2
Characterizing and Assessing Cognitive Aging
Many people are concerned about the effects that changes related to cognitive aging may have on their capacity for living independently and making autonomous choices. This chapter will clarify the concept of cognitive aging and explain how it differs from disease. The chapter will review the many different elements of cognition, how they are measured, what is known about the patterns of age-related changes in cognition in both humans and animals, the implications of these changes for everyday functioning, and important concepts such as adaptability and plasticity. The chapter then details the age-related changes observed in human cognition and discusses how research findings from non-human animal models may explain the biological basis for these changes. Next is a summary of the evidence concerning the neural mechanisms that contribute to age-related changes in cognition and the implications of these changes. The background offered in this chapter provides a framework to understand how cognition is assessed, with particular attention to the challenges of creating norms and applying them in cognitive tests, especially given that such tests are often used by non-experts outside of carefully monitored research settings.
Numerous age-related changes in cognitive abilities are highly relevant to everyday activities and have substantial importance to the public. For example, declines in cognitive abilities increase the risk that older adults will make errors in financial decisions, select options that have less than optimal financial rewards, and suffer financial fraud and abuse (Agarwal et al., 2009; Denburg et al., 2007; Samanez-Larkin et al., 2012). Older adults generally have limited opportunities for employment for a variety
of reasons, and they also tend to have less time to recover any financial losses. Age-related changes in cognition can impair driving performance, which has safety and public health implications (e.g., Ball et al., 1998; Clay et al., 2005; and see Chapter 6 for further discussion of these topics). Current research indicates that age-related changes in cognitive processes affect performance on technology-based tasks such as searching the Internet for health information (e.g., Czaja et al., 2013; Sharit et al., 2008) and using health care providers’ patient portals (Taha et al., 2013). Given the ubiquitous use of computers and the Internet for many routine interactions with businesses, public services, and social events, this has substantial implications for older adults’ ability to participate in many domains of life.
AGE-RELATED CHANGES IN HUMAN COGNITION
There is tremendous inter-individual and intra-individual variability in age-related changes in cognitive abilities. This vast heterogeneity among older adults increases the challenges associated with understanding cognitive aging. The trajectory is not the same for everyone, and an individual’s performance on measures of ability may change across evaluation occasions (see Figure 2-1). Differences in the degree to which individuals’ cognitive function changes with age are due in part to a lifetime of differences in experiences, health status, lifestyles, education, attitudinal and emotional factors, socioeconomic status, and genetics. The trajectory also varies for different cognitive functions. Some aspects of cognition decline with age while others show improvement or remain stable until the much later decades of life. In addition, performance on laboratory tasks is not always representative of performance in everyday functioning. While age-related declines on many standardized tests of cognitive abilities are well documented, older adults may still maintain high levels of competence on most everyday activities because they are often able to compensate for declines in cognitive abilities with expertise and experience or environmental cues or support. The age-related changes seen in several domains of cognition have implications for behavior and function. This section provides descriptions of the primary domains of cognition (also known as neurocognition) along with summaries of what is known about the age-related changes in each. Box 2-1 offers examples of how each domain of cognition can be evaluated.
Speed of Information Processing
Speed of information processing reflects the efficiency of cognitive operations. One of the hallmarks of cognitive aging is a generalized slowing of processing speed, which is reflected in both perceptual and cognitive operations (e.g., Birren, 1970). Generally, it takes longer for older people to process information and give a response. These age-related changes have
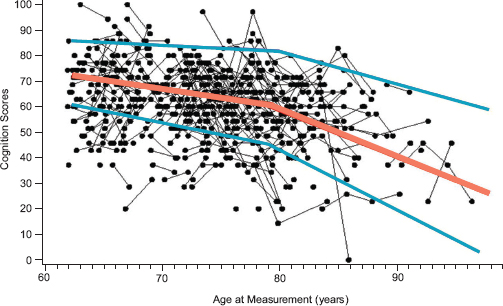
FIGURE 2-1 Intra-individual changes in cognition scores over time for a random sample of approximately 500 adults who were enrolled in the Health and Retirement Study.
NOTE: Lines connect dots that represent the scores of the same individual at different ages. The orange line represents the average score for all individuals, and the two blue lines represent 1 standard deviation above and below the average. Scores for some individuals increased over time even though, as indicated by the orange line representing the average score at each age, there was a general decline over time.
SOURCE: McArdle, 2011. Reprinted with permission.
an influence on the efficiency of other cognitive operations, such as working memory, attention, and speech processing, and have tremendous implications for behavior and interactions with others. For example, making the appropriate responses in driving, such as determining when to turn or when to stop at a red traffic light are heavily dependent on processing speed.
Declines in processing speed can also affect a person’s ability to remember spoken instructions, attend to important information, or perform tasks that have pacing demands. As will be discussed in more detail later in this chapter, age-related declines in processing speed can be offset to some extent by experience. For example, a study of skilled typists ranging in age from 19 to 72 years old found that although the older typists were slower on standard measures of reaction time and key tapping, they were not slower in typing speed (Salthouse, 1984). This was due to the older typists’ experience, which helped them anticipate the next characters that would have to be typed.
BOX 2-1
Examples of Cognitive Assessments
Numerous cognitive tests have been developed and validated. A few commonly used measures for key cognitive domains are
Speed of processing: The Pattern Comparison Processing Speed Test asks a person to determine whether a set of two pictures placed side by side are the same. The participant is given 90 seconds to evaluate as many sets of pictures as possible. The Digit Symbol Substitution Test, another timed measure of speed of processing, consists of nine digit–symbol pairs. The participant is given a list of digits and asked to write the corresponding symbol for each.
Sustained attention: The Connors Continuous Performance Test presents individuals with a repetitive task and asks them to maintain their focus for a period of time in order to respond to targets. For example, they will see or hear the number “1” or “2” and are told to respond when presented with a visual or auditory “1” but not when presented with “2.”
Selective attention: In visual search tasks, an individual is asked to search a visual display for a target, such as a letter, that is surrounded by other, non-target letters. The task can be made more difficult by making the target and non-target letters more similar or by increasing the density of the display. In the Stroop Test, another measure of attention, a person is asked to name the color of ink in which another color word is printed (e.g., the word “red” printed in the color green).
Episodic memory: The Picture Sequence Memory Test entails recalling increasingly long series of 6 to 18 illustrated objects and activities presented in a certain order on a computer screen. Participants are asked to recall the sequence of pictures presented over two learning trials.
Working memory: There are multiple common tests of working memory that include giving participants a list of items (letters, numbers, words) and asking them to repeat the items back in order. Performance on these tests is usually measured in terms of the longest sequence of letters, numbers, or words that are remembered correctly. For example, in the List Sorting Test, participants are shown pictures of different foods and animals along with the written text and audio recording that correspond to the item displayed. Then the participant is asked to recite the items back in order of size from smallest to largest, first within a single category (e.g., animals) and then in two categories (e.g., foods, then animals).
Semantic memory: In the Category Fluency Test, individuals are given a category, such as animals, and asked to name all of the items they can that belong to that category in 1 minute. In the Boston Naming Test, an individual is shown a series of pictures and asked to name each picture within 20 seconds (Kaplan et al., 1983).
Executive function: The Wisconsin Card Sorting Test is a measure of cognitive flexibility in which an individual is asked to match two sets of cards according to some characteristic—the color, shape, or number of items on the cards—but the participant is not told which characteristic is to be used. Feedback is provided after each match so that the participant can figure out the correct system of classifica-
tion. After a fixed number of correct matches, the classification is changed without notice, and the participant must learn the new rule of classification. In the Trail Making Test (Reitan, 1955)—a test of attention and set-shifting—the individual has to connect 25 consecutive items on paper or a computer screen. In the first part of the test, the participant is asked to connect numbers in sequential order (1, 2, 3, etc.), and in the second part of the test, the participant is asked to alternate between numbers and letters.
Reasoning: The Letter Sets Test consists of five sets of four letters. The participant has to decide which one of the five sets is dissimilar in the sense that it does not follow a rule used to generate the other four sets. Tests of inference are used to measure the ability to reason and draw conclusions from information presented in statements.
Language: There are a variety of tests that can be used to measure language skills depending on the particular aspect of language being assessed. For example, studies have used a word-by-word reading paradigm to assess comprehension of sentences (e.g., Kemtes and Kemper, 1997). Other studies use word-by-word reading time (e.g., Stine, 1990) or recall of text (Stine-Morrow et al., 1996). Another frequently used measure of language comprehension is the Token Test. A common test of word retrieval skills is the Boston Naming Test. The Category Fluency Test measures the ability to retrieve words rapidly from a semantic lexicon. The Boston Diagnostic Aphasia Examination is an example of a test of repetition of phrases and written communication skills. Finally, measures of prosody examine inflection and rhythm.
Spatial ability: In the Paper Folding Test, participants are shown a series of folds in a piece of paper through which a set of holes is then punched. The participants are asked to choose which of a set of unfolded papers with holes matches the original. In the Mental Rotations Test, participants are asked to compare several three-dimensional objects, often rotated on some axis, and state whether they are the same image or mirror images. A complex three-dimensional task is the Block Design subtest of the Wechsler Adult Intelligence Scale (WAIS). The participant is presented with a two-dimensional drawing in red and white of a target design and a set of three-dimensional blocks (some sides of the blocks are red, some sides are white, and some are half red and half white). The participant is asked to arrange the blocks within a specified time limit so that they mimic the drawing.
Intelligence: A common measure of intelligence used with older adults is the WAIS, 4th version. It consists of 10 core subtests and 5 supplemental subtests. The core subtests provide the intelligence quotient, which is derived from four index scores: Verbal Comprehension Index, Perceptual Reasoning Index, Working Memory Index, and Processing Speed Index. Sometimes a distinction is made between crystallized and fluid intelligence. The vocabulary and verbal subscale of the WAIS is often used as a measure of crystallized intelligence, whereas the other subtests are often used to assess fluid intelligence.
Additional measures: These measures provide an assessment of an individual’s mental status and include the Montreal Cognitive Assessment Battery, the Mini-Mental State Examination, and the Telephone Interview for Cognitive Status.
Attention
Attention is the capacity for processing information. Humans have limits in the amount of information they can process at any given time. Performing tasks at levels near full capacity for long periods of time can be tiring, especially for older adults (Kramer and Madden, 2008; Zanto and Gazzaley, 2014). When capacity limits are exceeded, performance tends to decline and be prone to error (Zanto and Gazzaley, 2014). There are several types of attention.
Selective attention refers to the ability to filter information and focus on select items despite the presence of other information. Examples include searching a visual display for a specific letter that is surrounded by other letters, identifying a road sign on a highway cluttered with billboards and advertisements, or finding relevant information on a highly cluttered website. This becomes more difficult with increasing amounts of clutter and irrelevant information, especially for older adults. In general, older adults have more trouble discriminating between relevant and irrelevant stimuli and locating relevant information in the presence of distracting background information (McDowd and Shaw, 2000).
Divided attention is the ability to split one’s focus between competing activities or multiple sources of information—also known as multitasking—and can involve the processing of multiple pieces of information or the performance of multiple tasks simultaneously. Reading an instruction manual while listening to music is one example of divided attention. A dangerous example is driving while typing a text message on a cell phone. Generally, older adults have more difficulty with multitasking than do younger adults (McDowd and Shaw, 2000; Tsang and Shaner, 1998; Verhaeghen and Cerella, 2002).
Sustained attention refers to the ability to maintain concentration on a task for a long period of time. It is required when a person must continuously monitor a situation in which important, but usually infrequent and unpredictable, events may occur. These types of tasks are typically referred to as vigilance tasks and are common in the work of air traffic controllers, inspection and quality control personnel, and others engaged in monitoring and surveillance. The literature suggests that sustained attention generally does not show age-related decrements (Berardi et al., 2001; Carriere et al., 2010).
Memory
Declines in memory are one of the most common complaints among older adults and can cause psychological distress and worry. Survey data from 21 states indicated that approximately 13 percent of Americans age 60 years and older reported confusion and memory loss (CDC, 2013).
As discussed below, there are many forms of memory. Some elements of memory are fairly stable in older adulthood, while others decline, and, as emphasized throughout this chapter, considerable variability exists among individuals. The aspects of memory discussed here were chosen because they are important to everyday functioning and have been thoroughly examined in the literature.
Working memory refers to the ability to temporarily hold information in one’s mind while it is processed or used. It encompasses the active manipulation of information or the maintenance of some information while dealing concurrently with further incoming information. For example, people listening to instructions on how to take a particular medication rely on working memory to hold the instructions in memory while they process the information for use at a later time. Working memory plays a central role in many activities, such as adherence to a medication schedule (e.g., Insel et al., 2006), and it is a fundamental element of other cognitive abilities such as processing language, solving problems, and making decisions. Working memory is also important to new learning. The rich literature available on the effects of aging on working memory indicates that working memory generally declines with age, especially for complex tasks (e.g., Salthouse, 1994; Zacks et al., 2000).
Long-term memory is the system for relatively permanent memory storage and is the repository of a person’s knowledge. There are multiple types of long-term memory.
Semantic long-term memory stores factual information acquired over a lifetime and is often not associated with a particular time or place. Semantic memory is used when a person provides answers to factual questions, such as naming the current president of the United States or a state capital. Older adults typically perform as well as young adults on tasks testing this type of memory (Craik and Jennings, 1992; Nilsson, 2003; Spaniol et al., 2006). In fact, an individual’s accumulated semantic knowledge and memory increase into the sixth and seventh decades of life and only a slight decline may be seen subsequently (Brickman and Stern, 2009). People may have some difficulty with retrieving semantic information if it has not been accessed for some time, but generally the information can be retrieved with the appropriate cues.
Episodic memory is the memory of autobiographical events, including times, places, associated emotions, and other contextual information. Episodic memory is relevant to events of both the recent and the distant past. This type of memory tends to decline with age, and the declines are greater when the task demands are more complex or when there are few environmental supports or cues available (e.g., writing a note to oneself about where the car was parked this morning) (Craik and McDowd, 1987; Mitchell, 1989).
Prospective memory is another type of long-term memory. It is the ability to remember to do something in the future, such as take a medication or pick up the dry cleaning, and it can be time-based or event-based. Time-based prospective memory is remembering to do something at a later time, such as to go to a doctor’s appointment next Tuesday at 2 p.m. Event-based prospective memory is remembering to do something after an event, such as meeting a friend after the appointment. Age-related declines occur in both types of prospective memory, but the declines are usually greater for time-based prospective memory (Henry et al., 2004; Maylor et al., 2002). The declines appear more pronounced for event-based tasks that require higher levels of controlled processing (e.g., effortful processing, which requires attentional capacity) than for those supported by relatively more automatic processes (e.g., very well-learned tasks, which require little attentional capacity). In addition, the effects of age on prospective memory appear to be greater when tested in laboratory tasks than when tested using more naturalistic tasks (Henry et al., 2004).
Procedural memory, also known as skill learning, refers to learning and remembering how to perform an activity such as driving a car, riding a bicycle, cooking a favorite recipe, or using a software program. Generally, this type of memory is built up gradually over time as a function of practice. Well-learned procedures such as driving or typing become automatic and can be performed without high levels of conscious processing or attentional resources. Older adults do not usually have trouble doing procedures that are automatic or well learned, and they can learn to do new procedures with practice (Backman et al., 2001). Distinguishing between performance time, learning, and memory for a task is important. For example, older adults may perform tasks more slowly when procedural memory is required, and they may learn procedural sequences at a slower rate than younger adults, but they can still maintain the procedural aspects of the task (e.g., how to type) and can learn new procedures (Brickman and Stern, 2009).
Finally, there is source memory, which relates to the context or details surrounding an event, particular fact, or piece of information. This is different from remembering the content of the information. For example, people might remember a news story but forget when they first received the information or whether they read it in a newspaper or heard it on television. Similarly, they might remember that a friend is retiring but forget who told them the information. Research findings indicate that source memory can decline with age and that aging has a greater effect on source memory than it does on the memory of content (Glisky et al., 2001; Spencer and Raz, 1995; Trott et al., 1997).
Executive Function
Executive function refers to the cognitive skills used to regulate behavior and modify responses based on environmental cues. This includes the ability to plan actions (e.g., paying bills or creating schedules), to organize information, to think abstractly, to allocate mental resources (cognitive flexibility), to reason, to solve novel problems, to adapt to new situations, and to act appropriately during social interactions. Generally, executive function declines with age (Zelazo et al., 2004).
Tests evaluating set-shifting (i.e., the ability to move back and forth between tasks) show significant changes with age. For example, performance on the Visual-Verbal Test (in which participants are asked to look at a series of cards and indicate how three of the four objects on each card are alike in one way and then indicate how three of the objects are alike in another way) declines substantially with age. These changes appear to be related to the difficulty that older participants have with switching from one abstract answer to another (e.g., they tend to get the first item correct but the second item wrong) (Albert et al., 1990).
Series completion tests also show substantial declines with age. These tests generally require the participant to examine a series of letters or numbers and to determine the rule that governs the sequencing of the items in the series. Cross-sectional and longitudinal data both demonstrate age-related declines on tasks of this sort (e.g., Lachman and Jelalian, 1984; Schaie, 1983).
Proverb interpretation tests, which require the participant to provide the general meaning of a proverb (e.g., “Barking dogs seldom bite.”), also demonstrate age-related declines (Albert et al., 1990). This is true whether participants are asked to provide the meaning of the proverb or are given alternative choices of interpretation.
Declines in executive functioning can affect a person’s ability to make decisions, to inhibit responses, and to simultaneously process relevant and irrelevant information. These declines have been linked to declines in ability to perform instrumental activities of daily living, such as medication management (Bell-McGinty et al., 2002).
Reasoning Abilities
Reasoning ability, which is sometimes considered an aspect of executive function, also declines with age. It reflects logical thinking, or the process of drawing conclusions from information to inform problem solving or make decisions, such as medical or financial decisions. (The societal implications of declines in reasoning ability are discussed in detail in Chapter 6.) There is a distinction between deductive and inductive reasoning. Deductive reason-
ing allows a person to draw conclusions about specific events or situations based on premises or general theories assumed to be true, whereas inductive reasoning allows a person to draw general conclusions based on specific observations. Overall, the literature from laboratory tests of reasoning indicates that both of these abilities decline with age in a fairly linear way, beginning in middle adulthood (e.g., Salthouse, 2004).
Language
Language function consists of an array of abilities, including understanding and producing speech, reading, writing, and naming. It is a fundamental component of human behavior and a primary mechanism for communication. Language processing is a critical element of cognitive tasks (e.g., the ability to understand written and spoken instructions) and social interactions. For example, older adults whose hearing loss impedes their understanding of spoken language may withdraw from social interaction (Mick et al., 2014). This is noteworthy not only because social interaction is important to quality of life for many people but also because limited social interaction may contribute to cognitive decline (see Chapter 4B).
Age-related effects on language abilities vary as a function of the ability being investigated: Some aspects of language function decline, while others do not. Although vocabulary does not decline until very old age (Burke and Shafto, 2008; Schaie, 1994, 2005), language production skills do decline with age. These are word-finding failures and language disfluencies, such as the phenomenon of “having a word on tip of the tongue” or pausing for longer intervals while speaking (Burke and Shafto, 2008; Kemper and Herman, 2006). The syntactic complexity of spoken language also tends to decline with age (Kemper and Sumner, 2001), and older adults generally produce sentences with lower idea density than younger adults (for a review, see Burke and Shafto, 2008). Older adults may also experience more difficulty spelling familiar words (Abrams and Stanley, 2004). However, the comprehension of the meaning of words is typically well-preserved in older age. With respect to speech comprehension, older adults generally have difficulty understanding spoken language that is distorted or too rapid (Wingfield and Grossman, 2006; Wingfield et al., 1999). Thus, it may be difficult for them to comprehend loudspeaker messages such as gate announcements in airports, or synthetic speech messages such as those used in interactive telephone messaging systems.
Spatial Ability
Spatial ability—the maintenance and manipulation of visual images—also generally declines with age (Techentin et al., 2014; Willis and Schaie,
1986). More specifically, spatial ability includes the abilities to produce figures, to recognize familiar faces, to form relationships among spatial locations, and to copy and match objects and pictures. Older adults do not perform as well as younger adults on spatial tasks requiring mental rotation, visualization abilities, or remembering the location of objects (e.g., Dobson et al., 1995; Hertzog and Rypma, 1991; Light and Zelinski, 1983). These abilities are important for tasks such as learning environmental layouts and routes, wayfinding, map reading, and translating directions. They also influence performance on computer tasks, such as editing text and using a spreadsheet, and searching map- and computer-based information (Pak et al., 2006b), and navigating telephone menu systems (Pak et al., 2006a).
Furthermore, compared to younger individuals, older people are less able to depict and perceive the three-dimensionality of drawings. In one study in which young and old adults (mean ages 21 and 67 years, respectively) were asked to draw a cube, the drawings by the older adults were rated as less accurate than those of the younger adults (Plude et al., 1986). The older participants were also less accurate in determining the adequacy of drawings of cubes that were distorted to varying degrees. They were, however, equally capable of copying an image of a cube when they were given cues about the size of the lines.
Intelligence
In the course of day-to-day activity, individuals combine many of these previously described cognitive domains in order to function. Intelligence is a multifaceted construct that refers to the ability to solve problems, plan, think abstractly, and adapt to and learn from everyday experiences. Cognitive abilities are the underlying processes or mechanisms of intelligence.
Intelligence is most commonly measured on one or more standardized tests that produce an intelligence quotient (IQ) score, which indicates how far an individual deviates from the average for that person’s age group. Different types of intelligence are discussed in the literature, including emotional intelligence (the ability to monitor one’s own and other people’s emotions, discriminate among different types of emotions, and use emotional information to guide behavior) and practical intelligence, or everyday competence.
Differentiating between crystallized intelligence and fluid intelligence can be useful when discussing aging and cognition. Crystallized intelligence, or crystallized abilities, reflects a person’s knowledge, such as language skills or knowledge about a particular topic, while fluid intelligence, or fluid abilities, is involved in processing current or new information, such as learning to play chess. Fluid abilities reflect a person’s capacity to think logically and solve problems in novel situations, and they aid in skill ac-
quisition and learning. Data from both cross-sectional (comparisons among different age groups at a single point in time) and longitudinal (examination of a change in a cohort over time) studies indicate that crystallized abilities tend to remain stable, with only modest age-related decline until the very latter decades, whereas declines in fluid abilities begin earlier and are more gradual across the life span (Schaie, 1996).
Wisdom
Like intelligence, wisdom, a construct that has a relatively short history of independent investigation within the realm of cognitive aging, is multidimensional and has been viewed from a number of theoretical perspectives and defined in a variety of ways. The definition most germane to this report considers wisdom to be an expert knowledge system and emphasizes the amount and use of knowledge that someone has accumulated in life and how that person is able to use and apply this knowledge (Baltes and Smith, 1990; Kunzmann and Baltes, 2003). Wisdom encompasses expertise and mastery of life matters that require insight, judgment, management of life circumstances and events, life planning, and personal conduct.
Wisdom goes beyond descriptive knowledge and entails a deeper interpretive understanding of knowledge (Ardelt and Hunhui, 2010). Overall, wisdom includes cognitive, reflective, and affective elements (Ardelt, 2004). For example, wisdom-related expertise is important in providing advice on relationship conflicts or in planning for retirement. With respect to aging, wisdom needs to be examined from a life span perspective in the sense that it begins developing in adolescence and early adulthood. Wisdom-related knowledge often remains stable in older adulthood (e.g., Baltes and Smith, 1990; Staudinger, 1999). However, studies comparing age differences in wisdom are relatively few and, just as there are differences in definitions of wisdom, the manner in which wisdom is measured also varies (for a review, see Jeste and Oswald, 2014).
Assessment of Cognitive Abilities
Examples of standard measures used to evaluate each domain of cognition are in Box 2-1. A wide variety of such measures is available for research and clinical use, and there is some controversy about which measures are optimal for each domain and population of interest. For example, people from racial or ethnic minority backgrounds might not do as well on certain tests as the rest of the population even though their cognitive ability being tested may be perfectly intact. The National Institutes of Health (NIH) has developed the NIH Toolbox for the Assessment of Neurological and Behavioral Function® to harmonize the measurement of functioning
across diverse study designs and settings. The NIH Toolbox® includes a set of brief measures (validated for use in individuals who are ages 3 to 85 years old) that can be used to assess cognitive, emotional, motor, and sensory function (Weintraub et al., 2013). The specific cognitive functions measured are executive function, episodic memory, language, processing speed, working memory, and attention.
At one time it was thought that specific cognitive functions were located within certain regions of the brain. For example, poor performance on the Wisconsin Card Sorting Test, a measure of executive function, was thought to be indicative of damage to the dorsolateral frontal lobes, but performance on this test is now known to be affected by damage to a number of different structures and neuronal pathways that serve this aspect of executive function. In fact, the vast majority of cognitive processes, such as memory, executive function, visuospatial abilities, and processing speed, are known to be related to highly sophisticated networks containing tens of millions of neurons. This emphasizes the need for continued development of sophisticated neurocognitive batteries as well as neuroimaging modalities that allow for structural analysis of brain connectivity. As will be discussed below, additional information can be derived from studying the associations between functional capabilities and performance on neuropsychological tests.
VARIABILITY IN COGNITIVE CHANGES
A few important caveats need to be noted concerning the above discussion. Life expectancy has increased due to changes in lifestyle and advances in medical care, and increasing numbers of people are living into their 80s, 90s, and beyond (see Chapter 1). People at 60 or 70 years of age are typically very different from people in their 80s, who are different from those 90 years or older. The prevalence of various diseases that affect cognition, such as cardiovascular diseases, diabetes, and dementia, increases greatly with advancing age, and sensory declines also may be magnified. Careful consideration needs to be given to what defines cognitive aging for younger older adults as compared to the oldest older adults. Even though there is limited research examining cognition in very old age, the available evidence shows deterioration in cognitive functioning in this population. A 6-year study examining changes in cognitive abilities in a sample of twin pairs age 80 years and older who did not have dementia found that all aspects of cognition that were assessed (memory, reasoning, processing speed, and verbal ability) declined in a linear fashion during that period (Johansson et al., 2004). The decline was evident even for those aspects of cognition that are less vulnerable to age-related effects, such as verbal abilities. Similarly, 6-year longitudinal data from a sample of adults age 70 years and older
who were enrolled in the Berlin Aging Study demonstrated that perceptual speed, memory, and fluency declined with age, while knowledge remained stable up to about 90 years of age and then declined thereafter (Singer et al., 2003).
Another key caveat, stated previously, is that the aging population is extremely heterogeneous, with older adults varying in their abilities and in the longitudinal course of their cognitive aging because of differences in genetics and experiences over the life span. Studies examining aging and cognition or aging and learning often differentiate between two types of variability: Inter-individual variability refers to the differences among people or groups of people, and intra-individual variability refers to the changes that occur within one person over time.
One type of inter-individual variability is the variability observed in comparisons of people in different age groups. When comparing younger and older adults on a variety of performance measures (e.g., processing speed or working memory), researchers often find that older adults, as a group, do not perform as well as younger people (e.g., Schaie, 1996). However, there is such tremendous variability in performance that some older people might perform as well as or better than some younger adults. For example, a study examining Internet search abilities among older and younger people indicated that, on average, the younger adults performed better on the search problems. However, some older participants performed at the same level as or better than the younger participants (e.g., Sharit et al., 2008). Also, sometimes differences found in these types of studies may not be entirely due to the effects of aging but may also be a function of cohort or generational differences. For example, skill using a computer or the Internet demonstrates a cohort difference between today’s younger and older adults; unlike younger adults, today’s older adults did not grow up using computers and therefore would not be expected to be as fluent with computer and Internet use.
Inter-individual variability also occurs among individuals within the same age group. Older adults vary widely in educational background, health status, literacy, culture, ethnicity, skills, abilities, and life experiences. These differences contribute to vast inter-individual variability among people of the same age and can make predicting performance based solely on age less precise as people increase in age and their differences in experience add up. Intra-individual variability, by contrast, is examined in studies that measure performance across two or more sessions. In longitudinal studies, these sessions are typically spread over a period of years, with the performance of the same people measured every 5 or 10 years. Intra-individual variability can also be seen over shorter periods of time, even days, due to such factors as fatigue, acute illness, distractions, or attention lapses.
A third caveat is that the cognitive trajectory over time is not neces-
sarily smooth or linear but rather may be a dynamic process with ups and downs due, for example, to environmental stressors, medications, or illnesses. Many of these factors are reversible, and there is much that can be done to prevent declines (see Chapters 4A, 4B, and 4C).
A fourth caveat is that aging is not just a picture of decline. As will be discussed later in this chapter, neural plasticity is retained as people age, so older adults can learn new skills and their performance can improve. For example, results from the ACTIVE trial, which examined the benefits of cognitive training on various aspects of cognitive ability, showed that training in speed of processing and reasoning resulted in cognitive improvements in a sample of older adults (e.g., Ball et al., 2002). Older adults also can and do successfully employ compensatory strategies to offset cognitive declines, and they have a wealth of knowledge, skills, and experience that younger adults may not have.
BOUNDARY CONDITIONS
As noted in Chapter 1, this report focuses on cognitive aging as opposed to the cognitive changes associated with age-related diseases of the brain, such as neurodegenerative dementias, including Alzheimer’s disease. The committee recognizes that much remains to be done to fully understand the boundary between cognitive aging and the initial phase of a neurodegenerative disorder, but it has concluded that a sufficient body of knowledge concerning cognitive aging exists to support distinguishing it from neurodegeneration and therefore to draw meaningful conclusions about cognitive aging and to make substantive recommendations for future directions.
The challenge of identifying boundaries between conditions is not unique. In the field of neurocognitive disorders, for example, the syndrome of mild cognitive impairment (MCI)1 is considered an interim clinical diagnostic phase between the time when an individual’s cognitive function falls within the normal range and the time when that person meets criteria for dementia. International studies have focused on participants with MCI (Petersen, 2004; Winblad et al., 2004), and consensus criteria have been established that are in wide use (Albert et al., 2011; Dubois et al., 2014). Nevertheless, it remains challenging to determine the boundary between cognitive aging and MCI. These diagnostic challenges remain for several reasons: (1) The clinical diagnosis of MCI is usually based not only on whether a person’s performance falls within the “normal range” on cognitive testing but also on how well that individual is functioning on a daily
____________
1The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, refers to MCI as “mild neurocognitive disorder” (APA, 2013).
basis, and information about this usually depends on inherently subjective self-reports or the reports of an informant who knows the individual well. (2) Medical illnesses or life stressors can have a temporary effect on cognition such that when the conditions are alleviated, a person previously thought to be on a downward trajectory may recover cognitive functions. (3) A range of disorders can cause progressive cognitive decline, and the symptoms of these disorders are quite varied, which means that the group of individuals that meets the criteria for MCI can be heterogeneous in terms of cognition and function. MCI is a syndromic label, capturing a variety of causes which include neurodegenerative diseases and cognitive aging. Differentiating between these causes can be challenging because the symptoms and signs are mild. It requires a clinical assessment and sometimes a series of assessments. Cognitive testing and imaging can help to distinguish among individuals with neurodegeneration or cognitive aging. For all of the foregoing reasons, not all individuals with MCI progress to dementia, and some may even improve over time. These diagnostic challenges are particularly problematic in epidemiological settings, where it may be difficult to obtain reliable information from an informant who knows the individual well. For similar reasons, it is often difficult to determine whether an individual is in the late phase of MCI or the early phase of dementia, as this distinction typically depends on the person’s degree of functional impairment in daily life, and functional impairment is often difficult to quantify because it depends on the subjective report of another person who knows the individual well. These diagnostic challenges can result in mislabeling an individual as irreversibly impaired.
Substantial efforts are under way to improve the accuracy of cognition-related diagnoses by using biological measures of underlying pathology, often referred to as “biomarkers.” For example, the revised diagnostic criteria for MCI and dementia due to Alzheimer’s disease now include the use of biomarkers to facilitate the determination of whether the underlying neurodegenerative process of Alzheimer’s disease is the cause of the observed cognitive decline. The use of biomarkers as criteria for diagnosis is an active and evolving area of research (Albert et al., 2011; McKhann et al., 2011; Sperling et al., 2011).
Similar challenges exist in the study of depression with, for example, the difficulty of distinguishing the boundaries between sadness, grief, and depression. Nevertheless, despite the difficulty in determining definitive boundaries between mental states, objective studies using operational criteria have permitted the study of depression in populations and led to improved treatments.
An emerging challenge to defining the boundaries between cognitive aging and disease is the more recent recognition of preclinical stages of neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases
(Sperling et al., 2011; Wu et al., 2011). The term describes a novel but not yet validated stage for which identification does not rely on a patient’s signs and symptoms, such as disabling impairments in cognition or movement, but instead measures pathobiology, such as the presence of amyloid as measured by imaging or the presence of autosomal dominant genes. If diagnosis at this stage of disease becomes part of clinical practice, then investigators of cognitive aging will need to decide whether to include otherwise healthy persons who have preclinical disease in future studies of cognitive aging. This issue is further examined later in this chapter in the section on norms.
AGE-RELATED CHANGES IN COGNITION—INSIGHTS FROM ANIMAL MODELS
The central premise of studying animal models is that if age-related changes in cognition found in humans are also found in other species, it increases the likelihood that the cognitive changes are related to aging and not to early signs of neurodegenerative disorders common in humans, such as Alzheimer’s disease. Studies in animals, like those in humans, have focused on subjects that are healthy, with the added advantage that animal models do not develop human neurodegenerative disorders. As a result, research in animal models has been particularly important in demonstrating that the cognitive changes seen with advancing age are related to aging, as opposed to disease.
The two areas of cognition that have been most widely studied across species are memory, as mediated by the hippocampus and other areas of the medial temporal lobe, and executive function, which is highly dependent on the prefrontal cortex. Given the extensive involvement of these two brain regions in the cognitive functions vulnerable to aging, most studies aimed at revealing the biological basis of cognitive aging have targeted these regions.
The following sections of the chapter review how animal models inform the neuropsychological and biological basis of age-related cognitive changes and the mechanisms that may explain these changes. While some cognitive functions, such as language, do not correlate well between humans and animals, others do allow comparable measures. This report focuses on three that do: attention, memory, and executive function.
Attention
Functions related to attention, including vigilance, orienting of attention, and cognitive flexibility, have a vulnerability to aging that can be observed in animal models. Aged rats show impairments in choice reaction time tasks that are thought to reflect vigilance (Jones et al., 1995; Moore et al., 1992; Muir et al., 1999), and these impairments are similar to age-
related declines in vigilance in humans (Parasuraman et al., 1989). The ability to focus attention on a designated spatial location in the environment appears to be largely spared in older monkeys (Baxter and Voytko, 1996), mirroring the relative preservation of this function in older adult humans without dementia (Greenwood et al., 1993). Cognitive flexibility and the capacity to shift attention to different sensory inputs in the course of problem-solving is impaired in aged rodents (Barense et al., 2002) as it is in older humans (Robbins et al., 1998). In short, a substantial correspondence in multiple domains of attention has been observed in both animal models and humans. (For a review, see Bizon et al., 2012.)
Memory
Episodic memory performance has been studied extensively in healthy monkeys and rodents across their life spans. The Delayed NonMatching to Sample task (DNMS) is used to assess recognition memory in the nonhuman primate. In this task, the monkey is required to indicate which of two objects was most recently presented. Monkeys may show either a deficit on learning the task, which likely involves the prefrontal cortex, or a deficit in memory as the delay gets longer, which is more likely to reflect hippocampal function. Studies using the DNMS have demonstrated that aged monkeys are impaired at learning the nonmatching principle but are only mildly impaired by increasing the delay (Arnsten and Goldman-Rakic, 1990; Bachevalier et al., 1991; Moss et al., 1988; Presty et al., 1987; Rapp and Amaral, 1989). Thus, as is the case for humans, as monkeys age they take longer to learn something new but retain the information reasonably well. Age-related changes can be seen on a very difficult memory task, such as the Delayed Recognition Span Test (DRST). This test requires a monkey to identify a new stimulus from an increasingly large selection of items with which the monkey is familiar. The goal is to keep track of as many stimuli as possible without making a mistake. Middle-aged monkeys are impaired on the spatial version of the DRST but not on the color version (Herndon et al., 1997). Older monkeys are impaired on both versions of the task (Moss et al., 1997). Thus, the performance of monkeys on the spatial version of the test may be functionally equivalent to the performance of humans on difficult delayed recall tests. As with aging humans, performance varies considerably in aging monkeys. Among the oldest animals, some perform as well as younger animals, but the performance average of the group declines substantially with age.
Rodents also display age-related declines in memory tasks. For example, older rats show less exploration than younger rats in a task similar to the visual paired comparison task (Cavoy and Delacour, 1993). Older rats are also impaired, relative to young and middle-aged rats, on the Morris
water maze task, in which animals are required to find a platform that is under water (Rapp et al., 1987). Rodents’ performance on this task has been shown to be highly dependent on the hippocampus (Morris et al., 1982). These deficits are highly stable over time when a specific combination of learning trials and probe trials are used. Moreover, as with humans and monkeys, there is considerable variability in performance among older rodents: A substantial subgroup shows age-related declines in performance, but some older rodents perform on par with young animals (Rapp et al., 1987).
Executive Function
Changes in executive function in non-human primates have been examined primarily by using reversal learning paradigms (Bartus et al., 1979; Rapp, 1990) or a form of delayed response (Bachevalier, 1993). Reversal learning entails responding to a change in reinforcement rules by first unlearning, or breaking, the initial stimulus–reinforcement bond that had been learned, and then shifting to a new one. In this way, reversal learning can be a measure of executive function and, by extension, a reflection of the integrity of the prefrontal cortex. Data show that when compared to young monkeys, older monkeys have trouble unlearning established stimulus–reward contingencies, particularly when they are based on spatial location, thus demonstrating impaired spatial reversal learning (Lai et al., 1995). Moreover, in tests of both spatial and object reversal learning, older adult monkeys tend to continue making the same response even though it is not rewarded.
Comparable findings have been reported on a task called the Conceptual Set Shifting Task, which was developed for non-human primates as an equivalent to the Wisconsin Card Sorting Test in humans (Moore et al., 2003). In the version for non-human primates, a pattern of responding is developed on the basis of rewards for responses to a specific visual pattern. The animal maintains this response pattern for a while, and then the reward contingency changes. One can therefore examine the number of errors prior to attainment of the initial abstraction rule as well as the number of perseverative responses after the rule changes. Older adult monkeys are impaired relative to young monkeys on both the concept formation and the set-shifting aspects of the task (Moore et al., 2003).
Executive function is dependent on working memory, which is also mediated by the prefrontal cortex. Working memory can be tested in monkeys by using a delayed response task, which requires the monkey to remember an initial visual stimulus over extended delays and to choose it in preference to a new stimulus when the two are presented simultaneously (Rapp and
Amaral, 1989). Monkeys show age-related declines in this task, particularly as the delays lengthen (Rapp and Amaral, 1989; Wang et al., 2011).
NEURAL MECHANISMS THAT CONTRIBUTE TO AGE-RELATED CHANGE IN COGNITIVE FUNCTION
Studies of brain tissue both in humans and in animal models have sought to examine the underlying neural mechanisms that may be responsible for the age-related changes in cognition described above. This research includes studies of neuronal number, synaptic integrity, and neurotransmitter changes. Overall, the studies show that while the number of neurons remains relatively stable, changes occur in their structure and in their neurotransmitter receptors—changes that likely explain the cognitive declines discussed above. The stability in the number of neurons number—that is, the lack of neuron death in areas supporting cognition—seen with aging in these studies is in contrast to the extensive neuron loss that occurs in Alzheimer’s disease.
Neuronal Number in Aging
A wealth of human anatomical data indicates that in the cortex neuronal loss with advancing age is either not significant or not as extensive as reports had suggested prior to 1984 (Anderson et al., 1983; Brody, 1955, 1970; Colon, 1972; Henderson et al., 1980; Shefer, 1973). Although large neurons appear to shrink, few are lost (Terry et al., 1987).
Interestingly, research in monkeys has produced comparable findings. The absence of neuron loss with increasing age in monkeys has been shown in multiple cortical areas, including the prefrontal cortex (O’Donnell et al., 1999; Peters et al., 1994; Vincent et al., 1989). These conclusions are based both on a comparison of the number of neurons in young and old monkeys and on an examination of the cortical tissue by electron microscopy (Peters et al., 1998). Studies in rodents have reported comparable findings (Rapp et al., 2002).
Post-mortem data from humans and monkeys show that age-related neuronal loss does not occur in the hippocampus (Amaral, 1993; GomezIsla et al., 1996; Rosene, 1993; West, 1993) or in the entorhinal cortex (Gazzaley et al., 1997), which is tightly linked to the hippocampus both structurally and functionally. Equivalent data have been reported in rodents: Even in the subset of animals with declines on a memory task that depends on the hippocampus, there was no decrease in the number of neurons in the various regions of the hippocampus (Rapp and Gallagher, 1996). Thus, while there are clear age-related declines in cognitive functions
mediated by the hippocampus and prefrontal cortex, they are not due to a loss of neurons.
These findings are in striking contrast to the extensive neuronal loss seen in Alzheimer’s disease, and they support the conclusion that age-related cognitive decline does not result simply from a milder form of neuron loss; rather, it is more likely to involve changes in neurons that are still living yet functionally compromised. (For a more detailed review of these issues, see Morrison and Hof, 1997.)
Synaptic Integrity
Even though there does not appear to be enough neuronal loss to account for age-related cognitive change, other changes in neuronal function—specifically, the number and function of synapses—may contribute to age-related cognitive changes. Studies in non-human primates have shown that with advancing age, specific subclasses of dendritic spines are selectively lost in the dorsolateral prefrontal cortex, and the density of these spines correlates with working memory performance (Dumitriu et al., 2010). The specific loss of the spines is important because they are known to be the most plastic spines, suggesting that it is not just a loss of synapses with aging that drives cognitive decline but, more specifically, it is the loss of synaptic plasticity that is key (Morrison and Baxter, 2012).
In contrast, in the hippocampus it is the largest, most stable, and most complex synapses that are selectively lost with age, and their number correlates with memory performance (Hara et al., 2012; Morrison and Baxter, 2012). Studies in rodents show comparable changes. For example, older rodents with spatial learning deficits display substantial decreases in the number of synapses (Smith et al., 2000) and alterations in synapse function, suggesting that a loss of synaptic plasticity with aging leads to memory deficits (Norris et al., 1996; Rosenzweig and Barnes, 2003).
Neurotransmitter Changes
The age-related synaptic alterations described above have been linked with neurotransmitter changes, particularly in the functioning of the AMPA receptor (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor). AMPA receptors have been linked to learning, memory, and synaptic plasticity. For example, within some synapses in the hippocampus of monkeys there is an age-related decrease in the number of AMPA receptors, and the extent of the decrease is predictive of declines in performance on a memory task (Hara et al., 2012). Additionally, alterations in certain regions that project to the cortex in humans and animals (e.g., the basal forebrain and the locus coeruleus) are likely responsible for decreases in the produc-
tion of neurotransmitters, such as norepinephrine, which is important for cognitive function (Robbins and Arnsten, 2009).
White Matter Changes
Studies have found age-related alterations in the brain’s white matter—the myelin sheath surrounding neuronal axons (Nielsen and Peters, 2000; Peters, 1996; Peters et al., 1994). Evidence from non-human primates suggests that the oligodendrocytes, which are responsible for forming white matter, may be less efficient with age. For example, a comparison of the oligodendrocytes of young and old monkeys showed that the myelin sheaths in the old monkeys were abnormal and appeared to be degenerating (Peters, 1996). However, when investigators compared the number of axons in old and young monkeys, the axon number was largely unchanged, and relatively few degenerating axons were found in the old monkeys (Nielsen and Peters, 2000). Because the myelin sheath enhances signal conduction down the axon, these age-related alterations may explain some of the age-related changes seen in neural processing speed.
Neuronal Proliferation in the Adult Brain
The adult brain has the capacity for neuronal replacement. Ample evidence indicates that new neurons are generated in the hippocampus and olfactory system in both monkeys (Gould et al., 1999; Kornack and Rakic, 1999) and humans (Eriksson et al., 1998). The proliferation of new neurons decreases with age. In rodents, there appears to be a gradual increase in total numbers of neurons; in primates, such an accumulation is less certain. It has been suggested that the number of neurons in the primate hippocampus is constant, with a balance maintained between the generation of new neurons and the rate of neuron death and cell removal (Kornack and Rakic, 1999).
Summary of Findings from Animal Models
When the findings in animal models are juxtaposed with observations from humans, together they suggest that the variability seen in cognitive changes with advancing age among healthy individuals is related to variations in synaptic integrity and synaptic plasticity in specific brain circuits that are tightly linked to cognitive functions, such as memory and executive function (see Morrison and Baxter, 2014, for a more detailed discussion). The fact that comparable changes are found among both healthy nonhuman primates and healthy rodents increases the likelihood that such
changes in humans are not the result of a neurodegenerative disorder such as Alzheimer’s disease.
These age-associated alterations have a clear effect on daily function, which will be discussed later in this chapter. Importantly, synapse loss is potentially reversible, whereas neuron death is not, which suggests a natural therapeutic target for sustaining synaptic and cognitive health. Synapse loss has been described in humans very early in the transition from normal cognitive function to cognitive decline that may represent the earliest stages of Alzheimer’s disease (Scheff et al., 2006). Synaptic alterations in humans may leave certain neurons vulnerable to the degeneration that occurs in Alzheimer’s disease, which suggests that early intervention at the synaptic level may be key to preventing the transition to Alzheimer’s disease.
While it appears that the loss of synapses without significant neuron loss contributes to cognitive decline, the cause of synapse loss is unknown, as is the extent to which such loss is linked to other age-related brain changes (e.g., vascular changes). In fact, pathologic alterations (e.g., tau and beta-amyloid accumulation) that have been linked to Alzheimer’s disease have also been seen in older adults with unimpaired cognition, although the changes in these cases are less clearly linked to neuron death than they are in Alzheimer’s disease dementia cases, and the degree to which this more moderate pathology promotes synapse loss is not known. In addition, pathology can differ by brain region, with virtually all adults approximately 70 years of age or older having some tau deposits in the entorhinal cortex (Bouras et al., 1994), but it is not clear how such restricted pathology affects cognitive function. It is also important to acknowledge that there are many protective factors that influence cognitive aging, as noted elsewhere in this report. Taken together, these findings suggest that cognitive aging represents a balance of lifelong risk and protective factors that include both neuropathologies and other factors that preserve or impair the organization and maintenance of brain structures and circuits. While the pathologic conditions associated with Alzheimer’s disease have been linked to neuron loss and dementia, the middle ground, where events lead to cognitive aging in the absence of neurodegeneration, remains poorly understood and requires further investigation both in humans and in animal models.
COGNITIVE RESERVE AND PLASTICITY
Though all brains age, they do not all age at the same rate or in the same way. For example, multiple studies have demonstrated that a person may function well and yet have the pathology characteristic of Alzheimer’s disease at autopsy (Crystal et al., 1988; Katzman et al., 1988; Morris et al., 1996; Price and Morris, 1999).
Why do some people have good cognitive function despite a level of
brain injury that impairs function in others? The concept of cognitive reserve has been proposed as an explanation for why some people are able to tolerate the brain alterations associated with dementia (and other illnesses) without exhibiting the associated symptoms (Scarmeas and Stern, 2004). Factors that may contribute to cognitive reserve include education, occupational attainment, physical activity, and engagement in intellectual and social activities (Tucker and Stern, 2011). Though some factors that increase risk for cognitive decline and dementia, such as genes, are not readily modified, a number of factors that contribute to cognitive reserve can be enhanced, even later in life. Various studies have shown a positive effect in older adults from maintaining an active social network (Graham et al., 2014; Magnezi et al., 2014) and keeping physically fit, although the quality of evidence in some studies is not strong (Anderson et al., 2014; Carvalho et al., 2014; also see Chapter 4A).
The proposed mechanism by which cognitive reserve operates is through enhancement of neural plasticity. Animal studies indicate that certain factors inhibit or promote the brain’s capacity to generate new neurons, even in adulthood. Negative factors include inflammation, damage from free radical forms of oxygen, and vascular changes associated with age, while positive factors include exercise and mental stimulation (Lee et al., 2012). People with greater cognitive reserve also appear more readily able to access alternate neural networks when their primary networks are damaged (Tucker and Stern, 2011).
In sum, cognitive function in an older person is not solely determined by the amount of pathology associated with brain-related diseases such as Alzheimer’s disease. Rather, the level of cognitive function may represent a balance between the extent of changes in the brain and the brain’s ability to compensate through cognitive reserve.
CHALLENGES AND OPPORTUNITIES IN DEFINING AND ASSESSING COGNITIVE AGING
Relationship Between Cognitive Aging and Functioning in Daily Life
Age-associated changes in cognitive abilities can challenge older people’s ability to perform everyday tasks such as managing medications or finances, negotiating complex environments, or learning something new. This is especially salient in a technology-driven world where people regularly need to learn and interact with new systems and new ways of performing routine tasks. Technology is integral to many aspects of life and is changing how people work, communicate, manage finances and health care, shop, and perform other routine activities. The rapid pace of technological innovation will require that people continue to learn and adapt to new ways
of doing things. Being able to successfully manage these adaptations and to learn new systems and tasks requires cognitive skills and abilities. In essence, cognitive function depends on one’s ability to meet the sensory and cognitive demands imposed by the system, tasks (requirements implicit in the use of the system), and the environment. A thorough understanding of these demands and how cognitive aging affects an older person’s ability to meet them is needed for the development of strategies to successfully negotiate the environment, perform routine tasks, learn new things, and live independently. This in turn requires outcome and performance measures that capture the relevant and critical elements of real-world tasks and environments while maintaining sound psychometric properties.
A broad array of neuropsychological measures can be used to assess and characterize age-related changes in cognition, as detailed earlier in this chapter (see Box 2-1). These measures provide information that is essential to understanding cognitive aging. Furthermore, strong relationships exist between performance on these measures and functional performance, such that individuals who demonstrate higher performance on measures of component cognitive abilities also generally demonstrate higher performance on functional tasks. For example, performance on measures that use working memory and reasoning is indicative of performance on computer-based tasks such as searching the Internet (e.g., Czaja et al., 2010) and the ability to manage medications (Insel et al., 2006; Stilley et al., 2010). Similarly, processing speed and attention are predictors of driving performance (Ball and Owsley, 2003; Ball et al., 1998; Edwards et al., 2008).
However, standard neuropsychological measures do not capture the complexity of real-world activities or a person’s knowledge of everyday tasks. Everyday activities involve a combination of component cognitive abilities and knowledge, and they occur within a context that shapes the demands of a task (Hertzog et al., 2009). For example, an older person using an automated teller machine (ATM) for a banking transaction uses such abilities as working memory and executive function to perform this task. However, using an ATM occurs within a certain context and requires some knowledge of how to use the machine. Performance on this task is likely to be less efficient if the person has limited familiarity with the use of ATMs, if the ATM is located in a sunny area where the display is difficult to read, or if there is a line of people waiting to use the machine, which can create social pressure and anxiety. More generally, performance in any situation is shaped by a number of factors, including the environmental and social contexts, knowledge and prior experience, health status, the demands of the activity, and available support.
Older adults with cognitive impairment are more likely to fall than those with no impairment. Specifically, attentional capacity, as measured by dual-task or time-sharing paradigms, is linked to gait impairment and
the risk of falls. Impairments in executive function may also increase risk of falling (for a review, see Segev-Jacubovski et al., 2011). With regard to spatial abilities, one study found a correlation between impaired spatial cognition and a history of falling, although the sample size was small (Newell et al., 2011). Multimodal interventions that focus on both mobility and cognition may be effective in reducing risk of falling (e.g., Segev-Jacubovski et al., 2011).
Numerous examples in the literature demonstrate that, despite age-related declines on measures of component cognitive abilities, performance on well-learned tasks often shows little decline. For example, although working memory—which typically declines with age—is important to chess performance, it is well-maintained in older adults who actively engage in chess playing (Roring and Charness, 2007). Additionally, studies have shown a relationship between measures of cognitive ability and job performance (e.g., Schmidt and Hunter, 1998, 2004), yet several meta-analyses of age and work performance have found little evidence that older workers are any less productive than younger workers (McEvoy and Cascio, 1989; Waldman and Avolio, 1986). Important factors in the relationship between age and work performance include the demands of the task (e.g., physical demands), worker experience, and the type of performance measures (e.g., supervisory ratings versus some objective measure of performance). Standardized measures of cognition do not capture the complexity of work situations, and for many work tasks older workers are able to use their knowledge, experience, and contextual support mechanisms to compensate for their age-related changes in cognitive ability. In most day-to-day tasks, people are rarely pressed to perform at their maximum level, in contrast to the standards for neuropsychological tests, where the typical expectation is to perform at one’s maximum potential.
Another approach to understanding functional competence is to gather information for a given individual from multiple sources. Individuals may overestimate their performance abilities or have inaccurate judgments regarding the presence of cognitive impairment. An informant, such as a spouse, can be another source of information about functional competence. One study showed that informants could reliably assess cognitive change in individuals with MCI, and their ratings correlated with ratings from objective measures of performance (Tsang et al., 2012). Additional sources of information may be especially important for obtaining reliable data because informant ratings may indicate a greater loss of everyday functional ability and cognitive competency than patient ratings. Informant ratings may also have a greater association with objective measures of cognitive performance than patient ratings (Schinka, 2010). Importantly, informant and self-ratings of performance may not always provide information as to sources of performance difficulties, as they tend to focus on global aspects of performance.
Understanding everyday cognition may require a more ecological approach and the identification of measures that have external and ecological validity. Ecological validity is the ability to generalize results to natural or real-world situations, and it depends on capturing the key elements of environments, tasks, and behaviors. In this case, ecological validity is the extent to which outcome measures capture the relevant features of real-world tasks and environments for activities such as driving, health care, financial management, and so on. There is an ongoing focus on developing and validating these types of measures. For example, the Everyday Cognition Battery (Allaire and Marsiske, 1999) is a set of paper-and-pencil tests that include assessment stimuli related to four everyday activities: medication use, financial planning, food preparation, and nutrition. The Everyday Problems Test is another paper-and-pencil measure designed to assess performance on instrumental activities of daily living (Willis and Marsiske, 1993), including problems with telephone use, shopping, meal preparation, housekeeping, transportation, medication use, and finance. The measure assesses a person’s ability to solve cognitively challenging everyday tasks related to these domains but does not assess the person’s actual ability to perform these tasks in a natural setting. The Revised Observed Tasks of Daily Living (OTDL-R) is a performance measure of everyday problem solving. It includes nine tasks involving medication use, telephone use, and financial management (Diehl et al., 2005). Performance on the OTDL-R has been found to be significantly associated with age, education, self-rated health, paper-and-pencil measures of everyday problem solving, and measures of basic cognitive functioning. However, while competence in basic cognitive abilities is necessary for successfully solving everyday problems, it is not sufficient.
Other investigators have developed ecologically valid simulations of common technology-based work tasks (e.g., Czaja et al., 2001; Sharit and Czaja, 1999) and have also shown that while cognitive abilities are important for performing tasks, other factors such as prior technology experience and the amount of task practice are also important predictors of performance. Researchers have developed a battery of computer-based simulations of common everyday activities, such as the use of an ATM, refilling a prescription, using a ticket kiosk, and medication management (Czaja et al., 2014). Preliminary data indicate that use of the battery is feasible with diverse populations of older adults: It is sensitive to individual differences in abilities, and performance on the battery correlates with performance on standard measures of cognition. And, because real-time performance measures are captured, use of the battery also permits the identification of sources of difficulties in task performance. Such data are critical to the development of strategies that can be used to improve performance. However, these measures need to be validated and normed with larger populations.
Norms and Norming for Cognitive Tests
The cognitive changes seen in aging humans have been documented through cognitive testing in research settings. In the coming years, as a result of public health and health care policy initiatives designed to preserve cognitive health and prevent cognitive losses, cognitive testing will likely become more common outside of these carefully supervised settings, especially among people age 65 years and older. The Centers for Disease Control and Prevention’s Healthy Brain Initiative includes action items to improve surveillance, monitoring, and public awareness of cognitive health and impairment (Alzheimer’s Association and CDC, 2013). Healthy People 2020, the nation’s roadmap for health, has among its goals improving the “health, function, and quality of life of older adults” (HHS, 2014).
Medicare beneficiaries have access to cognitive assessment through the Annual Wellness Exam that includes the detection of “any cognitive impairment” (CMS, 2013). Activities and health insurance benefits such as these will increase the likelihood that older adults will undergo cognitive testing, and the dissemination of computer-based, self-administered technologies will likely facilitate testing in settings where the expertise of a neuropsychologist is not available. Automated technologies to test cognition are increasingly common, such as computer-based, Internet-accessed cognitive testing and “brain exercises.” Typically, these technologies include feedback to participants about whether their performance is “normal.” Although not all of these tests are equally reliable, they may be used by consumers to assess whether they have had cognitive changes that require medical evaluation.
As cognitive testing becomes an increasing part of adults’ lives and of how they conceive of their health, society has a collective interest in ensuring that testing is performed responsibly and accurately. The failure to do so may result in people receiving inappropriate labels concerning their cognitive abilities—labels that can lead to stigma, demoralization, and discrimination, or, on the other hand, false reassurance. It may also thwart the ability of policy makers and public health officials to monitor cognitive health and to estimate the prevalence and severity of cognitive impairment and, therefore, the size and urgency of any problems and the kinds of interventions needed to address them. A foundational issue in ensuring the responsible and accurate use of cognitive testing is to develop and update cognitive norms. The following section examines norms and norming, which are essential to ensuring that evaluation is done responsibly and accurately.
Use of Norms to Interpret Cognitive Test Results
After a tester administers a cognitive test to an individual, such as a measure of memory, the tester has a test score result, also called a “raw score.” To transform this raw score into a description of the person’s cognition, the tester needs to interpret the raw score. Once interpreted, the score becomes a useful measure of that particular person’s cognitive ability.
The key to the interpretation is comparing the score to a standard, a process that is called “norming” (Brooks et al., 2011; Busch et al., 2006; Schretlen et al., 2008). Norming is done for the clinical interpretation of cognitive test results, such as the determination of whether someone has cognitive impairment and, if so, whether it aligns with a pattern seen in a neurodegenerative disease—for example, the amnesia seen in Alzheimer’s disease. However, norming is not always appropriate. For example, norming for age in research studies examining how aging contributes to cognition would confound the ability to detect the effects and thus would not be appropriate in that context.
The tester can use the score to inform answers to a test-taker’s questions, such as “Do I have normal memory?” or “Do I have a disease that is impairing my memory?” To norm a raw score, the tester finds where the individual’s score fits along the distribution of scores of a group of people similar to the individual, called a “normative sample.” A group is “similar to the individual” if it resembles the individual in one or more characteristics, typically health, age, gender, race, and years of education.
The need to create groups of people described by these characteristics follows from research observations that these characteristics are associated with cognitive performance in cognitively healthy people. Grouping people based on age is sensible because people experience changes in cognitive ability as they develop from infancy to adulthood. Similarly, because cognitive test performance correlates with education, tests typically group representative normative samples into tiers based on education. Gender and especially ethnicity and race are more complex characteristics by which to categorize people because they encompass a host of implicit but unmeasured environmental, economic, social, and cultural factors, such as the quality of education and childhood development, which are associated with cognitive performance (Romero et al., 2009). Cognitive tests designed to assist in the diagnosis of cognitive impairment or to understand how cognition changes along the life span typically use normative samples that exclude people with conditions that impair cognition. If these people were included in a normative sample, their presumably lower scores would decrease the ability of a test to either detect disease or measure changes that are not the result of disease.
Once normed, a test result has meaning—the performance on the test
might be interpreted as “average” or “unimpaired,” for example—but this meaning is not absolute. Instead, because of the many characteristics that can be used to group people—some of which, such as race and ethnicity, include other characteristics—a normed score is only a measure of a person’s “relative standing.” It is relative because its meaning comes from comparing the raw score to the normative group that the tester selected (Brooks et al., 2011). For example, a tester may want to assess the memory performance of a 66-year-old woman with MCI compared to other people with MCI. To do this, the tester compares the woman’s score to the scores of a group of people with MCI. The result may show that the woman has memory that is above average for a person with MCI, but when compared to a group of older adults without neurodegenerative disease, her memory is likely to be below average, and when compared to a group of young adults, it is likely to be markedly below average.
Challenges in Developing and Using Norms
The accurate interpretation of an individual’s cognitive test score requires quality data from an appropriate normative sample (Brooks et al., 2011). Achieving this presents challenges, especially in the cognitive assessment of older adults (Busch et al., 2006), and these challenges appear both in the design and in the interpretation of the norms.
Developing norms Creating a normative sample for a test requires a sample size large enough to limit errors from common statistical problems related to outliers and skewed distributions. This is particularly important in normative samples of older adults. Older adults, especially those more than 75 years old, have accumulated many of the age-related factors that increase variability in cognitive performance. Although having large sample sizes helps improve normative samples, the size of the normative samples for older adults is often small (Busch et al., 2006).
Excluding people with conditions that impair cognition is particularly important in the design of tests to diagnose cognitive impairment. However, this is especially challenging to do with older adults because they have medical, psychiatric, and neurological diseases, and sensory losses, and they take medications that can affect their cognitive performance. Overzealous exclusion of these people cuts off the lower end of normal performance, creating a distribution that lacks the full range of normative sampling. As a result, higher-functioning people may be categorized toward the low end of the distribution, leading to an overestimate of the prevalence of impaired functioning.
The challenge of determining whether to exclude people having conditions that impair cognition is compounded by changing diagnostic criteria.
For example, many cognitive tests developed for older adults did not exclude persons with MCI because this diagnosis was recognized only relatively recently. As a result, normative samples that formerly were considered to be free of people with impairments included people who would now be considered impaired (Busch et al., 2006). Changing concepts of disease support the need to revisit normative samples periodically to be sure that a given sample still represents the group it is supposed to represent.
An emerging issue in creating normative samples is whether the samples should include individuals who at the time of assessment were normal but subsequently were recategorized as impaired. This poses a particular challenge with the proposed criteria for preclinical stages of neurodegenerative diseases. Neurodegenerative diseases, such as Alzheimer’s disease, have a prodromal phase prior to onset of MCI or dementia, and perhaps diagnosis using genetic or biomarker tests might be helpful (Dubois et al., 2014; Sperling et al., 2011). Removing from the normative sample the people who later developed MCI or dementia—a practice that is referred to as robust norming—generally changes the original normative sample. These changes can cause people whose test scores are normed using the original sample to be mistakenly labeled as normal, when in fact they may have low normal or impaired cognition (De Santi et al., 2008; Holtzer et al., 2008; Pedraza et al., 2010). However, this outcome was not seen in a biracial sample of rural older adults with low education (Marcopulos and McLain, 2003), perhaps reflecting the challenges of norming cognition in healthy people who have multiple characteristics associated with low test scores independent of disease.
A final challenge in creating norms is that factors associated with cognitive performance themselves vary or undergo changes, which in turn change their association with cognition. Educational attainment is strongly associated with cognitive performance, but the quality of education is not the same from one state or time period to another. Hence, a 70-year-old in 2015 who reports the same number of years of education as a 70-year-old in 2005 may have a different relative standing.
Using norms When a tester is deciding what is the appropriate representative sample against which to norm an individual’s raw cognitive test score, the choice of which sample to use may be obvious, requiring little, if any, judgment. In some cases, however, the individual in question may have characteristics that differ from all of the available samples. Combinations of these differences may become complex—for example, a 75-year-old Latino man who reports a childhood of segregated poverty, learning English at 12 years old, followed by attending college and having a career as a doctor. A normative sample of people with these characteristics may not be available, requiring the tester to judge which group this man most closely resembles.
The failure to address these differences can result in an individual’s cognitive performance being misclassified, an error that can have notable consequences for an individual and even a population. California Verbal Learning Tests norms that used a well-educated sample have incorrectly classified more than 30 percent of people as “below average” or “impaired” (Brooks et al., 2011). The much-reported finding that African Americans are at greater risk of developing Alzheimer’s disease may in fact reflect the lack of adequate representative samples to norm their cognition instead of a greater prevalence of the disease in this population (Barnes and Bennett, 2014).
One solution to the challenges of developing norms and interpreting them for a particular person is repeat testing over time, such as every year. This allows people to serve as their own normative sample. If serial cognitive assessments become more routine—such as being conducted regularly during Annual Wellness Visits—some adults may receive repeated cognitive testing using the same test battery. Documenting their longitudinal performance might have considerable appeal to patients and their health care providers. However, repeat testing can present its own set of challenges. For example, if an individual repeats a test and the score increases, it might indicate cognitive improvement, or it might indicate enhanced performance as a result of previous exposure to the test. In this latter scenario, stable performance scores could mask declines resulting from aging or disease. In addition, feedback on test performance could affect an individual’s performance on subsequent tests. Finally, longitudinal assessment still requires norms so that a tester can determine how much change is expected within the parameters of normal variation as opposed to significant decline.
Future Directions for the Development of Norms
The tester’s selection of the “appropriate normative comparison group is one of the most important aspects of neuropsychological assessment, particularly as it pertains to older adults” (Busch et al., 2006, p. 134). It is important in older adults because they are likely to experience diseases that impair cognition, have age-related changes in cognition, and have accumulated a lifetime of exposures and experiences that affect cognition. Most fundamentally, the selection of a comparison group reflects values that shape how society thinks about and therefore defines cognitive aging. Using a normative sample that is entirely free of all diseases (both clinical and preclinical), conditions, and risk factors that affect cognition would result in the classification of most age-related changes as being “abnormal,” thus embedding the value that most change after development is abnormal. Such decisions need to be addressed directly and with knowledge of their potential implications, such as for the prevalence of disease and the social, cultural, and economic effects. These decisions can be informed by examin-
ing how other systems account for aging when using norms. For example, the normative values for hemoglobin, blood glucose, and bone mineral density are not adjusted for age. As a result, more people are diagnosed with diseases such as anemia, diabetes, and osteoporosis, respectively. The committee decided that some cognitive changes are to be expected with aging, and this is reflected in the committee’s definition of cognitive aging in Chapter 1.
Public health and health care policy initiatives to preserve cognitive health and prevent cognitive losses require a national investment to develop and update high-quality normative samples that are large and sufficiently representative of the demography of Americans—an investment similar to what the United States has made to discover and validate the biomarkers of neurodegenerative diseases (Weiner et al., 2010). These samples should be followed up in order to assess baseline factors that may affect norms, ways that practice effects influence test results, and the interactions between baseline factors and practice effects. This is especially important as researchers define preclinical stages of neurodegenerative diseases.
Measuring literacy rather than a simple count of the years of education may be especially useful in addressing challenges related to quality of education (Dotson et al., 2008; Manly et al., 2005). As measures are developed for widespread clinical and research application using remote technologies and without the supervision of a trained tester, individual users would benefit from knowing how well the measures are normed for people like them. A clearinghouse that compares how individuals perform on different batteries would be useful for assuring the reliable interpretation of results. This investment will allow testers to readily judge which group is the best comparison group for each person so that individuals can receive meaningful answers to important questions about their cognitive health and so that policy makers will have reliable data to inform the surveillance of the nation’s cognitive health and the need for interventions.
RECOMMENDATION
Recommendation 1: Increase Research and Tools for Assessing Cognitive Aging and Cognitive Trajectories
The National Institutes of Health, the Centers for Disease Control and Prevention, research foundations, academic research institutions, and private-sector companies should expand research on the trajectories of cognitive aging and improve the tools used to assess cognitive changes and their effects on daily function.
Specific needs include
- Studies using a range of assessments (e.g., neuronal injury biomarkers, neuroimaging, postmortem assessments of neu-
- ronal integrity) to explore the physiological and structural basis of cognitive aging;
- Non-human animal studies that examine the mechanisms and clinical correlates of cognitive aging and that are designed to inform human cognitive aging;
- Studies to examine the mechanisms underlying interventions that affect the cognitive trajectory;
- Studies to identify and validate novel tools and measures of function that capture the complexities of real-world tasks and are sensitive to early changes in cognition and function; and
- An update of the norms for cognitive function in older adults (including those in the most advanced age groups) to include the consideration of disease, literacy, language, racial and ethnic diversity, culture, and socioeconomic factors.
REFERENCES
Abrams, L., and J. H. Stanley. 2004. The detection and retrieval of spelling in older adults. In Advances in psychology research, Vol. 33, edited by S. P. Shohov. Hauppauge, NY: Nova Science Publishers. Pp. 87-109.
Agarwal, S., J. Driscoll, X. Gabaix, and D. Laibson. 2009. The age of reason: Financial decisions over the life cycle and implications for regulations. Washington, DC: Brookings Institution. http://www.brookings.Edu/~/media/Projects/BPEA/Fall%202009/2009b_bpea_agarwal.pdf (accessed February 24, 2015).
Albert, M. S., J. Wolfe, and G. Lafleche. 1990. Differences in abstraction ability with age. Psychology and Aging 5(1):94-100.
Albert, M. S., S. T. DeKosky, D. Dickson, B. Dubois, H. H. Feldman, N. C. Fox, A. Gamst, D. M. Holtzman, W. J. Jagust, R. C. Petersen, P. J. Snyder, M. C. Carrillo, B. Thies, and C. H. Phelps. 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3):270-279.
Allaire, J. C., and M. Marsiske. 1999. Everyday cognition: Age and intellectual ability correlates. Psychology and Aging 14(4):627-644.
Alzheimer’s Association and CDC (Centers for Disease Control and Prevention). 2013. The Healthy Brain Initiative: The public health road map for state and national partnerships, 2013-2018. Chicago, IL: Alzheimer’s Association. http://www.cdc.gov/aging/pdf/2013healthy-brain-initiative.pdf (accessed February 25, 2015).
Amaral, D. G. 1993. Morphological analyses of the brain of behaviorally characterized aged nonhuman primates. Neurobiology of Aging 14:671-672.
Anderson, D., C. Seib, and L. Rasmussen. 2014. Can physical activity prevent physical and cognitive decline in postmenopausal women?: A systematic review of the literature. Maturitas 79(1):14-33.
Anderson, J. M., B. M. Hubbard, G. R. Coghill, and W. Slidders. 1983. The effect of advanced old age on the neurone content of the cerebral cortex. Observations with an automatic image analyser point counting method. Journal of the Neurological Sciences 58(2):235-246.
APA (American Psychiatric Association). 2013. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association.
Ardelt, M. 2004. Wisdom as expert knowledge system: A critical review of a contemporary operationalization of an ancient concept. Human Development 47(5):257-285.
Ardelt, M., and O. Hunhui. 2010. Wisdom: Definition, assessment, and its relation to successful cognitive and emotional aging. In Successful cognitive and emotional aging, edited by D. Jeste and C. Depp. Washington, DC: American Psychiatric Publishing. Pp. 87-113.
Arnsten, A. F., and P. S. Goldman-Rakic. 1990. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiology of Aging 11(6):583-590.
Bachevalier, J. 1993. Behavioral changes in aged rhesus monkeys. Neurobiology of Aging 14(6):619-621.
Bachevalier, J., L. S. Landis, L. C. Walker, M. Brickson, M. Mishkin, D. L. Price, and L. C. Cork. 1991. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiology of Aging 12(2):99-111.
Backman, L., B. J. Small, and A. Wahlin. 2001. Aging and memory: Cognitive and biological perspectives. In Handbook of the psychology of aging, 5th ed., edited by J. E. Birren and K. W. Schaie. San Diego, CA: Academic Press. Pp. 348-376.
Ball, K., and C. Owsley. 2003. Driving competence: It’s not a matter of age. Journal of the American Geriatrics Society 51(10):1499-1501.
Ball, K., C. Owsley, B. Stalvey, D. L. Roenker, M. E. Sloane, and M. Graves. 1998. Driving avoidance and functional impairment in older drivers. Accident Analysis and Prevention 30(3):313-322.
Ball, K., D. B. Berch, K. F. Helmers, J. B. Jobe, M. D. Leveck, M. Marsiske, J. N. Morris, G. W. Rebok, D. M. Smith, S. L. Tennstedt, F. W. Unverzagt, and S. L. Willis. 2002. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA 288(18):2271-2281.
Baltes, P. B., and J. Smith. 1990. Toward a psychology of wisdom and its ontogenesis. In Wisdom: Its nature, origins, and development, edited by R. J. Sternberg. New York: Cambridge University Press. Pp. 87-120.
Barense, M. D., M. T. Fox, and M. G. Baxter. 2002. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learning and Memory 9(4):191-201.
Barnes, L. L., and D. A. Bennett. 2014. Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Affairs 33(4):580-586.
Bartus, R. T., R. L. Dean, 3rd, and D. L. Fleming. 1979. Aging in the rhesus monkey: Effects on visual discrimination learning and reversal learning. Journal of Gerontology 34(2):209-219.
Baxter, M. G., and M. L. Voytko. 1996. Spatial orienting of attention in adult and aged rhesus monkeys. Behavioral Neuroscience 110(5):898-904.
Bell-McGinty, S., K. Podell, M. Franzen, A. D. Baird, and M. J. Williams. 2002. Standard measures of executive function in predicting instrumental activities of daily living in older adults. International Journal of Geriatric Psychiatry 17(9):828-834.
Berardi, A., R. Parasuraman, and J. V. Haxby. 2001. Overall vigilance and sustained attention decrements in healthy aging. Experimental Aging Research 27(1):19-39.
Birren, J. E. 1970. Toward an experimental psychology of aging. American Psychologist 25(2):124-135.
Bizon, J. L., T. C. Foster, G. E. Alexander, and E. L. Glisky. 2012. Characterizing cognitive aging of working memory and executive function in animal models. Frontiers in Aging Neuroscience 4:1-19.
Bouras, C., P. R. Hof, P. Giannakopoulos, J. P. Michel, and J. H. Morrison. 1994. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: A quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cerebral Cortex 4(2):138-150.
Brickman, A. M., and Y. Stern. 2009. Aging and memory in humans. In Encyclopedia of neuroscience, Vol. 1, edited by L. Squire. Oxford: Academic Press. Pp. 175-180.
Brody, H. 1955. Organization of the cerebral cortex III: A study of aging in the human cerebral cortex. Journal of Comparative Neurology 102(2):511-516.
———. 1970. Structural changes in the aging nervous system. Interdisciplinary Topics in Gerontology 7:9-21.
Brooks, B., E. S. Sherman, G. Iverson, D. Slick, and E. Strauss. 2011. Psychometric foundations for the interpretation of neuropsychological test results. In The little black book of neuropsychology: A syndrome-based approach, edited by M. R. Schoenberg and J. G. Scott. New York: Springer. Pp. 893-922.
Burke, D. M., and M. A. Shafto. 2008. Language and aging. In The handbook of aging and cognition, edited by F. I. Craik and T. A. Salthouse. New York: Psychology Press. Pp. 373-443.
Busch, R., G. Chelune, and Y. Suchy. 2006. Using norms in neuropsychological assessment of the elderly. In Geriatric neuropsychology: Assessment and intervention, edited by D. K. Attix and K. A. Welsh-Bohmer. New York: Guilford Press. Pp. 133-157.
Carriere, J. S., J. A. Cheyne, G. J. Solman, and D. Smilek. 2010. Age trends for failures of sustained attention. Psychology and Aging 25(3):569-574.
Carvalho, A., I. M. Rea, T. Parimon, and B. J. Cusack. 2014. Physical activity and cognitive function in individuals over 60 years of age: A systematic review. Clinical Interventions in Aging 9:661-682.
Cavoy, A., and J. Delacour. 1993. Spatial but not object recognition is impaired by aging in rats. Physiology and Behavior 53(3):527-530.
CDC (Centers for Disease Control and Prevention). 2013. Self-reported increased confusion or memory loss and associated functional difficulties among adults aged ≥ 60 years—21 states, 2011. Morbidity and Mortality Weekly Report 62(18):347-350.
Clay, O. J., V. G. Wadley, J. D. Edwards, D. L. Roth, D. L. Roenker, and K. K. Ball. 2005. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: Current and future implications. Optometry and Vision Science 82(8):724-731.
CMS (Centers for Medicare & Medicaid Services). 2013. Quick reference information: The ABCs of providing the Annual Wellness Visit (AWV). http://www.cms.gov/Outreach-andEducation/Medicare-Learning-Network-MLN/MLNProducts/downloads/AWV_chart_ICN905706.pdf (accessed February 23, 2015).
Colon, E. J. 1972. The elderly brain: A quantitative analysis in the cerebral cortex of two cases. Psychiatria, Neurologia, Neurochirurgia 75(4):261-270.
Craik, F. I. M., and J. M. Jennings. 1992. Human memory. In The handbook of aging and cognition, edited by F. I. M. Craik and T. A. Salthouse. Hillsdale, NJ: Erlbaum. Pp. 51-110.
Craik, F. I. M., and J. M. McDowd. 1987. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition 13:474-479.
Crystal, H., D. Dickson, P. Fuld, D. Masur, R. Scott, M. Mehler, J. Masdeu, C. Kawas, M. Aronson, and L. Wolfson. 1988. Clinico-pathologic studies in dementia: Nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38(11):1682-1687.
Czaja, S. J., J. Sharit, R. Ownby, D. L. Roth, and S. Nair. 2001. Examining age differences in performance of a complex information search and retrieval task. Psychology and Aging 16(4):564-579.
Czaja, S. J., J. Sharit, M. A. Hernandez, S. N. Nair, and D. Loewenstein. 2010. Variability among older adults in internet health information-seeking performance. Gerontechnology 9(1):46-55.
Czaja, S. J., J. Sharit, C. C. Lee, S. N. Nair, M. A. Hernandez, N. Arana, and S. H. Fu. 2013. Factors influencing use of an e-health website in a community sample of older adults. Journal of the American Medical Informatics Association 20(2):277-284.
Czaja, S. J., P. D. Harvey, and D. Loewenstein. 2014. Development and evaluation of a novel technology-based functional assessment package. Paper presented at Society of Biological Psychiatry 69th Annual Scientific Meeting, New York.
De Santi, S., E. Pirraglia, W. Barr, J. Babb, S. Williams, K. Rogers, L. Glodzik, M. Brys, L. Mosconi, B. Reisberg, S. Ferris, and M. J. de Leon. 2008. Robust and conventional neuropsychological norms: Diagnosis and prediction of age-related cognitive decline. Neuropsychology 22(4):469-484.
Denburg, N. L., C. A. Cole, M. Hernandez, T. H. Yamada, D. Tranel, A. Bechara, and R. B. Wallace. 2007. The orbitofrontal cortex, real-world decision making, and normal aging. Annals of the New York Academy of Sciences 1121:480-498.
Diehl, M., M. Marsiske, A. L. Horgas, A. Rosenberg, J. S. Saczynski, and S. L. Willis. 2005. The Revised Observed Tasks of Daily Living: A performance-based assessment of everyday problem solving in older adults. Journal of Applied Gerontology 24(3):211-230.
Dobson, S. H., K. C. Kirasic, and G. L. Allen. 1995. Age-related differences in adults’ spatial task performance: Influences of task complexity and perceptual speed. Aging and Cognition 2:19-38.
Dotson, V. M., M. Kitner-Triolo, M. K. Evans, and A. B. Zonderman. 2008. Literacy-based normative data for low socioeconomic status African Americans. The Clinical Neuropsychologist 22(6):989-1017.
Dubois, B., H. H. Feldman, C. Jacova, H. Hampel, J. L. Molinuevo, K. Blennow, S. T. DeKosky, S. Gauthier, D. Selkoe, R. Bateman, S. Cappa, S. Crutch, S. Engelborghs, G. B. Frisoni, N. C. Fox, D. Galasko, M. O. Habert, G. A. Jicha, A. Nordberg, F. Pasquier, G. Rabinovici, P. Robert, C. Rowe, S. Salloway, M. Sarazin, S. Epelbaum, L. C. de Souza, B. Vellas, P. J. Visser, L. Schneider, Y. Stern, P. Scheltens, and J. L. Cummings. 2014. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurology 13(6):614-629.
Dumitriu, D., J. Hao, Y. Hara, J. Kaufmann, W. G. Janssen, W. Lou, P. R. Rapp, and J. H. Morrison. 2010. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. The Journal of Neuroscience 30(22):7507-7515.
Edwards, J. D., L. A. Ross, M. L. Ackerman, B. J. Small, K. K. Ball, S. Bradley, and J. E. Dodson. 2008. Longitudinal predictors of driving cessation among older adults from the ACTIVE clinical trial. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences 63(1):6-12.
Eriksson, P. S., E. Perfilieva, T. Bjork-Eriksson, A. M. Alborn, C. Nordborg, D. A. Peterson, and F. H. Gage. 1998. Neurogenesis in the adult human hippocampus. Nature Medicine 4(11):1313-1317.
Gazzaley, A. H., M. M. Thakker, P. R. Hof, and J. H. Morrison. 1997. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiology of Aging 18(5):549-553.
Glisky, E. L., S. R. Rubin, and P. S. Davidson. 2001. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition 27(5):1131-1146.
Gomez-Isla, T., J. L. Price, D. W. McKeel, Jr., J. C. Morris, J. H. Growdon, and B. T. Hyman. 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. Journal of Neuroscience 16(14):4491-4500.
Gould, E., A. J. Reeves, M. S. Graziano, and C. G. Gross. 1999. Neurogenesis in the neocortex of adult primates. Science 286(5439):548-552.
Graham, C. L., A. E. Scharlach, and J. Price Wolf. 2014. The impact of the “village” model on health, well-being, service access, and social engagement of older adults. Health Education and Behavior 41(1 Suppl):91s-97s.
Greenwood, P. M., R. Parasuraman, and J. V. Haxby. 1993. Changes in visuospatial attention over the adult lifespan. Neuropsychologia 31(5):471-485.
Hara, Y., M. Punsoni, F. Yuk, C. S. Park, W. G. Janssen, P. R. Rapp, and J. H. Morrison. 2012. Synaptic distributions of GluA2 and PKMzeta in the monkey dentate gyrus and their relationships with aging and memory. Journal of Neuroscience 32(21):7336-7344.
Henderson, G., B. E. Tomlinson, and P. H. Gibson. 1980. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. Journal of the Neurological Sciences 46(1):113-136.
Henry, J. D., M. S. MacLeod, L. H. Phillips, and J. R. Crawford. 2004. A meta-analytic review of prospective memory and aging. Psychology and Aging 19(1):27-39.
Herndon, J. G., M. B. Moss, D. L. Rosene, and R. J. Killiany. 1997. Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research 87(1):25-34.
Hertzog, C., and B. Rypma. 1991. Age differences in component of mental-rotation task performance. Bulletin of the Psychonomic Society 29:209-212.
Hertzog, C., A. F. Kramer, R. S. Wilson, and U. Lindenberger. 2009. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest 9(1):1-65.
HHS (U.S. Department of Health and Human Services). 2014. Healthy people 2020. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=7 (accessed October 24, 2014).
Holtzer, R., Y. Goldin, M. Zimmerman, M. Katz, H. Buschke, and R. B. Lipton. 2008. Robust norms for selected neuropsychological tests in older adults. Archives of Clinical Neuropsychology 23(5):531-541.
Insel, K., D. Morrow, B. Brewer, and A. Figueredo. 2006. Executive function, working memory, and medication adherence among older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences 61(2):102-107.
Jeste, D. V., and A. J. Oswald. 2014. Individual and societal wisdom: Explaining the paradox of human aging and high well-being. Psychiatry: Interpersonal and Biological Processes 77(4):317-330.
Johansson, B., S. M. Hofer, J. C. Allaire, M. M. Maldonado-Molina, A. M. Piccinin, S. Berg, N. L. Pedersen, and G. E. McClearn. 2004. Change in cognitive capabilities in the oldest old: The effects of proximity to death in genetically related individuals over a 6-year period. Psychology and Aging 19(1):145-156.
Jones, D. N., J. C. Barnes, D. L. Kirkby, and G. A. Higgins. 1995. Age-associated impairments in a test of attention: Evidence for involvement of cholinergic systems. Journal of Neuroscience 15(11):7282-7292.
Kaplan, E., H. Goodglass, and S. Weintrab. 1983. The Boston Naming Test. Philadelphia, PA: Lea and Febiger.
Katzman, R., R. Terry, R. DeTeresa, T. Brown, P. Davies, P. Fuld, X. Renbing, and A. Peck. 1988. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology 23(2):138-144.
Kemper, S., and R. E. Herman. 2006. Age differences in memory-load interference effects in syntactic processing. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences 61(6):327-332.
Kemper, S., and A. Sumner. 2001. The structure of verbal abilities in young and older adults. Psychology and Aging 16(2):312-322.
Kemtes, K. A., and S. Kemper. 1997. Younger and older adults’ on-line processing of syntactically ambiguous sentences. Psychology and Aging 12(2):362-371.
Kornack, D. R., and P. Rakic. 1999. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proceedings of the National Academy of Sciences of the United States of America 96(10):5768-5773.
Kramer, A. F., and D. J. Madden. 2008. Attention. In The handbook of aging and cognition, 3rd ed., edited by F. I. Craik and T. A. Salthouse. New York: Psychology Press. Pp. 189-249.
Kunzmann, U., and P. B. Baltes. 2003. Wisdom-related knowledge: Affective, motivational, and interpersonal correlates. Personality and Social Psychology Bulletin 29(9):1104-1119.
Lachman, M. E., and E. Jelalian. 1984. Self-efficacy and attributions for intellectual performance in young and elderly adults. Journal of Gerontology 39(5):577-582.
Lai, Z. C., M. B. Moss, R. J. Killiany, D. L. Rosene, and J. G. Herndon. 1995. Executive system dysfunction in the aged monkey: Spatial and object reversal learning. Neurobiology of Aging 16(6):947-954.
Lee, S. W., G. D. Clemenson, and F. H. Gage. 2012. New neurons in an aged brain. Behavioural Brain Research 227(2):497-507.
Light, L. L., and E. M. Zelinski. 1983. Memory for spatial information in young and old adults. Developmental Psychology 19(6):901-906.
Magnezi, R., Y. S. Bergman, and D. Grosberg. 2014. Online activity and participation in treatment affects the perceived efficacy of social health networks among patients with chronic illness. Journal of Medical Internet Research 16(1):e12.
Manly, J. J., N. Schupf, M. X. Tang, and Y. Stern. 2005. Cognitive decline and literacy among ethnically diverse elders. Journal of Geriatric Psychiatry and Neurology 18(4):213-217.
Marcopulos, B., and C. McLain. 2003. Are our norms “normal”? A 4-year follow-up study of a biracial sample of rural elders with low education. The Clinical Neuropsychologist 17(1):19-33.
Maylor, E. A., G. Smith, S. Della Sala, and R. H. Logie. 2002. Prospective and retrospective memory in normal aging and dementia: An experimental study. Memory and Cognition 30(6):871-884.
McArdle, J. 2011. Longitudinal dynamic analyses of cognition in the health and retirement study panel. Advances in Statistical Analysis 95(4):453-480.
McDowd, J. M., and R. J. Shaw. 2000. Attention and aging: A functional perspective. In The handbook of aging and cognition, edited by T. A. Salthouse and F. I. M. Craik. Mahwah, NJ: Erlbaum. Pp. 221-292.
McEvoy, G. M., and W. F. Cascio. 1989. Cumulative evidence of the relationship between employee age and job performance. Journal of Applied Psychology 74:11-17.
McKhann, G. M., D. S. Knopman, H. Chertkow, B. T. Hyman, C. R. Jack, Jr., C. H. Kawas, W. E. Klunk, W. J. Koroshetz, J. J. Manly, R. Mayeux, R. C. Mohs, J. C. Morris, M. N. Rossor, P. Scheltens, M. C. Carrillo, B. Thies, S. Weintraub, and C. H. Phelps. 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3):263-269.
Mick, P., I. Kawachi, and F. R. Lin. 2014. The association between hearing loss and social isolation in older adults. Otolaryngology-Head and Neck Surgery 150(3):378-384.
Mitchell, D. B. 1989. How many memory systems? Evidence from aging. Journal of Experimental Psychology: Learning, Memory, and Cognition 15(1):31-49.
Moore, H., P. Dudchenko, J. P. Bruno, and M. Sarter. 1992. Toward modeling age-related changes of attentional abilities in rats: Simple and choice reaction time tasks and vigilance. Neurobiology of Aging 13(6):759-772.
Moore, T. L., R. J. Killiany, J. G. Herndon, D. L. Rosene, and M. B. Moss. 2003. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiology of Aging 24(1):125-134.
Morris, J. C., M. Storandt, D. W. McKeel, Jr., E. H. Rubin, J. L. Price, E. A. Grant, and L. Berg. 1996. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46(3):707-719.
Morris, R. G., P. Garrud, J. N. Rawlins, and J. O’Keefe. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297(5868):681-683.
Morrison, J. H., and M. G. Baxter. 2012. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nature Reviews Neuroscience 13(4):240-250.
———. 2014. Synaptic health. JAMA Psychiatry 71(7):835-837.
Morrison, J. H., and P. R. Hof. 1997. Life and death of neurons in the aging brain. Science 278(5337):412-419.
Moss, M. B., D. L. Rosene, and A. Peters. 1988. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiology of Aging 9(5-6):495-502.
Moss, M. B., R. J. Killiany, Z. C. Lai, D. L. Rosene, and J. G. Herndon. 1997. Recognition memory span in rhesus monkeys of advanced age. Neurobiology of Aging 18(1):13-19.
Muir, J. L., W. Fischer, and A. Bjorklund. 1999. Decline in visual attention and spatial memory in aged rats. Neurobiology of Aging 20(6):605-615.
Newell, F. N., A. Setti, T. Foran, K. Burke, and R. A. Kenny. 2011. Reduced vision impairs spatial cognition in fall-prone older adults. INSIGHT: Research and Practice in Visual Impairment and Blindness 4(3):103-111.
Nielsen, K., and A. Peters. 2000. The effects of aging on the frequency of nerve fibers in rhesus monkey striate cortex. Neurobiology of Aging 21(5):621-628.
Nilsson, L. G. 2003. Memory function in normal aging. Acta Neurologica Scandinavica 179:7-13.
Norris, C. M., D. L. Korol, and T. C. Foster. 1996. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. The Journal of Neuroscience 16(17):5382-5392.
O’Donnell, K. A., P. R. Rapp, and P. R. Hof. 1999. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Experimental Neurology 160(1):300-310.
Pak, R., S. J. Czaja, J. Sharit, W. A. Rogers, and A. D. Fisk. 2006a. The role of spatial abilities and age in performance in an auditory computer navigation task. Computers in Human Behavior 24(6):3045-3051.
Pak, R., W. A. Rogers, and A. D. Fisk. 2006b. Spatial ability subfactors and their influences on a computer-based information search task. Human Factors 48(1):154-165.
Parasuraman, R., P. Nestor, and P. Greenwood. 1989. Sustained-attention capacity in young and older adults. Psychology and Aging 4(3):339-345.
Pedraza, O., J. A. Lucas, G. E. Smith, R. C. Petersen, N. R. Graff-Radford, and R. J. Ivnik. 2010. Robust and expanded norms for the dementia rating scale. Archives of Clinical Neuropsychology 25(5):347-358.
Peters, A. 1996. Age-related changes in oligodendrocytes in monkey cerebral cortex. Journal of Comparative Neurology 371:153-163.
Peters, A., D. Leahu, M. B. Moss, and K. J. McNally. 1994. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cerebral Cortex 4(6):621-635.
Peters, A., J. H. Morrison, D. L. Rosene, and B. T. Hyman. 1998. Are neurons lost from the primate cerebral cortex during normal aging? Cerebral Cortex 8(4):295-300.
Petersen, R. C. 2004. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 256(3):183-194.
Plude, D. J., W. P. Milberg, and J. Cerella. 1986. Age differences in depicting and perceiving tridimensionality in simple line drawings. Experimental Aging Research 12(4):221-225.
Presty, S. K., J. Bachevalier, L. C. Walker, R. G. Struble, D. L. Price, M. Mishkin, and L. C. Cork. 1987. Age differences in recognition memory of the rhesus monkey (Macaca mulatta). Neurobiology of Aging 8(5):435-440.
Price, J. L., and J. C. Morris. 1999. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of Neurology 45(3):358-368.
Rapp, P. R. 1990. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta). Behavioral Neuroscience 104(6):876-884.
Rapp, P. R., and D. G. Amaral. 1989. Evidence for task-dependent memory dysfunction in the aged monkey. Journal of Neuroscience 9(10):3568-3576.
Rapp, P. R., and M. Gallagher. 1996. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proceedings of the National Academy of Sciences of the United States of America 93(18):9926-9930.
Rapp, P. R., R. A. Rosenberg, and M. Gallagher. 1987. An evaluation of spatial information processing in aged rats. Behavioral Neuroscience 101(1):3-12.
Rapp, P. R., P. S. Deroche, Y. Mao, and R. D. Burwell. 2002. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cerebral Cortex 12(11):1171-1179.
Reitan, R. M. 1955. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology 19(5):393-394.
Robbins, T. W., and A. F. Arnsten. 2009. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annual Review of Neuroscience 32:267-287.
Robbins, T. W., M. James, A. M. Owen, B. J. Sahakian, A. D. Lawrence, L. McInnes, and P. M. Rabbitt. 1998. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. Journal of the International Neuropsychological Society 4(5):474-490.
Romero, H. R., S. K. Lageman, V. V. Kamath, F. Irani, A. Sim, P. Suarez, J. J. Manly, and D. K. Attix. 2009. Challenges in the neuropsychological assessment of ethnic minorities: Summit proceedings. The Clinical Neuropsychologist 23(5):761-779.
Roring, R. W., and N. Charness. 2007. A multilevel model analysis of expertise in chess across the life span. Psychology and Aging 22(2):291-299.
Rosene, D. L. 1993. Comparing age-related changes in the basal forebrain and hippocampus of the rhesus monkey. Neurobiology of Aging 14(6):669-670.
Rosenzweig, E. S., and C. A. Barnes. 2003. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Progress in Neurobiology 69(3):143-179.
Salthouse, T. A. 1984. Effects of age and skill in typing. Journal of Experimental Psychology: General 113(3):345-371.
———. 1994. The aging of working memory. Neuropsychology 8(4):535-543.
———. 2004. What and when of cognitive aging. Current Directions in Psychological Science 13(4):140-144.
Samanez-Larkin, G. R., S. M. Levens, L. M. Perry, R. F. Dougherty, and B. Knutson. 2012. Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. Journal of Neuroscience 32(15):5333-5337.
Scarmeas, N., and Y. Stern. 2004. Cognitive reserve: Implications for diagnosis and prevention of Alzheimer’s disease. Current Neurology and Neuroscience Reports 4(5):374-380.
Schaie, K. W. 1983. The Seattle Longitudinal Study: A twenty-one year exploration of psychometric intelligence in adulthood. In Longitudinal studies of adult psychological development, edited by K. W. Schaie. New York: Guilford Press. Pp. 64-135.
———. 1994. The course of adult intellectual development. American Psychologist 49(4): 304-313.
———. 1996. Intellectual development in adulthood: The Seattle Longitudinal Study. New York: Cambridge University Press.
———. 2005. What can we learn from longitudinal studies of adult development? Research in Human Development 2(3):133-158.
Scheff, S. W., D. A. Price, F. A. Schmitt, and E. J. Mufson. 2006. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiology of Aging 27(10):1372-1384.
Schinka, J. A. 2010. Use of informants to identify mild cognitive impairment in older adults. Current Psychiatry Reports 12(1):4-12.
Schmidt, F. L., and J. E. Hunter. 1998. The validity and utility of selection methods in personnel psychology: Practical and theoretical implications of 85 years of research findings. Psychological Bulletin 124(2):262-274.
———. 2004. General mental ability in the world of work: Occupational attainment and job performance. Journal of Personality and Social Psychology 86(1):162-173.
Schretlen, D. J., S. M. Testa, J. M. Winicki, G. D. Pearlson, and B. Gordon. 2008. Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. Journal of the International Neuropsychological Society 14(3):436-445.
Segev-Jacubovski, O., T. Herman, G. Yogev-Seligmann, A. Mirelman, N. Giladi, and J. M. Hausdorff. 2011. The interplay between gait, falls and cognition: Can cognitive therapy reduce fall risk? Expert Review of Neurotherapeutics 11(7):1057-1075.
Sharit, J., and S. J. Czaja. 1999. Performance of a complex computer-based troubleshooting task in the bank industry. International Journal of Cognitive Ergonomics and Human Factors 3:1-22.
Sharit, J., M. A. Hernandez, S. J. Czaja, and P. Pirolli. 2008. Investigating the roles of knowledge and cognitive abilities in older adult information seeking on the web. ACM Transactions on Computer-Human Interaction 15(1):3.
Shefer, V. F. 1973. Absolute number of neurons and thickness of the cerebral cortex during aging, senile and vascular dementia, and Pick’s and Alzheimer’s diseases. Neuroscience and Behavioral Physiology 6(4):319-324.
Singer, T., P. Verhaeghen, P. Ghisletta, U. Lindenberger, and P. B. Baltes. 2003. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin Aging Study (BASE). Psychology and Aging 18(2):318-331.
Smith, T. D., M. M. Adams, M. Gallagher, J. H. Morrison, and P. R. Rapp. 2000. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. Journal of Neuroscience 20(17):6587-6593.
Spaniol, J., D. J. Madden, and A. Voss. 2006. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition 32(1):101-117.
Spencer, W. D., and N. Raz. 1995. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging 10(4):527-539.
Sperling, R. A., P. S. Aisen, L. A. Beckett, D. A. Bennett, S. Craft, A. M. Fagan, T. Iwatsubo, C. R. Jack, Jr., J. Kaye, T. J. Montine, D. C. Park, E. M. Reiman, C. C. Rowe, E. Siemers, Y. Stern, K. Yaffe, M. C. Carrillo, B. Thies, M. Morrison-Bogorad, M. V. Wagster, and C. H. Phelps. 2011. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3):280-292.
Staudinger, U. M. 1999. Older and wiser? Integrating results on the relationship between age and wisdom-related performance. International Journal of Behavioral Development 23(3):641-664.
Stilley, C. S., C. M. Bender, J. Dunbar-Jacob, S. Sereika, and C. M. Ryan. 2010. The impact of cognitive function on medication management: Three studies. Health Psychology 29(1):50-55.
Stine, E. A. 1990. On-line processing of written text by younger and older adults. Psychology and Aging 5(1):68-78.
Stine-Morrow, E. A., M. K. Loveless, and L. M. Soederberg. 1996. Resource allocation in online reading by younger and older adults. Psychology and Aging 11(3):475-486.
Taha, J., S. J. Czaja, J. Sharit, and D. G. Morrow. 2013. Factors affecting usage of a personal health record (PHR) to manage health. Psychology and Aging 28(4):1124-1139.
Techentin, C., D. Voyer, and S. D. Voyer. 2014. Spatial abilities and aging: A meta-analysis. Experimental Aging Research 40(4):395-425.
Terry, R. D., R. DeTeresa, and L. A. Hansen. 1987. Neocortical cell counts in normal human adult aging. Annals of Neurology 21(6):530-539.
Trott, C. T., D. Friedman, W. Ritter, and M. Fabiani. 1997. Item and source memory: Differential age effects revealed by event-related potentials. Neuroreport 8(15):3373-3378.
Tsang, P. S., and T. L. Shaner. 1998. Age, attention, expertise, and time-sharing performance. Psychology and Aging 13(2):323-347.
Tsang, R. S., K. Diamond, L. Mowszowski, S. J. Lewis, and S. L. Naismith. 2012. Using informant reports to detect cognitive decline in mild cognitive impairment. International Psychogeriatrics 24(6):967-973.
Tucker, A. M., and Y. Stern. 2011. Cognitive reserve in aging. Current Alzheimer Research 8(4):354-360.
Verhaeghen, P., and J. Cerella. 2002. Aging, executive control, and attention: A review of meta-analyses. Neuroscience and Biobehavioral Reviews 26(7):849-857.
Vincent, S. L., A. Peters, and J. Tigges. 1989. Effects of aging on the neurons within area 17 of rhesus monkey cerebral cortex. The Anatomical Record 223(3):329-341.
Waldman, D. A., and B. J. Avolio. 1986. A meta-analysis of age differences in job performance. Journal of Applied Psychology 71:33-38.
Wang, M., N. J. Gamo, Y. Yang, L. E. Jin, X. J. Wang, M. Laubach, J. A. Mazer, D. Lee, and A. F. Arnsten. 2011. Neuronal basis of age-related working memory decline. Nature 476(7359):210-213.
Weiner, M. W., P. S. Aisen, C. R. Jack, Jr., W. J. Jagust, J. Q. Trojanowski, L. Shaw, A. J. Saykin, J. C. Morris, N. Cairns, L. A. Beckett, A. Toga, R. Green, S. Walter, H. Soares, P. Snyder, E. Siemers, W. Potter, P. E. Cole, and M. Schmidt. 2010. The Alzheimer’s Disease Neuroimaging Initiative: Progress report and future plans. Alzheimer’s & Dementia 6(3):202-211.
Weintraub, S., S. S. Dikmen, R. K. Heaton, D. S. Tulsky, P. D. Zelazo, P. J. Bauer, N. E. Carlozzi, J. Slotkin, D. Blitz, K. Wallner-Allen, N. A. Fox, J. L. Beaumont, D. Mungas, C. J. Nowinski, J. Richler, J. A. Deocampo, J. E. Anderson, J. J. Manly, B. Borosh, R. Havlik, K. Conway, E. Edwards, L. Freund, J. W. King, C. Moy, E. Witt, and R. C. Gershon. 2013. Cognition assessment using the NIH toolbox. Neurology 80(11 Suppl 3):S54-S64.
West, M. J. 1993. Regionally specific loss of neurons in the aging human hippocampus. Neurobiology of Aging 14:287-293.
Willis, S. L., and M. Marsiske. 1993. Manual for the Everyday Problems Test. University Park: Pennsylvania State University.
Willis, S. L., and K. W. Schaie. 1986. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychology and Aging 1(3):239-247.
Winblad, B., K. Palmer, M. Kivipelto, V. Jelic, L. Fratiglioni, L. O. Wahlund, A. Nordberg, L. Bäckman, M. Albert, O. Almkvist, H. Arai, H. Basun, K. Blennow, M. de Leon, C. DeCarli, T. Erkinjuntti, E. Giacobini, C. Graff, J. Hardy, C. Jack, A. Jorm, K. Ritchie, C. van Duijn, P. Visser, and R. C. Petersen. 2004. Mild cognitive impairment. Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine 256(3):240-246.
Wingfield, A., and M. Grossman. 2006. Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology 96(6):2830-2839.
Wingfield, A., P. A. Tun, C. K. Koh, and M. J. Rosen. 1999. Regaining lost time: Adult aging and the effect of time restoration on recall of time-compressed speech. Psychology and Aging 14(3):380-389.
Wu, Y., W. Le, and J. Jankovic. 2011. Preclinical biomarkers of Parkinson disease. Archives of Neurology 68(1):22-30.
Zacks, R. T., L. Hasher, and K. Z. H. Li. 2000. Human memory. In The handbook of aging and cognition, 2nd ed., edited by F. I. Craik and T. A. Salthouse. Mahwah, NJ: Erlbaum. Pp. 293-357.
Zanto, T. P., and A. Gazzaley. 2014. Attention and aging. In The Oxford handbook of attention, edited by A. C. Nobre and S. Kastner. Oxford: Oxford University Press. Pp. 927-971.
Zelazo, P. D., F. I. Craik, and L. Booth. 2004. Executive function across the life span. Acta Psychologica 115(2-3):167-183.












































