1
Introduction
Influenza poses a serious threat to health around the world. Seasonal influenza results in about 1 billion cases annually, leading to 3–5 million patients with severe illnesses (WHO, 2019), of whom an estimated 294,000–518,000 die (Paget et al., 2019). The effects of an influenza pandemic would be even greater. In 2019, when releasing its Global Influenza Strategy for 2019–2030, the World Health Organization (WHO) acknowledged that it is only a question of when, not whether, the next influenza pandemic will happen and that many experts believed a severe outbreak could be one of the most devastating global health events ever, with potentially far-reaching health, social, and economic consequences (WHO, 2019). As the world struggles to recover from the death and devastation caused since early 2020 by another respiratory virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), could nations, international organizations, and the private sector draw any useful lessons from the coronavirus disease 2019 (COVID-19) pandemic as they prepare for an influenza outbreak?
To strengthen countries’ preparedness for and response to seasonal and pandemic influenza, WHO’s Global Influenza Strategy (GIS) provides a comprehensive framework, from surveillance to prevention and control interventions. Building on the success of the Global Influenza Surveillance and Response System and the Pandemic Influenza Preparedness Framework, the GIS focuses on developing programs at the country level and investing in health systems strengthening as a means of enhancing pandemic preparedness. Yet, the response to COVID-19 has revealed gaps and opportunities for improvement in global efforts to prepare for a major outbreak of respiratory viral disease. As of May 18, 2021, 162,184,263 people worldwide had been
infected (WHO, 2021); 3,364,446 deaths have been recorded, but worldwide cases remain underreported by more than half, and based on the weekly excess death rate during the pandemic, the actual toll is estimated at 6.9 million deaths (IHME, 2021). While the response to COVID-19 continues, the global threat of emerging seasonal and pandemic influenza remains, underscoring the need to harness experiences garnered from COVID-19 and other previous influenza responses to update and advance preparedness efforts.
Coronaviruses and influenza viruses have a number of similarities and differences that factor into applying lessons from the COVID-19 pandemic to influenza events. Both infect the respiratory tract via surface proteins, result in similar symptoms, and have animal reservoirs (Abdelrahman et al., 2020). Influenza has a shorter incubation time, 1–4 days compared to 2–14 days. The variability in incubation time for SARS-CoV-2 has implications for public health strategies, such as the utility of testing and contact tracing. Both viruses are highly contagious and can remain on surfaces for more than 24 hours (ASM, 2020), yet research has shown that children shed influenza viruses longer and at higher levels (Heald-Sargent et al., 2020; Ng et al., 2016). However, as the Delta variant of SARS-CoV-2 became the dominant global strain in summer 2021, children were more likely to be affected. As new variants emerge, it will be important to maintain awareness of the similarities to and differences from influenza and other SARS-CoV-2 strains.
Non-vaccine control measures can be a vital defense during a respiratory virus pandemic—both before and after vaccines are available—and thus warrant special attention amidst efforts to strengthen preparedness. Once a new virus is identified, it can take at least 4–6 months to develop vaccines and many months more for clinical trials, regulatory processes, and eventual emergency use authorization or approval. Furthermore, producing and deploying vaccines can be constrained by variable and low to nonexistent supplies and limited manufacturing capacity across the world. On the other hand, non-vaccine control measures can be affordable, effective, and broadly implementable (PAHO, 2009). For example, a modeling study estimated that nearly 130,000 additional lives could have been saved from COVID-19 in the United States between September 2020 and February 2021 if 95 percent of the population wore face masks in public (Reiner et al., 2021). However, such measures globally have historically not been fully used during a pandemic: early case detection, contact tracing and isolation, quarantine, physical distancing, ventilation, hand hygiene, mask wearing, and travel restrictions are not always applied comprehensively or consistently enough to curb transmission, morbidity, and mortality (PAHO, 2009). Some interventions may be used too early or too late, delaying their impact and causing undue economic and social hardship, limiting the public health benefits, and reducing long-term public compliance and trust (Independent Panel for Pandemic Preparedness and Response, 2021).
Information has evolved during the COVID-19 pandemic on guidance for non-vaccine measures that can have implications for influenza control interventions. In fact, COVID-19 mitigation measures contributed to a marked decrease in influenza, with virtually no influenza season in fall 2020 and a 2019–2020 influenza season that was shortened by an estimated 4–7 weeks in the Northern hemisphere (Stojanovic et al., 2021). In the Southern hemisphere, influenza was almost absent as well in winter 2020 (Sullivan et al., 2020). While guidance on the use of non-vaccine public health measures has been widely published in many high-income countries, less attention has been directed toward understanding how to optimize such measures on a global scale in a way that accounts for unique social and political factors across the diverse contexts of low-, middle-, and high-income countries. Sustaining such levels of decreased influenza transmission may require ongoing compliance with COVID-19-era non-vaccine interventions to minimize the reservoir of viruses in populations where vaccination percentages remain below herd immunity rates (Solomon et al., 2020). Overall, the response to COVID-19—including both best practices and systematic gaps identified—offers an opportunity to reevaluate priorities for influenza and strengthen preparedness for seasonal and pandemic influenza.
PROJECT ORIGIN AND STATEMENT OF TASK
At the request of the U.S. Department of Health and Human Services’ Office of Global Affairs, the National Academies of Sciences, Engineering, and Medicine (the National Academies) created an initiative to advance pandemic and seasonal influenza vaccine preparedness and response by harnessing lessons from the efforts mitigating the COVID-19 pandemic. The National Academy of Medicine (NAM) convened a committee of domestic and international experts from across sectors (e.g., government, academia, industry, civil society, international public health organizations) and a variety of disciplines to provide an iterative process informed by experts for analyzing the impact that lessons learned during COVID-19, in particular with regard to the technologies, policies, and processes developed worldwide, could have on pandemic and seasonal influenza global preparedness and response. This committee developed the Statements of Task for four concurrent National Academies ad hoc committees.1
The Committee on Public Health Interventions and Countermeasures for Advancing Pandemic and Seasonal Influenza Preparedness and Re-
___________________
1 Information about the initiative and the other three studies can be found at https://www.nationalacademies.org/our-work/advancing-pandemic-and-seasonal-influenza-vaccine-preparedness-and-response-harnessing-lessons-from-the-efforts-to-mitigate-the-covid-19-pandemic (accessed November 18, 2021).
sponse (the committee) was convened to analyze the use of non-vaccine control measures for respiratory viruses, primarily during COVID-19. It was charged with recommending actions specifically related to non-vaccine public health interventions that could strengthen preparedness for seasonal and pandemic influenza. Box 1-1 provides the full charge to the committee, which included 12 members with academic and professional expertise in disease surveillance, therapeutics, non-vaccine public health and engineering interventions, communications, behavioral and social health, ethical aspects of public health, and other disciplines. Appendix A provides the biographies of the committee members and the staff who put together the report.
COMMITTEE APPROACH AND STUDY SCOPE
This study responds to a need to strengthen efforts to mitigate influenza, which was identified in the 2019–2030 WHO Global Influenza Strategy (WHO, 2019), the 2020–2030 U.S. National Influenza Vaccine Modernization Strategy (HHS, 2020), and other documents. In developing the report, the committee deliberated for approximately 4 months. Between March and early June 2021, the full committee met virtually three times, each time for 9 hours over multiple days. The first two full meetings included open sessions during which the committee heard from the sponsor and speakers to fulfill key information-gathering needs. Appendix B includes all of the public meeting agendas with speaker names and topics, and Appendix C provides further details of the study approach.
This study aims to provide a brief, high-level introduction to the many broad, complicated topics encompassed in the Statement of Task. Analysis of the study topics drew primarily from the rich and extensive expertise of the committee members. Staff initiated the analyses with literature searches (the terms of which are presented in Appendix C) to outline the key issues related to the Statement of Task, focusing on systematic reviews and highly cited articles. Identification of priorities, including further sources and subtopics to explore, was based on expert guidance from the committee. This study offers evidence from select sources; it is not intended to provide a comprehensive or systematic review of all available evidence or the many subtopics related to the points within the Statement of Task. The committee chose to prioritize the topics presented in the following chapters and predominantly focused on drawing lessons for future pandemics rather than seasonal events. The sources stemmed primarily from literature focused on the response to the COVID-19 pandemic that was published through early June 2021 in the form of select journal articles, case studies, examples, and news media articles. In addition to publications featuring original research
and evidence, the committee considered sources that examined the process of implementing non-vaccine control measures during COVID-19, explored critical opportunities to use therapeutics to mitigate disease progression, and reviewed surveillance-related successes, challenges, and innovations. With regard to the fourth point in the Statement of Task, upon initial analysis, most of the lessons learned and recommendations for best practice seemed to stem from inadequacies with core surveillance capacities. Given the challenge with defining what would qualify as an innovative approach and the dearth of evidence on the effectiveness of innovations, the committee focused on ways to strengthen core surveillance systems with consideration of innovative approaches, while not allowing such attention to detract from its primary focus. The committee considered both the level and strength of the evidence to provide specific recommendations on measures that could be used most effectively on a global scale and those with potential effectiveness but a lack of sufficient research or data. With the broad nature of the Statement of Task, the committee could not identify specific organizations that would have complete responsibility over particular areas of the study, so some of the recommendations likewise are broad. Given the study timeline and scope of the committee’s charge, the committee largely chose not to focus on the following issues: workforce training and capacity; interventions for health care workers (as opposed to the general population); and vaccine hesitancy.
The committee drew on the best science and expert testimony available in summer 2021. In the context of the ongoing global pandemic, which continues to evolve rapidly, we recognize that new data are continuing to emerge, especially related to new variants of the virus. Thus, this report reflects the state of the science during the period when the committee was working, and some points may become outdated as new studies are completed and new data become available.
ORGANIZATION OF THE REPORT
This report follows the Statement of Task, with the next four chapters corresponding to its first four points (see Figure 1-1). Chapter 2 explores the topics of the fourth point: surveillance-related lessons learned during COVID-19. Chapter 3 covers the evidence of effectiveness of non-vaccine control measures, defined in this chapter as their value in reducing virus transmission, which is followed in Chapter 4 by considering contextual factors that can affect implementation and population optimization of such measures. Chapter 5 explores opportunities to use therapeutic approaches. Each of these chapters also examines relevant research gaps and priorities, which is the fifth point in the Statement of Task. The study findings are discussed in the background sections of Chapters 2–5, while overarching
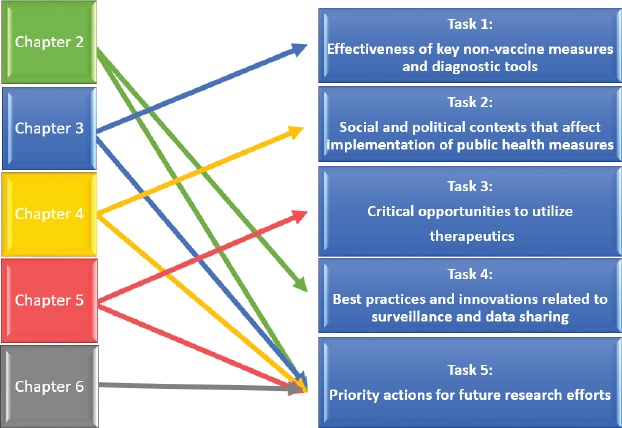
conclusions and recommendations from the findings appear at the end of each chapter. Closing thoughts are presented in Chapter 6, which summarizes the main conclusions and discusses the way forward and opportunities for research.
REFERENCES
Abdelrahman, Z., M. Li, and X. Wang. 2020. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Frontiers in Immunology 11:2309.
ASM (American Society for Microbiology). 2020. COVID-19 and the flu. https://asm.org/Articles/2020/July/COVID-19-and-the-Flu (accessed June 24, 2021).
Heald-Sargent, T., W. J. Muller, X. Zheng, J. Rippe, A. B. Patel, and L. K. Kociolek. 2020. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatrics 174(9):902–903.
HHS (U.S. Department of Health and Human Services). 2020. National influenza vaccine modernization strategy (NIVMS) 2020–2030. Public Health Emergency. https://www.phe.gov/Preparedness/planning/nivms/Pages/default.aspx (accessed June 24, 2021).
IHME (Institute for Health Metrics and Evaluation). 2021. COVID-19 has caused 6.9 million deaths globally, more than double what official reports show. http://www.healthdata.org/news-release/covid-19-has-caused-69-million-deaths-globally-more-double-what-official-reports-show (accessed May 17, 2021).
Independent Panel for Pandemic Preparedness and Response. 2021. Second progress report. https://theindependentpanel.org/wp-content/uploads/2021/01/Independent-Panel_Second-Report-on-Progress_Final-15-Jan-2021.pdf (accessed June 24, 2021).
Ng, S., R. Lopez, G. Kuan, L. Gresh, A. Balmaseda, E. Harris, and A. Gordon. 2016. The timeline of influenza virus shedding in children and adults in a household transmission study of influenza in Managua, Nicaragua. Pediatric Infectious Disease Journal 35(5):583–586.
Paget, J., P. Spreeuwenberg, V. Charu, R. Taylor, A. Iuliano, J. Bresee, L. Simonsen, and C. Viboud. 2019. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR project. Journal of Global Health 9:020421.
PAHO (Pan American Health Organization). 2009. Non-pharmaceutical interventions (NPIs): Actions to limit the spread of the pandemic in your municipality. https://www.paho.org/disasters/dmdocuments/RespToolKit_11_Tool%2004_NonPharmaceuticalInterventions(NPIs).pdf (accessed May 17, 2021).
Reiner, R. C., R. M. Barber, J. K. Collins, P. Zheng, C. Adolph, J. Albright, C. M. Antony, A. Y. Aravkin, S. D. Bachmeier, B. Bang-Jensen, M. S. Bannick, S. Bloom, A. Carter, E. Castro, K. Causey, S. Chakrabarti, F. J. Charlson, R. M. Cogen, E. Combs, X. Dai, W. J. Dangel, L. Earl, S. B. Ewald, M. Ezalarab, A. J. Ferrari, A. Flaxman, J. J. Frostad, N. Fullman, E. Gakidou, J. Gallagher, S. D. Glenn, E. A. Goosmann, J. He, N. J. Henry, E. N. Hulland, B. Hurst, C. Johanns, P. J. Kendrick, A. Khemani, S. L. Larson, A. LazzarAtwood, K. E. LeGrand, H. Lescinsky, A. Lindstrom, E. Linebarger, R. Lozano, R. Ma, J. Månsson, B. Magistro, A. M. M. Herrera, L. B. Marczak, M. K. Miller-Petrie, A. H. Mokdad, J. D. Morgan, P. Naik, C. M. Odell, J. K. O’Halloran, A. E. Osgood-Zimmerman, S. M. Ostroff, M. Pasovic, L. Penberthy, G. Phipps, D. M. Pigott, I. Pollock, R. E. Ramshaw, S. B. Redford, G. Reinke, S. Rolfe, D. F. Santomauro, J. R. Shackleton, D. H. Shaw, B. S. Sheena, A. Sholokhov, R. J. D. Sorensen, G. Sparks, E. E. Spurlock, M. L. Subart, R. Syailendrawati, A. E. Torre, C. E. Troeger, T. Vos, A. Watson, S. Watson, K. E. Wiens, L. Woyczynski, L. Xu, J. Zhang, S. I. Hay, S. S. Lim, C. J. L. Murray, and IHME COVID-Forecasting Team. 2021. Modeling COVID-19 scenarios for the United States. Nature Medicine 27(1):94–105.
Solomon, D. A., A. C. Sherman, and S. Kanjilal. 2020. Influenza in the COVID-19 era. JAMA 324(13):1342–1343.
Stojanovic, J., V. G. Boucher, J. Boyle, J. Enticott, K. L. Lavoie, and S. L. Bacon. 2021. COVID-19 is not the flu: Four graphs from four countries. Frontiers in Public Health 9:628479.
Sullivan, S. G., S. Carlson, A. C. Cheng, M. B. N. Chilver, D. E. Dwyer, M. Irwin, J. Kok, K. Macartney, J. MacLachlan, C. Minney-Smith, D. Smith, N. Stocks, J. Taylor, and I. G. Barr. 2020. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September, 2020. Eurosurveillance 25(47):pii=2001847.
WHO (World Health Organization). 2017. Up to 650 000 people die of respiratory diseases linked to seasonal flu each year. https://www.who.int/news/item/13-12-2017-up-to-650-000-people-die-of-respiratory-diseases-linked-to-seasonal-flu-each-year (accessed May 15, 2021).
WHO. 2019. Global Influenza Strategy 2019–2030. https://apps.who.int/iris/handle/10665/311184 (accessed June 24, 2021).
WHO. 2021. Weekly epidemiological update on COVID-19—8 May 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—18-may-2021 (accessed May 21, 2021).








