’s Health Initiative randomised trial. Journal of the American Medical Association, 289, 3243-3253.
Chu, K.C., Tarone, R.E., Kessler, L.G., Ries, L.A.G., et al. (1996). Recent trends in U.S. breast cancer incidence, survival, and mortality rates. Journal of the National Cancer Institute, 88, 1571-1579.
Ciccolallo, L., Capocaccia, R., Coleman, M.P., Berrino, F., et al. (2005). Survival differences between European and U.S. patients with colorectal cancer: Role of stage at diagnosis and surgery. Gut, 54(2), 268-273.
Cole, M.P., Jones, C.T.A., Todd, I.D.H. (1971). A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI 46474. British Journal of Cancer, 25, 270-275.
Collin, S.M., Martin, R.M., Metcalfe, C., Gunnell, D., et al. (2008). Prostate-cancer mortality in the USA and UK in 1975-2004: An ecological study. Lancet Oncology, 9, 445-452.
Commonwealth Fund Commission on a High Performance Health System. (2008). Why Not the Best? Results from the National Scorecard on U.S. Health System Performance, 2008. New York: Commonwealth Fund.
Constantinou, J., and Feneley, M.R. (2006). PSA testing: An evolving relationship with prostate cancer screening. Prostate Cancer and Prostatic Diseases, 9, 6-13.
Cotlar, A.M., Dubose, J.J., and Rose, D.M. (2003). History of surgery for breast cancer: Radical to the sublime. Current Surgery, 60(3), 329-337.
Crawford, E.D. (2003). Epidemiology of prostate cancer. Urology, 62(6 suppl 1), 3-12.
Cutler, D. (2004). Your Money or Your Life. New York: Oxford University Press.
Cutler, D.M., and Glaeser, E.L. (2006). Why Do Europeans Smoke More Than Americans? NBER working paper 12124. Cambridge, MA: National Bureau of Economic Research.
Cutler, D.M., Glaeser, E.L., and Shapiro, J.M. (2003). Why have Americans become more obese? Journal of Economic Perspectives, 17(3), 93-118.
D’Ambrosio, G., Samani, F., Cancian, M., and De Mola, C. (2004). Practice of opportunistic prostate-specific antigen screening in Italy: Data from the health search database. European Journal of Cancer Prevention, 13(5), 383-386.
Das, B., Feuer, E.J., and Mariotto, A. (2005). Geographic association between mammography use and mortality reduction in the US. Cancer Causes and Control, 16, 691-699.
De Koning, H.J., Liem, M.K., Baan, C.A., Boer, R., et al. (2002). Prostate cancer mortality reduction by screening: Power and time frame with complete enrollment in the European Randomised Screening for Prostate Cancer (ERSPC) Trial. International Journal of Cancer, 98(2), 268-273.
DeNavas-Walt, C., Proctor, B.D., and Smith, J. (2007). Income, Poverty, and Health Insurance Coverage in the United States: 2006. U.S. Census Bureau, Current Population Reports, P60-233. Washington, DC: U.S. Government Printing Office.
Department of Health. (1999). Breast Screening Programme, England 1997-1998. Available: http://www.dh.gov.uk/en/Publicationsandstatistics/Statistics/StatisticalWorkAreas/Statisticalhealthcare/DH_4016216 [accessed May 2010].
Early Breast Cancer Trialists’ Collaborative Group. (1995). Effects of radiotherapy and surgery in early breast cancer: An overview of the randomized trials. New England Journal of Medicine, 333(22), 1444-1455.
Early Breast Cancer Trialists’ Collaborative Group. (1998a). Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet, 352, 930-942.
Early Breast Cancer Trialists’ Collaborative Group. (1998b). Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet, 351, 1451-1467.
Eisinger, F., Blay, J.-Y., Morère, J.-F., Rixe, O., Calazel-Benque, A., Cals, L., Coscas, Y., Dolbeault, S., Namer, M., Serin, D., Roussel, C., and Pivot, X. (2008). Cancer screening in France: Subjects’ and physicians’ attitudes. Cancer Causes and Control, 19(4), 431-434.
Elmore, J.G., Armstrong, K., Lehman, C.D., and Fletcher, S.W. (2005). Screening for breast cancer. Journal of the American Medical Association, 293, 1245-1256.
Etzioni, R., Gulati, R., Falcon, S., and Penson, D. (2008). Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: A surveillance modeling approach. Medical Decision Making, 28, 323-331.
Feuer, E.J., Merrill, R.M., and Hankey, B.F. (1999). Cancer surveillance series: Interpreting trends in prostate cancer—Part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. Journal of the National Cancer Institute, 91(12), 1025-1032.
Fisher, B., Anderson, S., Bryant, J., Margolese, R., et al. (2002). Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine, 347(16), 1233-1241.
Fleshner, N., Rakovitch, E., and Klotz, L. (2000). Differences between urologists in the United States and Canada in the approach to prostate cancer. Journal of Urology, 163, 1461-1466.
Forey, B., Hamling, J., Lee, P., and Wald, N. (Eds.) (2002). International Smoking Statistics: A Collection of Historical Data from 30 Economically Developed Countries. London, England: Oxford University Press.
Garber, A. (2003). Comparing health systems from the disease-specific perspective. In Organisation for Economic Co-operation and Development, A Disease-Based Comparison of Health Systems: What Is Best and at What Cost? (pp. 95-106). Paris: Organisation for Economic Co-operation and Development.
Garber, A.M., and Skinner, J. (2008). Is American health care uniquely inefficient? Journal of Economic Perspectives, 22(4), 27-50.
Gatta, G., Capocaccia, R., Coleman, M.P., Gloeckler Ries, L.A., et al. (2000). Toward a comparison of survival in American and European cancer patients. Cancer, 89(4), 893-900.
Gibbons, L., and Waters, C. (2003). Prostate cancer—Testing, incidence, surgery and mortality. Health Reports, 14(3), 19-20.
Gøtzsche, P.C., and Nielsen, M. (2009). Screening for breast cancer with mammography (review). Cochrane Database of Systematic Reviews, Issue 2, 1-90.
Haldorsen, T., and Grimsrud, T.K. (1999). Cohort analysis of cigarette smoking and lung cancer incidence among Norwegian women. International Journal of Epidemiology, 28(6), 1032-1036.
Halliday, A., Mansfield, A., Marro, J., Peto, C., et al. (2004). Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet, 363(9420), 1491-1502.
Hanks, G.E., Pajak, T.F., Porter, A., Grignon, D., et al. (2003). Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The radiation therapy oncology group protocol 92-02. Journal of Clinical Oncology, 21(21), 3972-3978.
Harris, R., and Lohr, K.N. (2002). Screening for prostate cancer: An update of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 137(11), 917-929.
Holden, C.A., Jolley, D.J., McLachlan, R.I., Pitts, M., Cumming, R., Wittert, G., Handelsman, D.J., and de Kretser, D.M. (2006). Men in Australia Telephone Survey (MATeS): Predictors of men’s help-seeking behaviour for reproductive health disorders. Medical Journal of Australia, 185(8), 418-422.
Howard, D., Richardson, L., and Thorpe, K. (2009). Cancer screening and age in the U.S. and Europe. Health Affairs, 28(6), 1838-1847.
Hsing, A.W., Tsao, L., and Devesa, S.S. (2000). International trends and patterns of prostate cancer incidence and mortality. International Journal of Cancer, 85(1), 60-67.
Hughes, M. (2003). Summary of results from breast cancer disease study. In Organisation for Economic Co-operation and Development, A Disease-Based Comparison of Health Systems: What Is Best and at What Cost? (pp. 77-94). Paris: Organisation for Economic Co-operation and Development.
Humphrey, L.L., Helfand, M., Chan, B.K.S., and Woolf, S.H. (2002). Breast cancer screening: A summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 137(5, 1), 347-360.
Ilic, D., O’Connor, D., Green, S., and Wilt, T. (2006). Screening for prostate cancer. Cochrane Database of Systematic Reviews, Issue 3.
Institute of Medicine. (2001). Coverage Matters: Insurance and Health Care. Washington, DC: National Academy Press.
International Agency for Research on Cancer. (2008). World Health Organization Mortality Data. Lyon, France: Author.
Jaiyesimi, I.A., Buzdar, A.U., Decker, D.A., and Hortobagyi, G.N. (1995). Use of tamoxifen for breast cancer: Twenty-eight years later. Journal of Clinical Oncology, 13, 513-529.
Jatoi, I., and Miller, A.B. (2003). Why is breast-cancer mortality declining? Lancet Oncology, 4, 251-254.
Kapteyn, A., Smith, J.P., and van Soest, A. (2007). Vignettes and self-reports of work disability in the United States and the Netherlands. American Economic Review, 97(1), 461-473.
Kaul, P., Armstrong, P., Chang, W., Naylor, C., Granger, C., Lee, K., Peterson, I., Califf, R., Topol, E., and Mark, D. (2004). Long-term mortality of patients with acute myocardial infarction in the United States and Canada. Circulation, 110, 1754-1760.
Klimont, J., Ktir, J., and Leitner, B. (2007). Österreichische gesundheitsbefragung 2006/2007: hauptergebnisse und methodische Dokumentation. Statistik Austria. Federal Ministry of Health, Family and Youth. Available: http://www.bmgfj.gv.at [accessed July 2008].
Kvåle, R., Auvinen, A., Adami, H.-O., Klint, Å., et al. (2007). Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. Journal of the National Cancer Institute, 99(24), 1881-1887.
Lazovich, D., Solomon, C.C., Thomas, D.B., Moe, R.E., et al. (1999). Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the Treatment of Patient with Early Stage Invasive Breast Carcinoma. Cancer, 86(4), 628-637.
Levi, F., La Vecchia, C., Randimbison, L., Erler, G., Te, V.-C., and Franceschi, S. (1998). Incidence, mortality, and survival from prostate cancer in Vaud and Neuchatel, Switzerland, 1974-1994. Annals of Oncology, 9(1), 31-35.
Levi, F., Lucchini, F., Negri, E., and La Vecchia, C. (2000). Recent trends in prostate cancer mortality in the European Union. Epidemiology, 11(5), 612.
Levi, F., Bosetti, C., Lucchini, F., Negri, E., et al. (2005). Monitoring the decrease in breast cancer mortality in Europe. European Journal of Cancer Prevention, 14, 497-502.
Li, H., Stampfer, M., Hollis, J., Mucci, L., Gaziano, J., Hunter, D., Giovannucci, E., and Ma, J. (2007). A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. Public Library of Science, 4(3), 562-571.
Lousbergh, D., Buntinx, F., Geys, G., Du Bois, M., Dhollander, D., and Molenberghs, G. (2002). Prostate-specific antigen screening coverage and prostate cancer incidence rates in the Belgian province of Limburg in 1996-1998. European Journal of Cancer Prevention, 11(6), 547-549.
Luengo, S., Lazrao, P., Madero, R., Alvirac, F., et al. (1996). Equity in the access to mammography in Spain. Social Science and Medicine, 43(8), 1263-1271.
Lumey, L.H., Pittman, B., Zang, E.A., and Wynder, E.L. (1997). Cigarette smoking and prostate cancer: No relation with six measures of lifetime smoking habits in a large case-control study among U.S. whites. Prostate, 33(3), 195-200.
Lu-Yao, G., Albersen, P., Moore, D., Shih, W., Lin, Y., DiPaola, R., and Yao, S. (2008). Survival following primary androgen deprivation therapy among men with localized prostate cancer. Journal of the American Medical Association, 300(2), 173-181.
Macinko, J., Starfield, B., and Shi, L. (2003). The contribution of primary care systems to health outcomes within Organisation for Economic Co-operation and Development (OECD) Countries, 1970-1998. Health Services Research, 38(3), 831-865.
Mariotto, A., Feuer, E.J., Harlan, L.C., Wun, L.M., et al. (2002). Trends in use of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States: 1975-1999. Journal of the National Cancer Institute, 94, 1626-1634.
McKinsey Global Institute. (2008). Accounting for the Cost of U.S. Health Care: A New Look at Why Americans Spend More. Available: http://www.mckinsey.com/mgi/publications/us_healthcare/ [accessed May 2010].
Melia, J., Coulson, P., Johns, L., and Moss, S. (2003). Report to the Department of Health: Study to Assess the Rate of PSA Testing in Men with No Previous Diagnosis of Prostate Cancer. Surrey, England: Cancer Screening Evaluation Unit.
Michaud, P.C., Goldman, D., Lakdawalla, D., Gailey, A., and Zheng, Y. (2009). International Differences in Longevity and Health and Their Economic Consequences. Santa Monica, CA: RAND.
Moise, P. (2003). The heart of the health care system: Summary of the ischaemic heart disease part of the OECD Ageing-Related Diseases Study. In Organisation for Economic Co-operation and Development, A Disease-Based Comparison of Health Systems: What Is Best and at What Cost? (pp. 27-52). Paris: Organisation for Economic Co-operation and Development.
Møller, B., Weedon-Fekjær, H., Hakulinen, T., Tryggvadóttir, L., et al. (2005). The influence of mammographic screening on national trends in breast cancer incidence. European Journal of Cancer Prevention, 14(2), 1117-1128.
National Cancer Institute. (2008). Surveillance, Epidemiology and End Results. Available: http://seer.cancer.gov/ [accessed May 2010].
National Center for Health Statistics. (2000). Health, United States, 2000. Hyattsville, MD: Author.
National Center for Health Statistics. (2002). Deaths: Final data for 2000. National Vital Statistics Reports, 50(15).
National Center for Health Statistics. (2008). Deaths: Final data for 2005. National Vital Statistics Reports, 56(10).
Nolte, E., and McKee, C.M. (2008). Measuring the health of nations: Updating an earlier analysis. Health Affairs, 27(1), 58-71.
Ogawa, K., Nakamura, K., Sasaki, T., Onishi, H., et al. (2008). Radical external beam radiotherapy for prostate cancer in Japan: Differences in the patterns of care among Japan, Germany, and the United States. Radiation Medicine, 26, 57-62.
Ohsfeldt, R.L., and Schneider, J.E. (2006). The Business of Health: The Role of Competition, Markets, and Regulation. Washington, DC: AEI Press.
Organisation for Economic Co-operation and Development. (2003). Stroke Care in OECD Countries: A Comparison of Treatment, Costs, and Outcomes in 17 Countries. Health Working Paper #5. Paris: Author.
Organisation for Economic Co-operation and Development. (2006). Health Care Quality Indicators Project: Initial Indicators Report. OECD health working paper #22. Paris: Author.
Organisation for Economic Co-operation and Development. (2007). Health Care Quality Indicators Project 2006: Data Collection Update Report. Working paper #29. Paris: Author.
Organisation for Economic Co-operation and Development. (2008). OECD Health Data 2008: How Does the United States Compare? Paris: Author.
Osborne, C.K. (1998). Tamoxifen in the treatment of breast cancer. New England Journal of Medicine, 339(22), 1609-1618.
Otto, S.J., Van der Crusijen, I.W., Liem, M.K., Korfage, I.J., Lous, J.J., Schröder, F.H., and De Koning, H.J. (2003). Effective PSA contamination in the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. International Journal of Cancer, 105(3), 394-399.
Páez, A., Luján, M., Llanes, L., Romero, I., de la Cal, M.A., Miravalles, E., and Berenguer, A. (2002). PSA-use in a Spanish industrial area. European Urology, 41, 162-166.
Pilepich, M.V., Winter, K., Lawton, C.A., Krisch, R.E., et al. (2005). Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: Long-term results of Phase III. RTOG 85-31. International Journal of Radiation Oncology*Biology*Physics, 61(5), 1285-1290.
Pollack, A., Zagars, G., Starkschall, G., Antolak, J., Lee, J., Huang, E., von Eschenback, A., Kuban, D., and Rosen, I. (2002). Prostate cancer radiation dose response: Results of the M.D. Anderson Phase III randomized trial. International Journal of Oncology, Biology, Physics, 53(5), 1097-1105.
Post, P.N., Damhuis, R.A.M., van der Meyden, A.P.M., and the EUROCARE Working Group. (1998). Variation in survival of patients with prostate cancer in Europe since 1978. European Journal of Cancer, 34(14), 2226-2231.
Potosky, A.L., Feuer, E.J., and Levin, D.L. (2001). Impact of screening on incidence and mortality of prostate cancer in the United States. Epidemiologic Reviews, 23(1), 181-186.
Preston, S., and Wang, H. (2006). Changing sex differentials in mortality in the United States: The role of cohort smoking patterns. Demography, 43(4), 413-434.
Quinn, M.J. (2003). Cancer trends in the United States—A view from Europe. Journal of the National Cancer Institute, 95(17), 1258-1261.
Ragaz, J., Jackson, S.M., Le, N., Plenderleith, I.H., et al. (1997). Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. New England Journal of Medicine, 337(14), 956-962.
Ross, L.E., Berkowitz, Z., and Ekwueme, D.U. (2008). Use of the prostate-specific antigen test among U.S. men: Findings from the 2005 National Health Interview Survey. Cancer Epidemiology Biomarkers & Prevention, 17(3), 636-644.
Sandblom, G., Dufmats, M., Nordenskjöld, K., and Varenhorst, E. (2000). Prostate carcinoma trends in three counties in Sweden 1987-1996: Results from a population-based national cancer register, south-east region prostate cancer group. Cancer, 88(6), 1445-1453.
Sant, M., Allemani, C., Berrino, F., Coleman, M.P., et al. (2004). Breast carcinoma survival in Europe and the United States: A population-based study. Cancer, 100(4), 715-722.
Schootman, M., Jeffe, D., Reschke, A., and Aft, R. (2004). The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast Cancer Research and Treatment, 85(3), 219-222.
Schröder, F.H., Hugosson, J., Roobol, M.J., Tammela, T.L.J., et al. (2009). Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine, 360, 1320-1328.
Sennfalt, K., Carlsson, P., and Varenhorst, E. (2006). Diffusion and economic consequences of health technologies in prostate cancer care in Sweden, 1991-2002. European Urology, 49, 1028-1034.
Shampo, M. (2002). Development of the PSA test. Journal of Pelvic Surgery, 8(2), 123-126.
Shapiro, S., Coleman, E.A., Breeders, M., Codd, M., et al. (1998). Breast cancer screening programmes in 22 countries: Current policies, administration and guidelines. International Journal of Epidemiology, 27, 735-742
Sirovich, B.E., Schwartz, L.M., and Woloshin, S. (2003). Screening men for prostate and colorectal cancer in the United States: Does practice reflect the evidence? Journal of the American Medical Association, 289, 1414-1420.
Smith, D.P., and Armstrong, B.K. (1998). Prostate-specific antigen testing in Australia and association with prostate cancer incidence in New South Wales. Medical Journal of Australia, 169, 17-20.
Snider, J., Beauvais, J., Levy, I., Villeneueve, P., and Pennock, J. (1997). Trends in mammography and Pap smear utilization in Canada. Chronic Diseases in Canada, 17(3), 1-15.
Stattin, P., Johansson, R., Lodnert, R., Ove, A., et al. (2005). Geographical variation in incidence of prostate cancer in Sweden. Scandinavian Journal of Urology and Nephrology, 39(5), 372-379.
Tarone, R.E., Chu, K.C., and Brawley, O.W. (2000). Implications of stage-specific survival rates in assessing recent declines in prostate cancer mortality rates. Epidemiology, 11(2), 167-170.
Technological Change in Health Care (TECH) Research Network. (2001). Technological change around the world: Evidence from heart attack care. Health Affairs, 20(3), 25-42.
Thompson, I.M., Tangen, C.M., Paradelo, J., Lucia, M.S., et al. (2006). Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized clinical trial. Journal of the American Medical Association, 296(19), 2329-2335.
Thorpe, K.E., Howard, D.H., and Galactionova, K. (2007a). Differences in disease prevalence as a source of the U.S.-European health care spending gap. Health Affairs, 26(6), 678-686.
Thorpe, K.E., Howard, D.H., and Galactionova, K. (2007b). Technical appendix. On-line data supplement to Thorpe et al. (2007a).
Torti, D.C., and Matheson, G.O. (2004). Exercise and prostate cancer. Sports Medicine, 34(6), 363-369.
Trock, B., Han, M., Freedland, S., Humphreys, E., DeWeese, T., Partin, A., and Walsh, P. (2008). Prostate cancer-specific survival following salvage radiotherapy vs. observation in men with biochemical recurrence after radical prostatectomy. Journal of the American Medical Association, 299(23), 2760-2769.
Ugnat, A.-M., Xie, L., Semenciw, R., Waters, C., et al. (2005). Survival patterns for the top four cancers in Canada: The effects of age, region and period. European Journal of Cancer Prevention, 14, 91-100.
U.S. Department of Health, Education, and Welfare. (1964). Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Public Health Service Publication No. 1103. Washington, DC: Office of the Surgeon General.
U.S. Preventive Services Task Force. (2008). Screening for prostate cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine, 149(3), 185-191.
Vainio, H., and Bianchini, F. (2002). Breast Cancer Screening. IARC Handbooks of Cancer Prevention, Vol. 7. Lyon, France: International Agency for Research on Cancer Press and World Health Organization.
Vercelli, M., Quaglia, A., Marani, E., and Parodi, S. (2000). Prostate cancer incidence and mortality trends among elderly and adult Europeans. Critical Reviews in Oncology/ Hematology, 35, 133-144.
Verdecchia, A., Francisci, S., Brenner, H., Gatta, G., et al. (2007). Recent cancer survival in Europe: A 2000-2002 period analysis of EUROCARE-4 data. Lancet Oncology, 8, 784-796.
Veronesi, U., Cascinelli, N., Mariani, L., Greco, M., et al. (2002). Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. New England Journal of Medicine, 347(16), 1227-1232.
Veronesi, U., Boyle, P., Goldhirsch, A., Orecchia, R., et al. (2005). Breast cancer. Lancet, 365, 1727-1741.
Vutuc, C., Haidinger, G., and Waldhoer, T. (1998). Prevalence of self-reported screening mammography and impact on breast cancer mortality in Austria. Wien Klin Wsch, 110, 485-490.
Wells, J. (1998). Mammography and the politics of randomised controlled trials. British Medical Journal, 317(31), 1224-1230.
Wilt, T.J., MacDonald, R., Rutks, I., Shamliyan, T.A., et al. (2008). Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer. Annals of Internal Medicine, 148(6), 435-448.
Wolf-Maier, K., Cooper, R., Kramer, H., Banegas, J., Giampaoli, S., Joffres, M., Poulter, N., Primastesta, P., Stegmayr, B., and Thamm, M. (2004). Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension, 43(10), 10-17.
Wong, Y.-N., Mitra, N., Hudes, G., Localio, R., et al. (2006). Survival associated with treatment vs. observation of localized prostate cancer in elderly men. Journal of the American Medical Association, 296, 2683-2693.
Wood, W.C. (1994). Progress from clinical trials on breast cancer. Cancer, 74(S9), 2606-2609.
World Health Organization. (2009). World Health Organization Statistical Information System. Available: http://www.who.int/whosis/whostat/en/ [accessed May 2010].
Writing Group for the Women’s Health Initiative Investigators. (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. Journal of the American Medical Association, 288, 321-333
Zappa, M., Visioli, C., Crocetti, E., Buonamici, C., Baccini, A., Taddei, S., and Ciatto, S. (2003). Practice of opportunistic PSA screening in the Florence District. Cancer Prevention, 12, 201-204.
Zhou, E.H., Ellis, R.J., Cherullo, E., Colussi, V., et al. (2009). Radiotherapy and survival in prostate cancer patients: A population-based study. International Journal of Radiation Oncology*Biology*Physics, 73(1), 15-23.








































