1
Introduction
The Office of the Assistant Secretary for Planning and Evaluation (ASPE), in partnership with other agencies and divisions of the U.S. Department of Health and Human Services (HHS), coordinates a portfolio of projects that build data capacity for conducting patient-centered outcomes research (PCOR). The PCOR data infrastructure provides decision makers with objective, scientific evidence on the effectiveness of treatments, services, and other interventions used in health care. This research is frequently focused on analyzing existing data to address questions and provide objective information for the purpose of informing real-world health care decisions.
BACKGROUND
The legal framework that established funding for research on the outcomes and effectiveness of treatments and health care interventions dates back to the 2003 Medicare Prescription Drug, Improvement, and Modernization Act. This act provided authorization for the Agency for Healthcare Research and Quality (AHRQ) to support research comparing the outcomes and effectiveness of treatments and clinical approaches and to disseminate the findings from this research. In 2009, the American Recovery and Reinvestment Act provided additional funding to AHRQ, the National Institutes of Health, and HHS for research that compares the effectiveness of medical options. In 2010, the Patient Protection and Affordable Care Act provided further authorization for research that assists patients, clinicians, purchasers, and policy makers in making informed health decisions.
To facilitate PCOR, in 2010 Congress established the Patient-Centered Outcomes Research Trust Fund (PCOR Trust Fund) with the U.S. Department of the Treasury. The goals of the PCOR Trust Fund are to fund PCOR research, disseminate research findings, and develop a data infrastructure for PCOR. The PCOR Trust Fund has been reauthorized through 2029, through H.R.1865 of the Further Consolidated Appropriations Act of 2020. The most recent statute specified intellectual and developmental disabilities, as well as maternal mortality, as research priorities. The statute also called for PCOR studies to include consideration of the full range of outcomes data. Specifically, the law states that:
Research shall be designed, as appropriate, to take into account and capture the full range of clinical and patient-centered outcomes relevant to, and that meet the needs of, patients, clinicians, purchasers, and policymakers in making informed health decisions. In addition to the relative health outcomes and clinical effectiveness, clinical and patient-centered outcomes shall include the potential burdens and economic impacts of the utilization of medical treatments, items, and services on different stakeholders and decision-makers respectively. These potential burdens and economic impacts include medical out-of-pocket costs, including health plan benefit and formulary design, non-medical costs to the patient and family, including caregiving, effects on future costs of care, workplace productivity and absenteeism, and healthcare utilization.1
The bulk of the PCOR Trust Fund funding (80%) is allocated for research and is made available through the Patient-Centered Outcomes Research Institute (PCORI), a nongovernmental organization established by Congress for this purpose. Approximately 16 percent of the PCOR Trust Fund funding is set aside for disseminating research findings, incorporating findings into clinical practice, and training researchers in PCOR. The agency overseeing this work is AHRQ.
The remaining funding, which constitutes 4 percent of the PCOR Trust Fund, is allocated for building data capacity for PCOR and is overseen by ASPE. Specifically, Section 937(f) of the Public Health Service Act instructed the Secretary of HHS to:
… provide for the coordination of relevant Federal health programs to build data capacity for comparative clinical effectiveness research, including the development and use of clinical registries and health outcomes research networks, in order to develop and maintain a comprehensive, interoperable data network to collect, link, and analyze data on outcomes and effectiveness from multiple sources including electronic health records.2
___________________
1https://www.ssa.gov/OP_Home/ssact/title11/1181.htm.
2https://aspe.hhs.gov/collaborations-committees-advisory-groups/os-pcortf/about-os-pcortf.
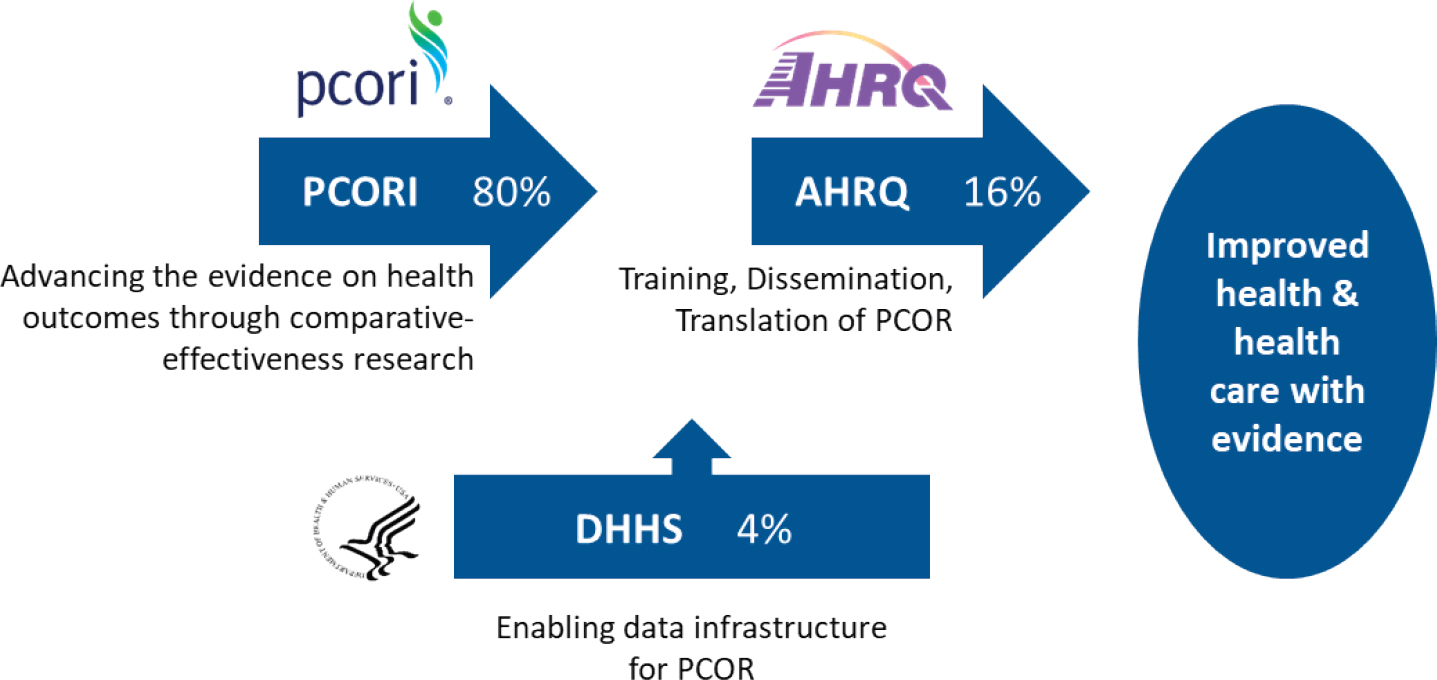
NOTE: AHRQ = Agency for Healthcare Research and Quality; DHHS = U.S. Department of Health and Human Services; PCOR = patient-centered outcomes research; PCORI = Patient-Centered Outcomes Research Institute.
SOURCE: Workshop presentation by ASPE, May 3, 2021.
Figure 1-1 shows how the PCOR funding and work is allocated across the three entities. This National Academies of Sciences, Engineering, and Medicine study is focused on issues relevant to ASPE’s continued work on the PCOR data infrastructure, in other words, on the priorities for the use of the 4 percent of the funding that is allocated to HHS for work related to the data infrastructure for PCOR.
As the coordinating agency for the data infrastructure investment portfolio across HHS agencies, ASPE guides the PCOR data infrastructure’s strategic framework and vision, sets funding priorities, and coordinates interagency workgroups. ASPE’s work is assisted by a Leadership Council for the PCOR Trust Fund, which includes representatives from other HHS agencies, including the Administration for Children and Families, the Administration for Community Living, the Assistant Secretary for Preparedness and Response, AHRQ, the Centers for Disease Control and Prevention (CDC), the Centers for Medicare & Medicaid Services, the U.S. Food and Drug Administration (FDA), the Health Resources and Services Administration, the Indian Health Service, the National Institutes of Health, the Office of the Chief Technology Officer, the Office of the National Coordinator for Health Information Technology, and the Substance Abuse and Mental Health Services Administration. The Leadership Council provides input on priorities for the portfolio, including projects to fund. During the period
2010 to 2019, the PCOR Trust Fund funded 53 projects, which translated to 76 agency awards, totaling approximately $131 million.
Figure 1-2 is a visual representation of ASPE’s current framework for the PCOR data infrastructure. The bottom row shows the main data sources feeding into the PCOR infrastructure. Data collected as part of clinical care include data collected for health care delivery and for billing purposes. Examples of primary data collected as part of research studies include data from clinical trials and national health surveys. Other examples of data sources include Medicare or Medicaid claims data; quality or outcomes data collected by health care providers for the purposes of improving health care value; FDA data on the safety of medications and medical devices; and CDC data on births and deaths provided by state public health authorities.
The framework describes the relationship between the data sources and the current key functionalities and focus areas (middle row) that support the research. The key functionalities are described in further detail in Box 1-1. Major building blocks are the services, standards, policies, and governance that enable the use of the data for research, described in further detail in Box 1-2. The top row shows the key data users and contributors of data. A more detailed overview of ASPE’s work and the projects funded to date will be included in the final report, at the conclusion of the committee’s review.
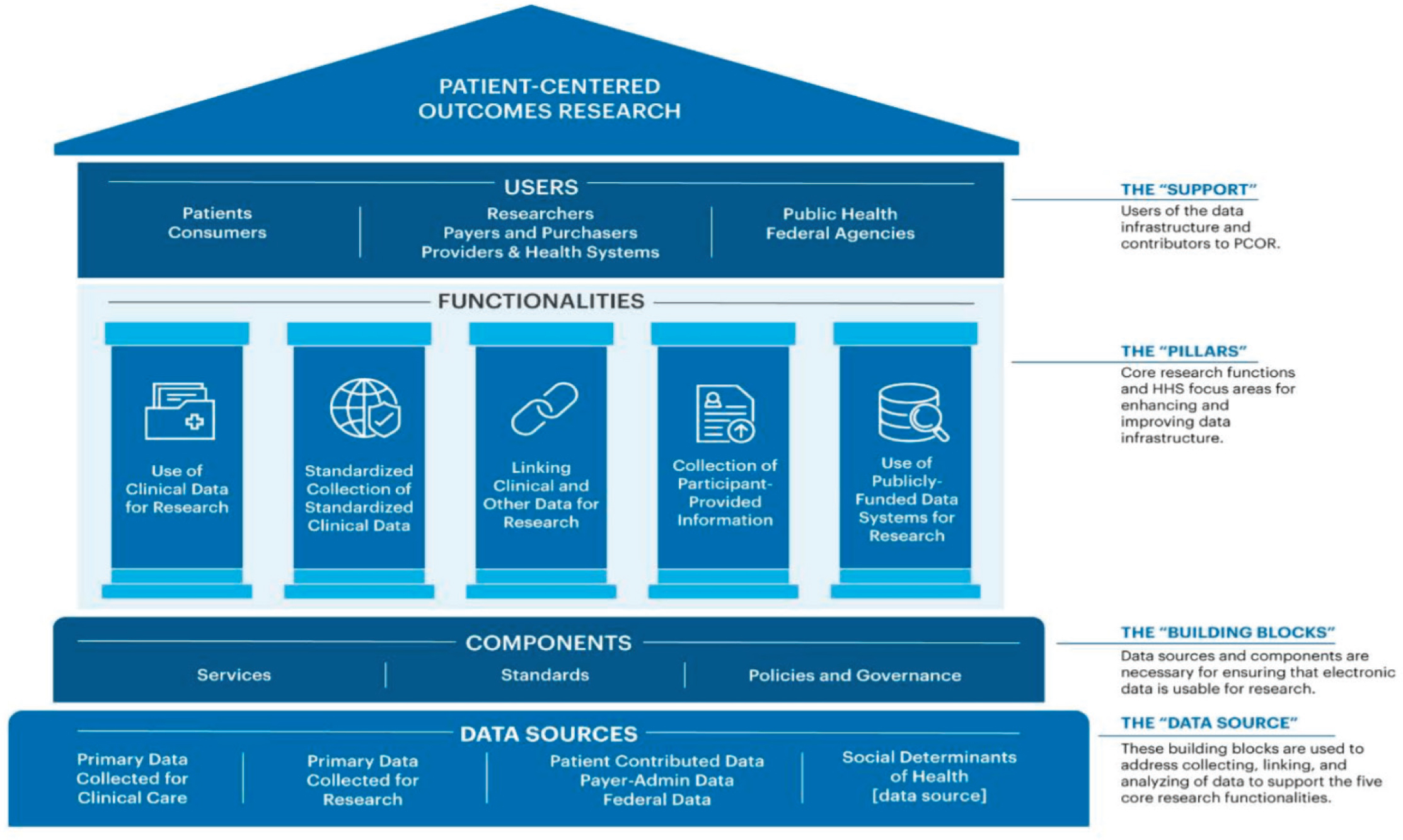
SOURCE: Workshop presentation by ASPE, May 3, 2021.
ISSUES FOR THE COMMITTEE
ASPE asked the National Academies of Sciences, Engineering, and Medicine to appoint a consensus study committee and identify issues critical to building data capacity for PCOR and for generating new evidence to inform health care decisions. The input provided by the committee will contribute to ASPE’s strategic planning for their work related to the data infrastructure over the next decade. The study is part of a broader initiative by ASPE intended to update the strategic plan in light of the reauthorization
of the PCOR Trust Fund and advances in health information technology and interoperability tools in recent years.
The study is a collaboration of three units of the National Academies: the Committee on National Statistics, the Board on Health Care Services, and the Computer Science and Telecommunications Board. The consensus study committee has a diverse membership; its 15 members include experts with decades of experience, as well as emerging leaders, in the broad fields of (1) PCOR; (2) research methods, statistics, and demography; (3) computer science and data infrastructure; and (4) patient engagement and patient perspectives. Appendix A contains the biographical sketches of the committee members.
As part of its information-gathering activities, the committee was asked to organize three workshops to collect input from stakeholders on aspects of the charge developed in consultation with ASPE. The workshops focused on key topics that the committee believed would particularly benefit from broad input from a variety of data users and other stakeholders. The committee’s conclusions from each workshop are summarized in a series of interim reports, of which the first centered on emerging data needs. This first interim report summarizes the discussion and committee conclusions from the first workshop, which focused on looking ahead at data user needs over the next decade. The second workshop in the series centered on data standards, methods, and policies that could make the PCOR data infrastructure more useful. The third workshop discussed research and data collaborations. This report summarizes the discussion and committee conclusions from the second workshop, which focused on data standards, methods, and policies that could make the PCOR data infrastructure more useful. The third report will discuss research and data collaborations.
As an interim report focused on one in a series of information-gathering activities, the scope of this report is limited to a subset of the topics relevant to the committee’s charge and the conclusions reached by the committee are, at this stage, fairly high level. Some aspects of the topics discussed are examined in further detail in other workshops. After completing all of its information-gathering activities, the committee will issue a final report, which will integrate and examine these topics in further detail.
Box 1-3 shows the committee’s Statement of Task for the overall study. The committee will address this charge in its final report, integrating what was learned from the workshops and from all other forms of input, including public meetings with HHS staff and background documentation available on the history and operations of the PCOR Trust Fund. The final report will contain overall findings and conclusions from the study, on the basis of the committee’s further deliberations and integrated judgment on the input received and materials reviewed.
Appendix B shows the agenda for the workshop, which was held on May 24, 2021. The committee’s goal for this event was to bring together researchers and policy experts to
- Identify data standards and methods that can make the PCOR data infrastructure more useful for research and other data needs.
- Identify data policies that are needed to facilitate the continued development and operation of the PCOR data infrastructure.
- Discuss what HHS is best positioned to address and support, and how the agency could maximize resources available for the PCOR
data infrastructure (representing 4% of the PCOR Trust Fund), in the context of the HHS public mission, authorities, programs, and data resources.
Invited speakers in each of the sessions were asked to reflect on the general topics above. The specific questions for each session are described in Chapters 2 through 4. An obvious limitation of an activity of this type is that only a small number of stakeholders can be invited to speak. To compensate for this limitation, the invited participants included diverse experts working in a variety of areas and on a range of types of projects, including both early career researchers and experts with decades of experience. A recording of the workshop as well as the presentation slides used by the speakers are available on the National Academies website at www.nationalacademies.org/PCORData.
Prior to the workshop, information about the event was disseminated through National Academies mailing lists and on the project website. To collect additional stakeholder input, members of the public were invited to provide comments on topics related to the workshop (or any other topic related to the committee’s charge), using a public input form available on the National Academies website.
OVERVIEW OF THE REPORT
This report is organized around the three main sessions of the workshop: Chapter 2 discuses data standards, Chapter 3 is centered on research methods, and Chapter 4 describes discussions focused on data policies and related infrastructure considerations. The points conveyed by the workshop participants do not necessarily reflect the views of the committee. In each chapter, a summary of the input received is followed by the committee’s conclusions. The conclusions are based primarily on the input collected as part of the workshop, background documentation received from ASPE and other public sources, and the committee members’ synthesis and expert judgment. Because this is an interim report, the committee’s conclusions at this stage are big-picture conclusions, which will be integrated with additional input over the course of the study.










