Tremendous progress in understanding the key role glutamate receptors play in regulating synaptic plasticity has fueled the development of novel, rapidly acting antidepressants—ketamine and esketamine—that have shown effectiveness against treatment-resistant depression, said Husseini Manji. Moreover, he said, the therapeutic effects of these medications appear to persist even when the drug has cleared from the body, allowing for intermittent administration.
A BRIEF HISTORY OF KETAMINE AND ESKETAMINE
Ketamine has been used as an dissociative anesthetic since the mid-1960s. The use of ketamine as a treatment for depression began in the early 2000s when investigators at Yale conducted a small study of intravenous ketamine in seven patients with depression (Berman et al., 2000), said workshop speaker Carla Canuso, vice president of clinical development at Janssen Research & Development, LLC. Ketamine was known to be a potent antagonist of the NMDA glutamate receptor. and there was a growing understanding of the role of glutamate and the NMDA receptors in the pathophysiology of depression, she said. In this initial proof of concept study, researchers demonstrated that a single subanesthetic dose of ketamine produced a rapid, robust antidepressant effect, said Canuso (see Figure 3-1). A few years later, a randomized controlled trial in 18 subjects with treatment-resistant depression showed a similar rapid, robust response after a single dose that waned over the course of 1 week (Zarate et al., 2006), she said.
Following these initial studies, many clinical trials have confirmed the efficacy of ketamine and esketamine1 administered orally, intravenously, or intranasally (McIntyre et al., 2020). Importantly, Canuso added, a moderate to very large effect size was seen in all of these trials. Rates of remission
___________________
1 Esketamine is one of two racemic (mirror-image) structural chemical forms of ketamine.
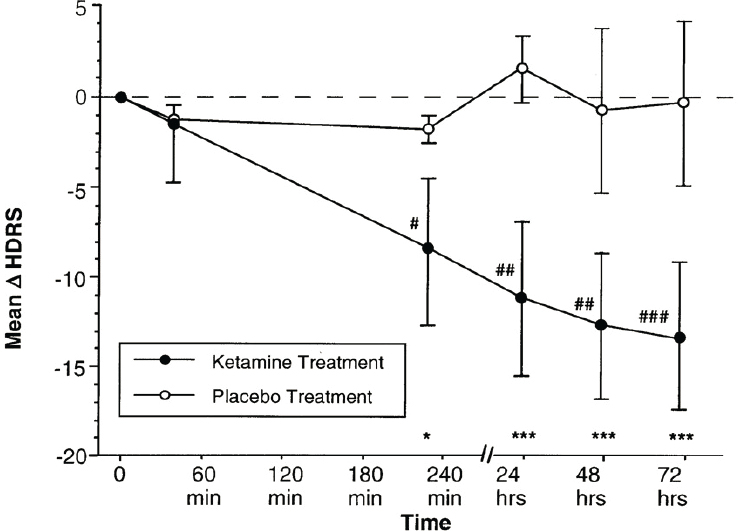
SOURCES: Presented by Carla Canuso, March 8, 2021; adapted from Berman et al., 2000.
achieved with ketamine and esketamine have been shown to be similar, about 25–30 percent, which is much higher than what had been seen in previous studies of standard antidepressants in treatment-resistant depression (Correia-Melo et al., 2020). The extent to which ketamine caused feelings of dissociation, a well-described side effect broadly defined as altered consciousness and awareness of the self, environment, and reality (Ballard and Zarate, 2020), were also similar across clinical trials, said Canuso.
CLINICAL AND PATIENT EXPERIENCES WITH INTRANASAL ESKETAMINE
Canuso leads the team at Janssen Research & Development, LLC that developed intranasal esketamine (Spravato®) for treatment-resistant depression in adults. Spravato® was granted FDA approval with Fast Track and Breakthrough Therapy designations in March 2019 for use in
treatment-resistant depression and for patients with major depressive disorder accompanied by acute suicidal ideation and behavior. Canuso added that intranasal esketamine has also shown promise for other conditions such as bipolar depression, posttraumatic stress disorder (PTSD), and substance use disorder.
Canuso reviewed the evidence that led to the approval of intranasal esketamine, including data from two Phase 3 studies and other Phase 2 and 3 studies demonstrating safety and efficacy (Singh et al., 2020). Subgroup analyses confirmed a favorable treatment response across all patient subgroups whether based on gender, baseline depression scores, history of previous treatment, background antidepressants, or functional status, said Canuso. A key question of interest concerned whether ketamine’s dissociative effects are necessary for its antidepressant effects (Ballard and Zarate, 2020). To address this issue, the Janssen team’s clinical trials demonstrated that neither esketamine- nor ketamine-induced dissociation was required to achieve an antidepressant response, said Canuso.
Preventing relapse was another important issue explored in the drug development process, said Canuso. In previous studies of antidepressant treatments, relapse rates were very high when drugs were withdrawn after achieving remission (Rush et al., 2006). Similarly, high relapse rates were also seen when esketamine was removed from the treatment regimen, said Canuso. However, relapse was avoided in about 70 percent of patients who transitioned to every-other-week dosing instead of weekly dosing (Daly et al., 2019).
Because ketamine is a controlled substance, the long-term safety and abuse potential of esketamine in the therapeutic context of treatment-resistant depression treatment remains a concern. Canuso noted that FDA approval was contingent on a risk evaluation and mitigation strategy (REMS), which requires the continued long-term study of patients. So far, she said, no changes in cognition have been seen, other than slightly slowed reaction times in patients older than 65. There have been no observations of abuse, dependence, withdrawal, or problems related to bladder, kidney, liver, or blood pressure health. She added that no signs of tolerance to the antidepressant effects of intranasal esketamine were observed.
Because of ketamine’s rapid antidepressant effects (Grunebaum et al., 2018; Wilkinson et al., 2018), Canuso’s team was particularly interested in studying intranasal esketamine in patients with acute suicidal ideation, when quickly bringing symptoms under control is essential. She noted that patients who are acutely suicidal are typically excluded from clinical treatment trials of antidepressants, in part because these patients often receive extensive clinical care that can confound study outcomes. In addition, because of methodological issues—including the difficulty of measuring suicidality and its rapidly fluctuating nature—studies of esketamine’s effect
on suicidality have produced mixed results, said Canuso. Nevertheless, she said much has been learned by including patients who meet the criteria for acute suicidality in esketamine’s clinical trials. Ninety percent of the patients studied in Janssen’s pivotal trials had moderate to severe suicidality, and 60 percent reported a prior suicide attempt, said Canuso. She called for more research in diverse groups of patients, including adolescents, as well as comparative research across different drug formulations.
KETAMINE’S MECHANISM OF ACTION ON SYNAPSES, CIRCUITS, AND SYSTEMS
Precisely how ketamine alleviates depression remains unclear, said John Krystal, the Robert L. McNeil, Jr. Professor of Translational Research and professor of psychiatry and neuroscience at the Yale School of Medicine. One relevant observation, he said, is that ketamine exerts its antidepressant effects across a very narrow range of doses. Dose increases above the plasma concentration of 0.5 micromolar, which is the level produced by the standard antidepressant dose of ketamine (0.5 mg/kg administered intravenously over 40 minutes) increase the intensity of dissociative and psychotic side effects, he said, without improving antidepressant efficacy. At anesthetic doses, beginning about 4-fold higher than the antidepressant dose, ketamine does not appear to be an effective antidepressant. Therefore, he said, the underlying mechanisms relevant to ketamine’s antidepressant effects are most likely molecular targets that are engaged within a 2- to 4-fold range from the target antidepressant plasma level. The molecular targets engaged at higher doses are probably not relevant to the antidepressant effects of ketamine, although they may play a role in mediating its anesthetic effects.
As mentioned earlier, ketamine primarily NMDA glutamate receptors, said Krystal. Figure 3-2 illustrates the many types of glutamate receptors present in glutamate synapses. In NMDA glutamate receptors, the binding of glutamate and its co-agonists glycine and D-serine opens an ion channel within the receptor, allowing calcium to enter neurons. However, when ketamine binds to the NMDA glutamate receptor, the ion channel within the receptor remains closed. Ketamine is most potent at blocking NMDA receptors containing the GluN2A-GluN2C subunits. Besides NMDA receptors, ketamine binds to many other targets in the brain, albeit at concentrations that are in excess of a typical anesthetic dose in humans.
Ketamine’s blockage of NMDA receptors could affect microcircuits through different mechanisms depending on whether the receptors are on inhibitory or excitatory neurons, for example, said Krystal. Studies suggest that ketamine increases glutamate release, he continued, and the signatures associated with the increased glutamate are related to the antidepressant
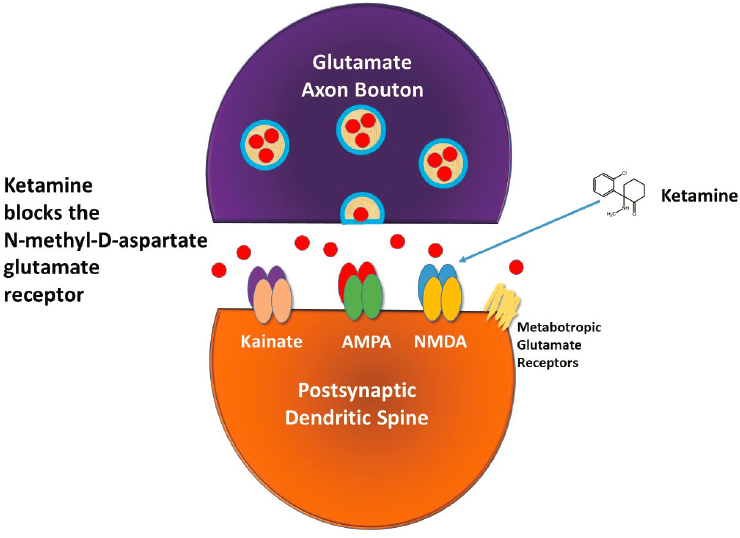
SOURCE: Presented by John Krystal, March 8, 2021.
response in depressed patients (Abdallah et al., 2018; Esterlis et al., 2018; Gilbert and Zarate, 2020; Stone et al., 2012).
In animal models, he said, the early phase of ketamine administration causes the cortical expression of genes associated with neural activation, such as Arc, phosphor-ERK, and Akt/mTORC. Later, beginning at about 6 hours after ketamine administration, there is an increased expression of genes associated with new synapses, including genes expressed by axon terminals, such as synapsin 1 and SV2A, and genes that are expressed by the postsynaptic dendritic spine, such as PSD95 and GluR1, said Krystal. Yale scientists Ronald Duman and George Aghajanian demonstrated that ketamine triggers the rapid regrowth of dendritic spines that had previously undergone pruning, he continued.
One model that has emerged to explain this phenomenon posits that when ketamine blocks NMDA receptors on GABA neurons, there is a reduction in GABA release and an increase in glutamate release, said Krystal. Increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation by glutamate induces brain-derived trophic factor (BDNF) gene expression, local BDNF release, and its binding to TrkB receptors.
This triggers a shuttling of intracellular AMPA receptors to the cell surface, thus increasing the efficacy of synaptic communication and increasing neuroplasticity. Meanwhile, the stimulation of TrkB receptors activates the Akt/mTOR pathway, resulting in increased protein synthesis and the recreation of pruned dendritic spines, he said. This conceptual framework could be viewed as a presynaptic model for ketamine efficacy (see Figure 3-3).
A different approach to understanding ketamine’s mechanism of action emerged from the work of Vanderbilt University scientists Lisa Monteggia and Ege Kavalali. Their model proposes that the spontaneous glutamate release from glutamate nerve terminals stimulates synaptic NMDA receptors. Under normal circumstances, BDNF levels are suppressed and AMPA trafficking is curtailed in response to the stimulation of synaptic NMDA receptors, he said. Monteggia and Kavalali proposed that ketamine antago-
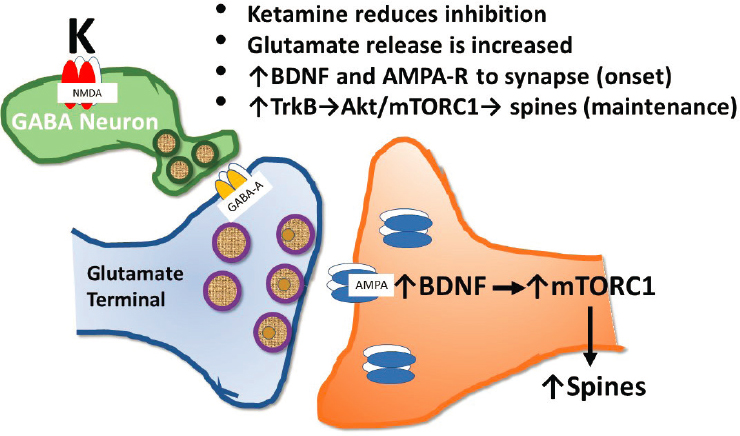
SOURCE: Presented by John Krystal, March 8, 2021.
nism at NMDA receptors relieves the brake on BDNF levels while upregulating AMPA receptor trafficking to the nerve terminal.
Together, this leads to a rapid increase in synaptic efficacy, stimulation of TrkB receptors, mTORC1 activation, and the reformation of pruned dendritic spines. This is known as the postsynaptic model (see Figure 3-4). There is also the possibility, Krystal added, that ketamine may stimulate TrkB receptors directly.
The work of Weill Cornell Medicine scientist Conor Liston highlighted that there might be different mechanisms underlying the initiation and maintenance of ketamine’s antidepressant effects, with initiation caused by enhanced AMPA receptor function and trafficking to the cell surface and maintenance caused by newly regrown dendritic spines, said Krystal. If interfering with the maintenance of recreated dendritic spines shortens the therapeutic effect of ketamine, he said it raises the question of whether it is possible to increase the duration of the antidepressant effects by increasing the duration of the newly restored dendritic spines.
STRATEGIES TO IMPROVE THE ANTIDEPRESSANT EFFECTS OF KETAMINE
As noted earlier, ketamine or esketamine treatment for depression can cause a remittance in symptoms that lasts for about 1 week after a single
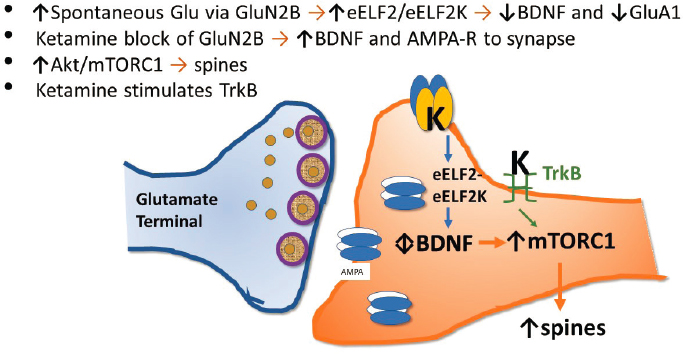
SOURCE: Presented by John Krystal, March 8, 2021.
dose. As Krystal said, a deeper understanding of the mechanism of action raises the possibility that different pharmacological strategies could be developed to increase the duration of the antidepressant effects of ketamine. Clinicians and researchers have explored different strategies for extending the antidepressant effect of ketamine, said Carlos Zarate, chief of the Section on Neurobiology and Treatment of Mood Disorders and Section of Experimental Therapeutics and Pathophysiology Branch at NIMH and clinical professor of psychiatry at The George Washington University.
These include adjunctive drugs such as glutamatergic modulators; psychotherapies such as cognitive-behavioral therapy or motivation enhancement therapy; non-invasive neurostimulation; repeated racemic ketamine2 infusions; and continued use of intranasal esketamine, said Zarate. Of these approaches, only continued intranasal esketamine treatment demonstrated the ability to maintain ongoing antidepressant effects, he said. A study conducted by Krystal and colleagues demonstrated that a low dose of the powerful anti-inflammatory drug rapamycin given before dosing with ketamine extended the duration of ketamine’s antidepressant effects (Abdallah et al., 2020). However, the mechanism underlying this remains unclear, said Krystal.
Different molecular mechanisms may also underlie the variable symptomatology and responses to therapy in different patients, said Krystal. For example, he noted that by using positron emission tomography (PET) to detect the presence of synaptic vesicles at presynaptic terminals, investigators demonstrated that reduced synaptic density correlated with reduced functional connectivity between the dorsolateral prefrontal cortex and the posterior cingulate cortex and also correlated with increased severity of depression (Holmes et al., 2019). Ketamine’s ability to increase synaptic density may thus explain its rapid antidepressant effects, said Krystal. He added that another aspect of ketamine treatment that is not well understood at a neurobiological level is the effect of repeated exposure to ketamine over time and why some patients can eventually taper down to less frequent dosing while maintaining clinical benefits.
Krystal added that because ketamine interferes with learning and other forms of neuroplasticity, it might be possible to increase the magnitude of the therapeutic effect by combining ketamine treatment with behavioral interventions designed to “rewrite” maladaptive memories that may be contributing to mental unwellness (Das et al., 2019). In addition, because ketamine appears to be implicated in a cascade of intracellular events that contribute to its antidepressant properties and that enhance neuroplasticity, it is possible that behavioral interventions might have enhanced efficacy during this period, said Krystal. Lastly, Krystal dis-
___________________
2 Racemic ketamine contains both mirror-image structures (isomers) of ketamine.
cussed how different aspects of the signaling mechanisms implicated in the antidepressant effects of ketamine might be directly targeted with novel treatments. These targeted interventions might have the possibility of recapitulating aspects of the antidepressant efficacy of ketamine while increasing safety and tolerability.
Morgan Sheng, co-director of the Stanley Center for Psychiatric Research at the Broad Institute of the Massachusetts Institute of Technology (MIT) and Harvard University and professor of brain and cognitive science at MIT, agreed that developing a “better ketamine” will require more research to understand how ketamine works. Rather than modifying ketamine, he suggested pursuing drugs with different molecular mechanisms of action that mimic the neurobiological effects of ketamine upstream at the circuit or brain system level or that modulate a downstream pathway. Zarate added, however, that it will likely be difficult to develop a drug that has ketamine’s broad therapeutic effects across multiple mental health conditions (e.g., PTSD, obsessive-compulsive disorder, anhedonia, suicidality).
Krystal suggested that ketamine may, at least in part, rely on interneuron-dependent effects. Pathology in interneuron populations could explain why patients respond differentially to ketamine, he said. Drugs such as 5-HT2A agonists and psychedelics, which directly stimulate excitatory pyramidal neurons rather than interneurons, might prove more effective in these patients, he said.
Moving upstream of the synapse to the circuit or systems level will also require a better understanding of the circuits and brain regions that are affected by ketamine, noted Manji. Technologies such as structural and functional imaging, magnetoencephalography, electroencephalography, proteomics, and metabolomics may be useful to interrogate these systems, said Zarate. For example, Abdallah and colleagues used functional magnetic resonance imaging to show that ketamine treatment normalizes prefrontal global brain connectivity in patients with treatment-resistant depression (Abdallah et al., 2017), explained Zarate. He added that investigators are also studying how ketamine and other treatments affect three networks implicated in depression: the default mode network, which is involved in rumination and self-referential thoughts (Zhou et al., 2020); the salience network, which identifies emotionally salient stimuli (Seeley et al., 2007); and the central executive network, which is involved in the regulation of emotions, behaviors, and thoughts (Miller et al., 2018). In addition to studying these networks, scientists are using animal models to study which cell types (e.g., inhibitory interneurons, excitatory pyramidal neurons, etc.) are involved. To do this, researchers leverage in vivo single-cell recordings of many cells simultaneously during treatment with ketamine, said Sheng. He suggested further studies using single-cell RNA-Seq to identify the transcriptomic responses of different cell types. Sheng also suggested the use of
spatial transcriptomics to study how antidepressants exert cell-specific and region-specific effects on gene expression.
In the search for more effective treatments, Zarate supported continued exploration of preclinical targets that overlap with ketamine’s targets, while still trying to harness ketamine’s therapeutic effect. This approach will help elucidate which of ketamine’s molecular targets exert broad versus selective effects, he said. Treatments that target synaptic plasticity and synapse growth make intuitive sense as a broader spectrum strategy not only for treating depression, but for other disorders as well, added Sheng.
Krystal added that there are a number of molecular mechanisms that are engaged in the presynaptic and postsynaptic models of ketamine efficacy discussed earlier, and some of these may be targets to potentiate the antidepressant effect of ketamine. These include muscarinic antagonists, psychedelic agents, 5-HT2A agonists, GABA-A receptor partial inverse agonists, AMPAkines, metabotropic glutamate receptor-2 antagonists, and ketamine metabolites.
IDENTIFYING A BIOMARKER TO HELP PREDICT RESPONSES TO KETAMINE
While ketamine’s efficacy and rapid onset of effectiveness have provided dramatic benefits for patients like Ashley Clayton, not all patients with depression experience these strong effects, as Krystal mentioned earlier. Why some patients respond to ketamine while others do not is unclear. Researchers are hoping to find a reliable biomarker that would help predict how likely it is for a particular patient to benefit from treatment before that patient enrolls in clinical trials. This patient stratification biomarker could enable more productive clinical studies as well as more individualized treatment, yet no such biomarkers have been identified in the depression field, said Zarate. Krystal and colleagues have been exploring the use of PET to assess levels of SV2A, a protein that can be used to measure synaptic density; the hypothesis is that patients with low synaptic density in certain brain regions may be good candidates for ketamine. However, the high cost of PET scans limits the feasibility of using this tool for patient stratification. In addition, said Sheng, SV2A PET measures have limited predictive value. He suggested that brain oscillations assessed using EEG might prove to be a more useful predictive or diagnostic stratification biomarker.
This page intentionally left blank.












