1
Introduction1
Regenerative medicine products, which are intended to repair or replace damaged cells or tissues in the body, include a range of therapeutic approaches such as cell- and gene-based therapies, engineered tissues, and non-biologic constructs. It is often challenging to properly characterize these products for a number of reasons. The mechanisms of action of these products (i.e., the biochemistry that results in their therapeutic result) is incompletely understood, the complexity of intracellular and biochemical networks is confounding, and complicated patient responses to therapies involve variable interactions within patients’ cellular and physiological microenvironments. As a result, there is often not a definitive correlation between what is measured and the clinical outcome for these complex products. Additional complexity is introduced by the manufacturing process and by the difficulty in predicting the biological effect of these processes. It is important to understand which measurements accurately and reliably characterize products in a way that is predictive of their clinical efficacy and safety for patients.
The quality attributes of regenerative medicine products was a topic of discussion at a previous public workshop of the Forum on Regenerative
___________________
1 The planning committee’s role was limited to planning the workshop, and the Proceedings of a Workshop was prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not necessarily endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
Medicine (NASEM, 2017). These attributes can include identity, quantity, purity, sterility, viability, and potency, and are measured and validated as part of regulatory submissions. Reliable characterization of regenerative medicine products is required to ensure quality, safety, and efficacy; these characteristics are identified as the products’ critical quality attributes (CQAs) (Burke and Zylberberg, 2019). However, to the extent that each product has unique features, it is likely that each product requires unique assays for characterization. The exact mechanisms of action for many regenerative medicine products are not well understood, making it difficult to determine which factors or analytes need to be measured to assess biological activity or identity (Tsokas et al., 2019). An additional challenge in defining CQAs is that in vitro metrics are not always predictive of in vivo activity. One approach to characterization might be to measure numerous assay endpoints, but this is a potentially expensive and unsustainable approach. Furthermore, collecting large amounts of data in the absence of an appropriate theoretical construct or clear understanding of mechanism of action may not elucidate the most important characteristics of a given product or system that provide the predictive information needed by researchers, manufacturers, and regulators.
A systems-focused approach is intended to better define the mechanistic parameters involved in the biological outcome and allows for the collection and use of more relevant data by recognizing those analytes that are most indicative of providing the most important predictive and actionable information about a product. Systems thinking2 involves the consideration of data acquisition, data analysis, and theoretical frameworks in order to develop a predictive understanding of complex systems. It is a multidisciplinary effort and can incorporate tools and knowledge from the fields of data science, biology, engineering, manufacturing, regulatory science, and clinical research and therefore requires the use of disparate data sources. Systems thinking for regenerative medicine might involve consideration of the characteristics of starting materials, ancillary materials, manufacturing processes, and patient characteristics in models that consider the physical factors that control the regulatory pathways associated with a given therapeutic outcome.
___________________
2 The term “systems thinking” does not currently have a widely accepted definition. However, the concept of systems thinking is closely associated with “developing coherent understanding of complex biological processes and phenomena from the molecular level to the level of ecosystems” (Verhoeff et al., 2018). According to one proposed definition, “Systems thinking is a set of synergistic analytic skills used to improve the capability of identifying and understanding systems, predicting their behaviors, and devising modifications to them in order to produce desired effects. These skills work together as a system” (Arnold and Wade, 2015, p. 675). See Chapter 2 for additional discussion of the definition of systems thinking by the workshop speakers.
Given these considerations, the Forum on Regenerative Medicine convened experts across disciplines for a 2-day virtual public workshop to explore systems thinking approaches and how they may be applied to support the identification of relevant quality attributes that can help in the optimization of manufacturing and streamline regulatory processes for regenerative medicine. The Statement of Task for the workshop can be found in Box 1-1. A broad array of stakeholders participated in the workshop, including data scientists, physical scientists, industry researchers, regulatory officials, clinicians, and patient representatives.
In opening the workshop, the planning committee members Anne Plant, a National Institutes of Standards and Technology (NIST) fellow and the former chief of the Biosystems and Biomaterials Division at NIST, and Krishnendu Roy, the Robert A. Milton Chair Professor, the director of the National Science Foundation’s Engineering Research Center for Cell Manufacturing Technologies, and the director of the Marcus Center for Therapeutic Cell Characterization and Manufacturing at the Wallace H. Coulter Department of Biomedical Engineering at the Georgia Institute of Technology and Emory University, described the workshop’s motivation and focus. The primary objective in convening the workshop, Plant said, was to explore the challenges that could lead the field to identifying meaningful predictive CQAs (e.g., determining what should be measured about a product to best characterize it and predict how well the product will
work in a patient). This is a challenging task from the perspectives of both regulators and practitioners. Given the complexity of cell-based therapies, no single measure will suffice, yet it is difficult to determine which combination of measures will be predictive of the efficacy and safety of a product. Even for successful therapies with known benefit, measurements have not yet been sufficiently correlated with patients’ clinical outcomes (Xue et al., 2017). New approaches and tools are needed to better understand the functional characteristics of the types of products emerging in the field of regenerative medicine, she said.
Another objective of the workshop was to explore challenges related to the increasing volumes of data that are now being collected, primarily through omics-type technologies. These data need to be parsed, analyzed, and integrated into theoretical frameworks that provide context for how to evaluate measurements in ways that provide predictive capability, Plant said. Moreover, the ecosystem of cell therapy product development in regenerative medicine is expansive, comprising multi-scale, dynamic, complex systems nested within each other (see Figure 1-1). Identifying the theoretical approaches that will bring these disparate data together in the appropriate context must be addressed. A systems approach is needed to analyze large-scale data from various sources (e.g., omics data, clinical indicators, starting materials) and begin to contextualize it using modeling frameworks, said Plant (see Figure 1-2). If one has not identified which measurements capture a product’s critical quality attributes, it will be impossible to identify the critical manufacturing process parameters that are required to achieve those CQAs.
In addition to a focus on data, data structures, data modeling, and how data modeling and theory can help cultivate the understanding and development of regenerative medicine products, Roy encouraged workshop participants to consider several additional issues. In particular, he mentioned challenges related to data-intensive analytical methods and theoretical models, strategies to influence the regulatory process using data and theoretical approaches, and the use of systems modeling and artificial intelligence to optimize and manage the complex supply chain for regenerative medicine products. He asked participants to consider best practices for data sharing, data collection, and clinical trial design (e.g., collecting longitudinal data from patients) to enable systems thinking across the regenerative medicine ecosystem. Other considerations he mentioned related to strategies in education and workforce development to prepare the next generation of scientists, technicians, and leaders to understand big data and systems thinking.
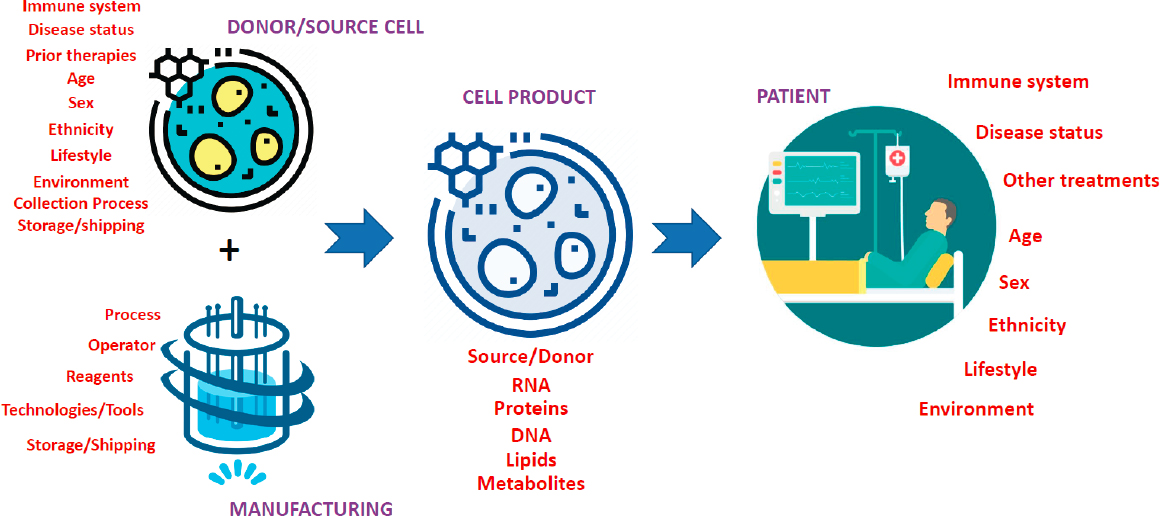
SOURCE: Krishnendu Roy workshop presentation, October 22, 2020.
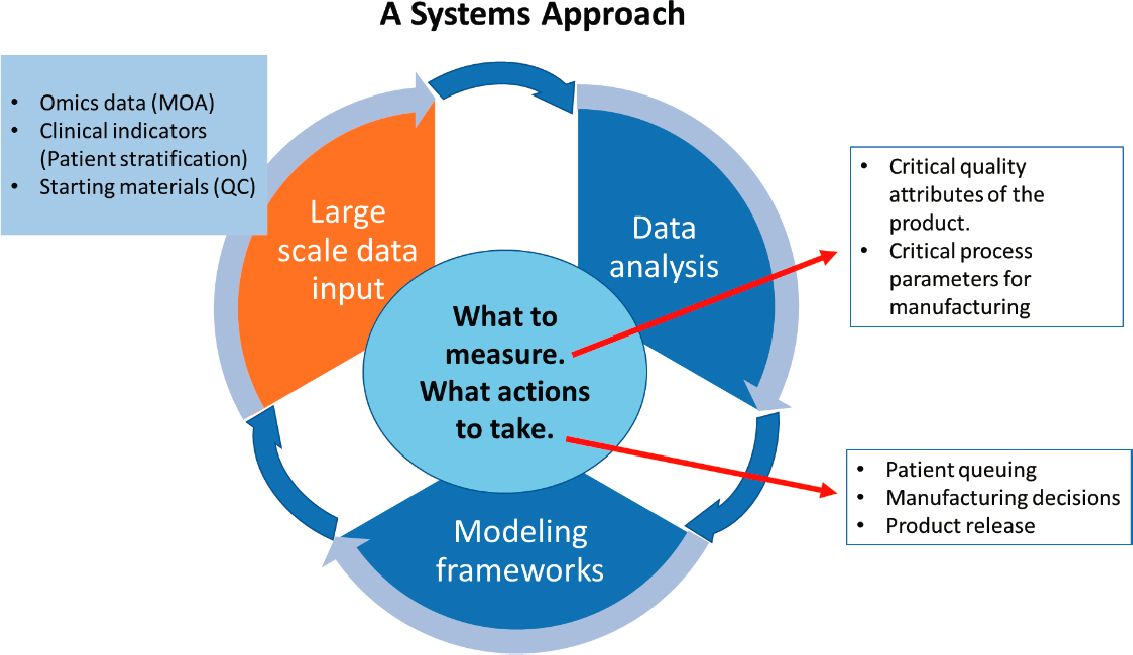
NOTE: MOA = mechanism of action; QC = quality control.
SOURCE: Anne Plant workshop presentation, October 22, 2020.
ORGANIZATION OF THE PROCEEDINGS
This Proceedings of a Workshop summarizes the presentations and discussions that took place at the workshop on October 22–23, 2020. The first session provided an introduction to systems thinking concepts (Chapter 2). Speakers discussed systems thinking, its application to the development of regenerative medicine, and computational approaches for systems-level data collection. The second session explored the role of systems thinking in addressing challenges related to CQAs (Chapter 3). It included a fireside chat on systems thinking and the regulation of regenerative medicine products as well as a panel discussion on the costs of not implementing systems thinking approaches. The third session looked at challenges associated with data collection, aggregation, and sharing (Chapter 4). Its presentations examined data challenges with omics analysis and disease modeling, the use of big data for clinical stratification of patents, and opportunities to use large datasets to predict patient outcomes. The fourth session focused on challenges and opportunities associated with systems-level analysis and modeling (Chapter 5). Speakers discussed the development of algorithms for single-cell genomics, strategies for modeling dynamic data to identify a reduced variable space, and the adoption of metabolic modeling tools in the biopharmaceutical industry. The fifth session considered strategies to address regenerative medicine manufacturing and supply chain challenges with systems-level approaches (Chapter 6). Speakers discussed the use of artificial intelligence in cell and gene therapies, modeling the manufacturing process in regenerative medicine, and supply chain and cost modeling. The sixth session explored issues in workforce development related to systems thinking (Chapter 7). Panelists in that session discussed challenges and opportunities for training and workforce development in data science, artificial intelligence, and computational biology.
This page intentionally left blank.








