1
Introduction
The leading cause of reportable waterborne disease outbreaks in the United States today is Legionnaires’ disease, a pneumonia caused by the Legionella bacterium. Legionella was first documented as a cause of human disease in 1976, after an outbreak of pneumonia of unknown origin was described among members of the American Legion who had attended a conference at the Bellevue-Stratford Hotel in Philadelphia. Of the nearly 2,000 conference attendees, 182 people developed clinical disease and 29 died from their illness. This large outbreak generated national alarm, as public health experts, laboratory scientists, and clinicians raced to define the pathogen (Winn, 1988). A subsequent epidemiological investigation revealed a relationship between the attack rate and the time spent in the hotel lobby and consequently, the route of exposure was surmised to be airborne. The high attack rate coupled with disease severity may have reflected the prevalence of pre-existing conditions among those exposed: of the 94 hospitalized cases, 58 had pre-existing conditions (Fraser et al., 1977). Once the etiologic agent was identified, antibody titers of hotel employees suggested they had been exposed to the bacterium intermittently over a long period.
In 1978 Dr. Joseph McDade and colleagues at the U.S. Centers for Disease Control and Prevention (CDC) discovered the etiologic agent of the Philadelphia outbreak (McDade et al., 1979). The bacterium responsible was named Legionella pneumophila, reflecting the patients initially diagnosed with disease and the respiratory complications seen with infection. The first to isolate Legionella species (spp.) is thought to be Hugh Tatlock in 1943 (Tatlock, 1944); subsequently, in 1959 F. Marilyn Bozeman isolated these bacteria from individual human cases. Retrospectively, the Philadelphia infectious agents were determined to be similar to the L. pneumophila seen previously by Bozeman in 1959 (Bozeman et al., 1968), as judged by immunological assay and similarity of guanine-cytosine DNA composition (McDade et al., 1979).
As news of the Philadelphia outbreak and the identification of L. pneumophila as a human pathogen spread, legionellosis became recognized throughout the world. Legionella spp. were retrospectively linked to previous enigmatic outbreaks of respiratory disease, including one dating back to 1957 in Minnesota. Thus, legionellosis was present in the United States
for years prior to its detection in 1978 (Osterholm et al., 1983; Thacker et al., 1978). Another retrospective study linked L. pneumophila to a previously undiagnosed cluster of patients in a county health department facility in Pontiac, Michigan, struck by a “flu-like,” less severe form of the illness associated with fever, headaches, and myalgias—a syndrome termed Pontiac fever (Glick et al., 1978). As culture techniques were improved and adopted by the microbiology community, the number of reported Legionella spp. grew to include L. micdadei, L. bozemanii, and L. longbeachae, among others. At the time of this publication, more than 61 Legionella spp. have been identified, of which more than half are associated with human disease (Cunha et al., 2016).
In the period following the Philadelphia outbreak, knowledge surrounding legionellosis expanded rapidly (see Figure 1-1). In the first few years after the outbreak, scientists identified the pathogen, procedures critical for laboratory isolation, common environmental sites of exposure (e.g., air conditioning units, cooling towers, potable water), and the underpinning to new methods for diagnosis (e.g., the urinary antigen test) (Berdal et al., 1979; Feeley et al., 1978; Politi et al., 1979; Shands et al., 1985). Importantly, epidemiologic and laboratory animal models demonstrated the benefits of macrolides and flouroquinolones as antibiotic therapy for legionellosis (Fraser et al., 1978). Epidemiologic studies also identified Legionella spp. as primarily waterborne pathogens. In the 1976 Philadelphia outbreak, the hotel’s cooling tower was the source of water droplets contaminated with Legionella that spread through the air in and around parts of the hotel. Subsequently, many Legionella outbreaks have been linked to water exposures, including clusters caused by potable water sources in hospitals (Broome and Fraser, 1979; Tobin et al., 1980, 1981). A timeline of Legionella-related events since 1943 is provided by Figure 1-1. Medical and epidemiological terms related to Legionnaires’ disease and used throughout this report are defined in Box 1-1.
CLINICAL DISEASE AND EPIDEMIOLOGIC STUDIES
Legionnaires’ disease is caused by bacteria of the Legionella genus—small aerobic Gram-negative rods that are facultative intracellular pathogens. Humans are primarily exposed to Legionella through inhalation into the respiratory system, after which the organism replicates in pulmonary macrophages and monocytes. Incubation periods are thought to range from two to twelve days, but may be longer, particularly in immunosuppressed patients. Patients with Legionella pneumonia have fever, cough, shortness of breath, and myalgias (i.e., soreness or aching of the muscles)—common symptoms in other respiratory infections. Unlike most people with community-acquired pneumonias, however, patients with Legionnaires’ disease more frequently have gastrointestinal symptoms and altered mental status and neurologic abnormalities. Patients with Pontiac fever present with fever, myalgias, chills, and headache, but by definition do not have pneumonia; most patients recover without treatment. Because both diseases have symptoms that are similar to other infections, the legionellosis diagnosis may be delayed or missed, which can lead to severe consequences in those with Legionella pneumonia.
Legionellosis is most common among the elderly and those who are immunosuppressed. Incidence is also higher in men and in people who smoke cigarettes. While disease from non-pneumophila Legionella spp. is more common in immunosuppressed patients, the majority of reported cases are caused by L. pneumophila and most frequently serogroup 1 (although this varies by country). Legionella spp. have been found on every continent (Aranciba et al., 2014; Beauté, 2017; Carvalho et al., 2008; Chaudhry et al., 2017; Chedid et al., 2005; Guo et al., 2015; Wolter et al., 2016; Yu et al., 2002), but most currently available epidemiologic data focus on legionellosis in large metropolitan areas in developed regions.
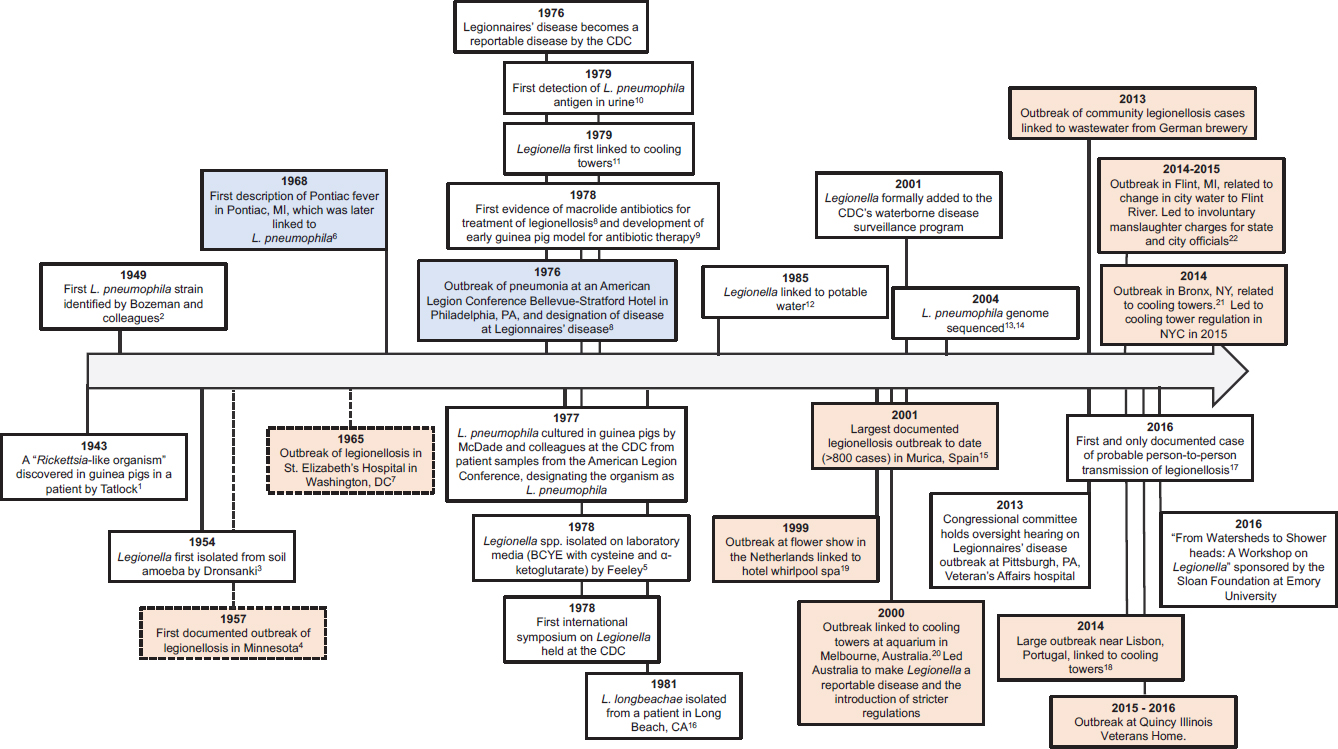
NOTES: Broken lines are outcomes determined through retrospective analysis. First Legionella spp. reported by Tatlock was Tatlockia micdadei, a synonym for Legionella micdadei. Blue indicates first reported cases of Legionella pneumonia and Pontiac fever respectively; orange indicates a large and historically significant outbreak. Citations indicated are listed at the end of the chapter. Timeline not to scale.
In the United States, incidence of Legionnaires’ disease increased more than six-fold from 2000 to 2018 (see Figure 1-2).
Legionnaires’ disease is acquired by exposure to contaminated aerosols of water generated by manufactured devices such as showerheads and faucets, cooling towers, fountains, hot tubs, and other building water systems. Despite numerous reports of common-source outbreaks in the community, through travel or through hospital exposures, and despite improvements in epidemiologic and laboratory tools, the vast majority of Legionella cases remain sporadic, community-acquired cases for which the primary exposure source is never identified.
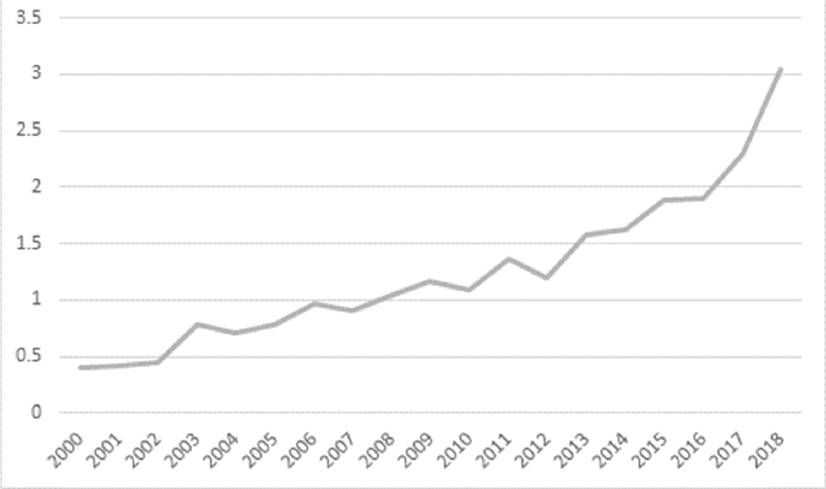
SOURCES: 2000-2002 data adapted from Shah et al. (2018); 2003-2013 data from Adams et al. (2015); 2014 data from Adams et al. (2016); 2015 data from Adams et al. (2017); 2016, 2017, and 2018 data from the National Notifiable Disease Surveillance System.
The medical establishment has not optimized the diagnosis of Legionnaires’ disease for many reasons. When patients present with pneumonia, most physicians choose empiric therapies that adequately treat the disease, so these cases are never counted. If a patient is tested using the urinary antigen test on site, results for infections caused by L. pneumophila serogroup 1 can be received in one day. However, when a patient is culture-tested for Legionella, it can be at least a week or longer before the results are known, making it difficult to diagnose cases in a timely manner. Worldwide, the actual burden of Legionnaires’ disease is generally acknowledged to be underreported by as much as eight- to ten-fold (Dooling et al., 2015; Mercante and Winchell, 2015; Phin et al., 2014; St-Martin et al., 2013; von Baum et al., 2008).
Among common waterborne pathogens, Legionella is now the most common cause of reported drinking water-associated outbreaks (see Figure 1-3). Etiologic shifts from the 1970s to the modern era likely reflect successful efforts mandated by the Safe Drinking Water Act of 1974 to control fecal and enteric bacterial pathogens and parasites (primarily Cryptosporidium spp. and Giardia lamblia).
Waterborne infections account for $3–4 billion in excess costs in the United States per year (Adam et al., 2017). Nearly $1 billion per year goes to the top five primarily waterborne diseases (i.e., giardiasis, cryptosporidiosis, Legionnaires’ disease, otitis externa, and non-tuberculous mycobacterial infections), including $430 million in hospitalization costs to Medicare and Medicaid (Collier et al., 2012). Proven Legionella cases are estimated to lead to a median cost of $26,000 to $38,000 per admission (Collier et al., 2012). European data paint a more ominous picture; Cassini and colleagues (2018) suggest that Legionella spp. are one of the top five pathogens leading to the most disability-adjusted life years (DALYs) and one of only four infections (including HIV, tuberculosis, and invasive pneumococcal disease) considered to have both a high population and high individual burden of disease (see Figure 1-4). Numerous sources worldwide have documented increasing incidence of Legionella cases, suggesting little progress in decreasing risk for Legionella.
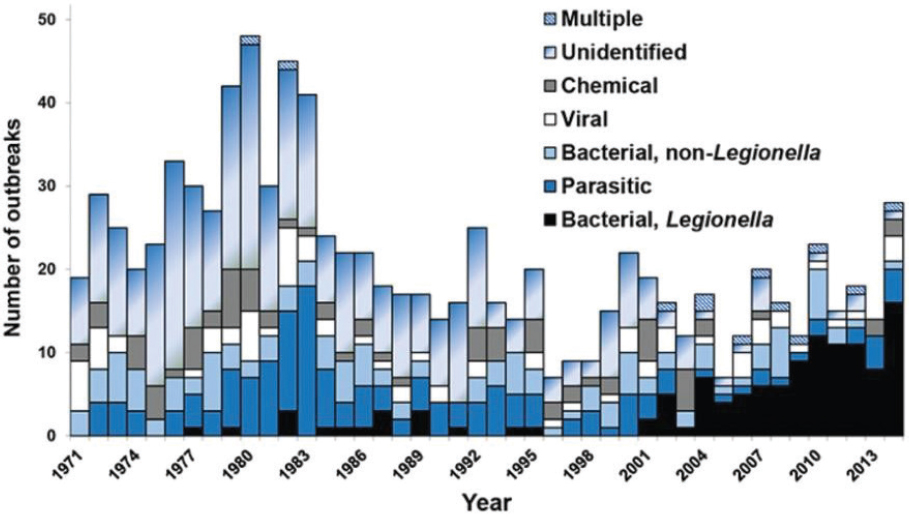
SOURCE: Benedict et al. (2017).
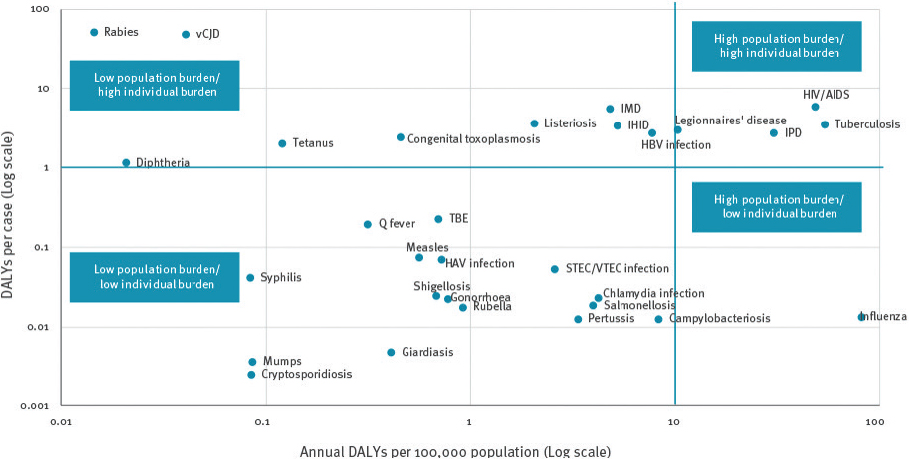
SOURCE: Cassini et al. (2018).
NOTE: EU/EAA: European Union/European Economic Area; HAV: Hepatitis A virus; HBV: Hepatitis B virus; HIV/AIDS: Human immunodeficiency virus infection; IHID: Invasive Haemophilus Influenzae disease; IMD: Invasive meningococcal disease; IPD: Invasive pneumococcal disease; STEC/VTEC: Shiga toxin/verocytotoxin-producing Escherichia coli; TBE: Tick-borne encephalitis; vCJD: variant Creutzfeldt-Jakob disease. Diseases were arbitrarily subdivided according to burden in DALYs per 100,000 population and DALYs per case.
Incidence is generally thought to be underestimated, such that the true financial and human costs of legionellosis are also likely underestimated.
Table 1-1 lists waterborne bacterial pathogens associated with human sinopulmonary disease. Acquiring pneumonia from waterborne pathogens is uncommon, as most lead to gastrointestinal illness. Nonetheless, pneumonia can occur from waterborne pathogens, most notably Legionella spp., Pseudomonas spp., and non-tuberculous mycobacteria. Some of the listed bacteria grow opportunistically in building water systems, but only Legionella causes a reportable illness. Hundreds of organisms associated with waterborne disease are not discussed in detail in this report, including common waterborne pathogens such as Pseudomonas spp., non-tuberculous mycobacteria, Campylobacter spp., Cryptosporidium, and some E. coli infections. Nor does this report discuss other clinical illnesses linked to water, including otitis externa (an ear infection involving the external ear canal), diarrheal disease and their etiologic agents, and skin and soft-tissue infections, among others. Finally, Table 1-1 does not include a large number of important viruses, fungal spp., and parasites causing sinopulmonary infections that have been linked to water sources.
BUILT ENVIRONMENT IS A MAJOR ECOLOGICAL NICHE
Legionella bacteria naturally reside in many freshwater and soil environments, such as lakes, streams, and sediments, and many different species potentially cause disease. However, it is the unchecked growth of pathogenic legionellae in human-made water systems that typically leads to human exposures and causes disease. Humans are exposed to Legionella after inhaling or aspirating contaminated water aerosolized from a variety of sources.
L. pneumophila appears to grow poorly as individual, free-living cells in natural environments; instead, its growth is optimal within amoebae (Kuiper et al., 2004) and other free-living protozoa that are associated with biofilms (Buse et al., 2012; Hellinga et al., 2015). Indeed, the bacterial growth requirements are consistent with a natural, parasitic lifestyle. For example, replicating Legionella require external sources of certain amino acids and minerals (Reeves et al., 1981; States et al., 1985), low levels of oxygen (optimum below 1 mg/L) (Mauchline et al., 1992), and a temperature range between 25°C and 43°C (Garrity et al., 2005). During the Legionella life cycle, its physiological state switches between infectious and replicative forms as well as more hardy, dormant cell forms. Although Legionella can survive and persist in the absence of a host cell, in nature significant amplification appears to require protozoan hosts (Fields et al., 2002). Sometimes pathogenic legionellae replicate within free-living amoebae to levels that elevate risks to the people who are exposed (Ashbolt, 2015b; Declerck, 2010).
Accordingly, the ecology of L. pneumophila is directly linked to that of protozoa, whose primary habitat is biofilm (Declerck, 2010). A biofilm is a community of microorganisms within a self-produced hydrated gel matrix attached to moist soil, sediment, and other solid surfaces that accumulates organic and inorganic material (Characklis and Marshall, 1990). Biofilms typically form on all moist surfaces, including engineered surfaces such as pipes, tanks, appurtenances, filters, and gaskets—virtually everything that contacts water. Biofilm communities growing on pipes can include bacteria/archaea (including round, rod-shaped, filamentous, and appendaged forms), fungi, and higher organisms such as amoebae, ciliates, nematodes, larvae, and crustaceans (see Figure 1-5). The pipe material can exert a strong influence on the composition and activity of the biofilm’s microbial community. Both surface materials and temperature influence the complex interactions among Legionella, host amoebae, and biofilm community members. (More detailed discussion on this ecology is found in Chapter 2.) In general, at moderate to warm temperatures, surfaces wetted with water
TABLE 1-1 Common Waterborne Bacterial Pathogens Associated with Human Sinopulmonary Disease
| Pathogen | Associated Disease(s) | Source* | Comments |
|---|---|---|---|
| Acinetobacter spp. | bacteremia, pneumonia, skin and soft-tissue infections | Healthcare-acquired |
|
| Aeromonas spp. | bacteremia, pneumonia, skin and soft-tissue infections | Community-acquired |
|
| Burkholderia cepacia complex | bacteremia, pneumonia | Healthcare-acquired |
|
| Burkholderia pseudomallei | bacteremia, pneumonia, skin and soft-tissue infections | Healthcare-acquired |
|
| E. coli and other selected Enterobacteraciae spp. | bacteremia, pneumonia, diarrhea, gastroenteritis | Both |
|
| Legionella spp. | pneumonia | Both |
|
| Methylobacterium spp. | bacteremia, pneumonia | Healthcare-acquired |
|
| Non-tuberculous mycobacteria | bacteremia, pneumonia | Both |
|
| Pseudomonas spp. | bacteremia, pneumonia, skin and soft-tissue infections | Both |
|
| Stenotrophomonas maltophilia | bacteremia, pneumonia, skin and soft-tissue infections | Healthcare-acquired |
|
* Probable source location designation (community- or healthcare-acquired) is based on published data regarding documented water source links.
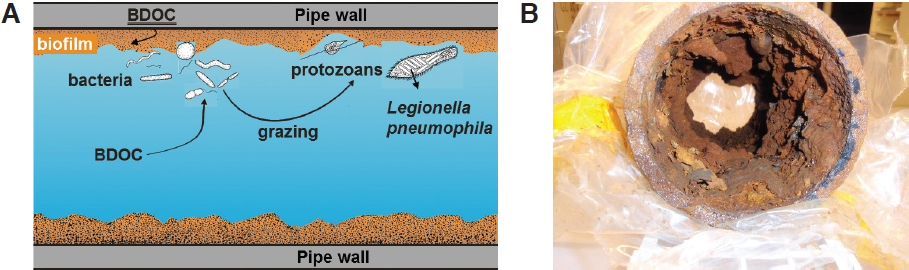
NOTE: BDOC = biodegradable dissolved organic carbon. BDOC can leach out of plastic pipe walls and can also be provided by microorganisms.
SOURCE: Courtesy of Paul van der Wielen.
that contains nutrients provide a favorable habitat for biofilm growth, grazing protozoa, and Legionella growing within the protozoa.
The main mode of human exposure to Legionella is via inhalation of aerosols. Aerosols are small (typically <100 µm) drops of liquid formed by the action of turbulence on fluids, although only those less than 10 µm can reach deep into the human lung. Any materials suspended within the liquid, such as bacteria and protozoa, can be transported within these droplets. The aerosol particles have a large surface area-to-volume ratio and may selectively accumulate hydrophobic materials, including bacteria (Parker et al., 1983). Aerosolization is distinct from volatilization, which is a chemical phase change wherein either a dissolved solute, or the solvent itself, exits the liquid to form a true vapor state.
Sites with both biofilm growth and potential for aerosolization are possible sources of Legionnaires’ disease risk. Many such areas exist in the built environment, including components of heating, ventilation, and air conditioning (HVAC) systems such as cooling towers and humidifiers; indoor plumbing (called premise plumbing) including outlets such as showerheads and faucets; and spas, hot tubs, and Jacuzzis (collectively called hot tubs in this report) (see Figure 1-6). Additional known sources of infection are fountains, misters, nebulizers, car washes, and industrial wastewater treatment plants.
Building- and industrial-scale (e.g., power plants, industries) wet cooling towers have been implicated in many outbreaks of Legionnaires’ disease (see Figure 1-7). Cooling towers remove heat from recirculating water used in water-cooled chillers, heat pumps, air compressors, and other equipment. Heat is rejected from recirculating water in the cooling tower primarily through evaporation. Under certain conditions, biofilms can develop within water-associated piping, heat exchangers, and other component surfaces. Furthermore, the warm temperatures of the bulk water in cooling towers are also conducive to Legionella growth. These towers may generate bacteria-laden aerosols that drift away from the building or facility and then are inhaled by people working and living in the building as well as passersby. Exposure can also occur indoors if the downdraft from cooling towers is transported into building interiors, via air intakes or infiltration. In the United States, there are estimated to be two million cooling towers including both individual and industrial towers.1
___________________
1 See https://energytrendswatch.com/2017/11/21/cooling-towers-not-so-cool/.
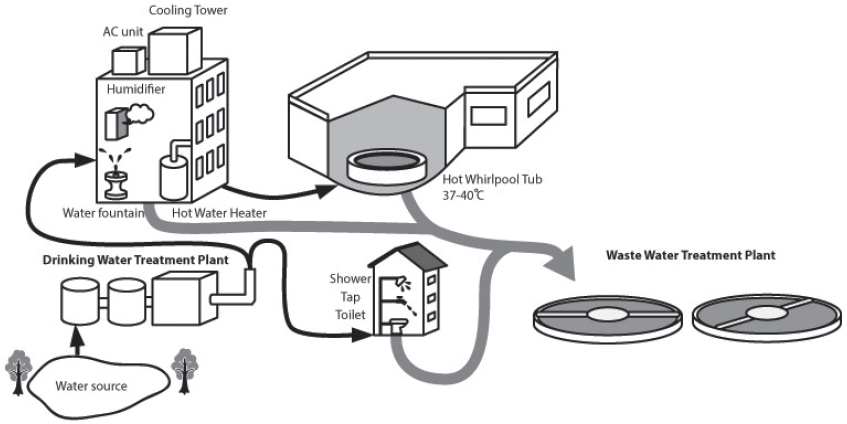
NOTE: Thinner black arrows indicate water pathways to premise plumbing and thicker grey arrows indicate wastewater.
SOURCE: Adapted from Exner (2018) by Kyoko Kurosawa.
Legionella can also contaminate drinking water, either in distribution systems or premise plumbing. In the United States, more than 322 million people are served by 152,000 public drinking water systems with more than 1.2 million miles of water supply mains. The total length of premise plumbing, which refers to all piping downstream of the service line connection and within buildings, is thought to be more than 6 million miles (NRC, 2006). Compared with the main distribution system, premise plumbing uses relatively long sections of small diameter tubing with about ten times more surface area per unit length (NRC, 2006). Thus, premise plumbing provides extensive interior surface area for biofilm growth. Moreover, because of stagnation, premise plumbing is frequently devoid of a disinfectant residual.
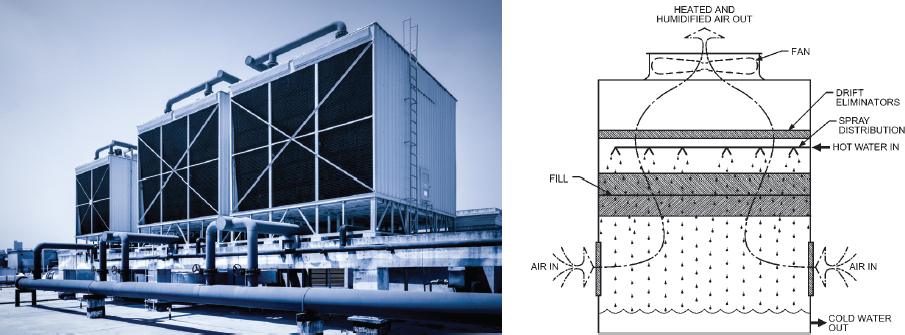
SOURCES: Shutterstock and ASHRAE (2016).

SOURCE: Shutterstock.
If water in the pipes becomes enriched with pathogens such as Legionella, occupants may be exposed to aerosols created by showerheads or faucets (see Figure 1-8). Other home appliances such as hot-water heaters (see Figure 1-9) and humidifiers (see Figure 1-10) can also provide habitats for biofilm growth and enrichment. In 2018, there were estimated to be more than 127 million households in the United States.2
Biofilm and Legionella growth can also be enhanced by water age, which depends on the building type and use, occupancy, and water use. Indeed, the water residing in premise plumbing has a much wider age distribution than the water entering a home from the distribution system (NRC, 2006). Although in the United States the average hotel occupancy is about 66 percent, it can fluctuate seasonally between less than 50 percent to more than 75 percent,3 creating significant potential for water stagnation. There are more than 5 million hotel rooms in the country.4 Green buildings may provide additional areas for growth of pathogens because of lower hot-water temperatures, lower flows, and longer building water ages (Rhoads et al., 2016).
In addition to the premise plumbing and fixtures typical of residential and commercial buildings, healthcare and medical facilities provide additional opportunities for both biofilm colonization and aerosolization due to specialized medical devices, such as dental units and hydrotherapy units. Moreover, compared with the general public, their susceptible patient populations are at risk of Legionnaires’ disease of greater intensity or severity. In the United States there are more than 6,200 hospitals, with more than 930,000 beds.5 In 2015 there were about 1.7 million nursing home beds in the United States.6 According to the American Dental Association, there are almost 200,000 dentists in the United States.7
Another major type of built environment that can generate Legionella risk are recreational water features, both outdoor and indoor. These include swimming pools, hot tubs,
___________________
2 See https://www.statista.com/statistics/183635/number-of-households-in-the-us.
3 See https://www.statista.com/statistics/206546/us-hotels-occupancy-rate-by-month.
4 See https://www.statista.com/statistics/245864/us-hotel-rooms-by-chain-scale-segment.
5 See https://www.aha.org/statistics/fast-facts-us-hospitals.
6 See https://www.statista.com/statistics/323196/number-of-licensed-nursing-home-beds-in-the-us.
7 See https://www.ada.org/en/science-research/health-policy-institute/data-center/supply-and-profile-of-dentists.
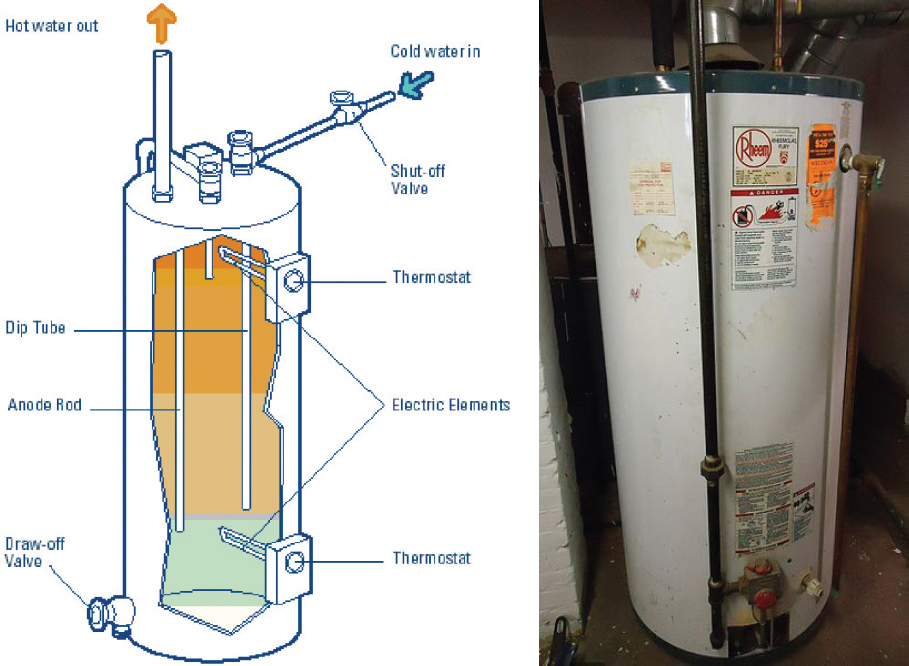
SOURCES: https://buildingsfieldtest.nrel.gov/electric_resistance_water_heaters. © Government of Yukon (diagram) and WikiCommons (photo).
hot-spring baths, fountains (see Figure 1-10), and water parks, as biofilms can form on surfaces and contaminated aerosols can be generated. Recreational sources have resulted in several Legionnaires’ disease outbreaks (Leoni et al., 2018). In the United States, there are an estimated 9 million swimming pools and 5 million hot tubs.8
Other less common locales in the built environment can provide conditions suitable for biofilm growth, and hence colonization by pathogens such as Legionella and their protozoan hosts. Among these locales are interior water features such as green walls and waterfalls (den Boer et al., 2002; Haupt et al., 2012) and external building features that introduce additional wetted and irrigated surfaces including green walls or roofs, particularly when collected or harvested water is used for domestic purposes (Hamilton et al., 2017). Irrigating, lawn sprinkling, and spray washing with stagnant water from hoses or fixtures may be of concern as well, especially when using recycled water treated to non-potable standards (Hamilton et al., 2018; Johnson et al., 2018). In several countries, wastewater treatment plants receiving industrial wastewaters with temperatures higher than 25°C have also been identified as sources of Legionnaires’ disease (e.g., Loenenbach et al., 2018; Olsen et al., 2010) and Pontiac fever (e.g., Castor et al., 2005; Gregersen et al., 1999).
___________________
8 See http://www.apsp.org/Portals/0/2016%20Website%20Changes/2015%20Industry%20Stats/2015%20Industry%20Stats.pdf.

SOURCE: Shutterstock.
ORIGIN OF THE STUDY
In the 40 years since the discovery of L. pneumophila, much has been learned about its ecology; in contrast, less progress has been made toward preventing Legionnaires’ disease or understanding the ecology of different species of pathogenic Legionella and their protozoan hosts. Most knowledge regarding Legionnaires’ disease comes from investigations of disease outbreaks, in which two or more people are infected at the same time by the same source. Yet, only 4 percent of Legionnaires’ disease cases are associated with known outbreaks and are thoroughly investigated (Hicks et al., 2011). Whether outbreaks accurately represent the exposure route of the numerous sporadic cases that go unreported remains unclear. Thus, improved monitoring of Legionnaires’ disease in patient populations and of Legionella presence in water systems is critical to better understanding the disease burden and the likelihood for particular water systems to be sources of infection.
Though monitoring the disease incidence and the presence of L. pneumophila in water samples has evolved considerably over the past 20 years, controversies associated with each persist. For Legionnaires’ disease, a urine antigen test or a (more difficult) sputum test are considered diagnostic, and yet such tests are not commonly performed for hospital patients with pneumonia. Furthermore, the urine antigen test only detects one of the 14 known serogroups of L. pneumophila. Because of these reasons, among others, the number of cases of Legionnaires’ disease is grossly underestimated in the United States as well as in other countries that conduct surveillance. Water systems have traditionally been sampled using culture-based methods, which can take many days to detect growth and can be biased toward certain bacterial types. Polymerase chain reaction (PCR) methods exist, but their
ability to differentiate between viable and nonviable organisms is still evolving. If routine monitoring of water systems for Legionella is to become standard practice for determining the risk of Legionnaires’ disease for a given building or water system, accurate and quantitative microbiologic environmental testing is needed. Finally, although there is general agreement about the levels of detected Legionella that require remedial actions within a water system, there is no consensus on whether there is a threshold level of detected Legionella below which there is no risk of infection.
The treatment of water systems to reduce colonization by Legionella is further complicated by the bacterium’s complex ecology. Legionella have developed multiple strategies to survive in the environment, including entering into a viable-but-non-culturable-like state, multiplying within a variety of protozoa including amoebae, and persisting within microbial biofilms. Indeed, its evolution of mechanisms to avoid digestion by its natural predatory hosts is thought to account for the virulence of certain strains of Legionella within human lung macrophages. Compared with bacteria in suspension, biofilms are relatively resistant to biocides and disinfectants, a primary means of treating built water systems. Other treatment methods involve raising water temperature beyond the growth range for the bacterium and host protozoa, reducing organic carbon levels in source water, altering pipe materials to discourage biofilm formation, and maintaining flow regimes in premise plumbing.
At best, a patchwork of laws, codes, policies, and guidance documents dictate how Legionella is managed in U.S. water systems and buildings. Healthcare facilities that are part of the Veterans Health Administration (VHA) system or that receive funding from the Centers for Medicare & Medicaid Services (CMS) must manage for Legionella contamination, but with varying requirements and oversight. Regulations to register, monitor, and treat cooling towers were put in place in New York City in the wake of the Bronx legionellosis outbreaks in the summer of 2015. New York State regulations now require all general hospitals and residential healthcare facilities to perform an environmental assessment, prepare and implement a sampling and management plan to test their potable water systems for Legionella, and institute control measures in the event of an exceedance. Similar and more widespread regulations for both cooling towers and buildings have existed in Australia, Canada, and some European countries for the past few years, and some are thought to have reduced the risk of Legionnaires’ disease.
Beyond those buildings affected by the VHA and CMS requirements, management of legionellosis in the United States is mainly dictated via voluntary guidance documents from the American Industrial Hygiene Association, the American Society of Heating, Refrigerating, and Air-Conditioning Engineers, and the National Sanitation Foundation International, among others. Hence, Legionella management can be enforced only for a small subset of vulnerable buildings across the country. There is no federal law specifically targeting Legionella. The Surface Water Treatment Rule of the Safe Drinking Water Act only indirectly addresses Legionella via the requirement for maintaining a disinfectant residual in public water supply distribution systems that use surface water sources, and it does not extend to groundwater supplies or to building premise plumbing.
STUDY PURPOSE AND APPROACH
Following a May 2016 workshop on Legionella attended by representatives from around the world with expertise in public health, microbiology, and environmental engineering (Emory University, 2016), participants representing the National Academies of Sciences, Engineering, and Medicine sought to commence a consensus study on Legionella that could address the shortcomings previously mentioned. CDC, VHA, the U.S. Environmental
Protection Agency (EPA), and the Sloan Foundation provided the funding for a project to address the Committee on Management of Legionella in Water Systems’ statement of task (see Box 1-2).
ORGANIZATION OF THE REPORT
Chapter 2 discusses the diagnosis of Legionnaires’ disease, the life history and complex ecology of Legionella in both natural and built water environments, and common exposure pathways. These active areas of research will require continued investment in order to improve the management of Legionella in water systems.
Chapter 3 focuses on the surveillance of Legionnaires’ disease in the United States and Europe, as well as the environmental monitoring of Legionella that is becoming more common in built water systems. The need for a quantitative threshold of Legionella concentration above which action must be taken, and the role of quantitative microbial risk assessment, are extensively discussed in this chapter.
Chapter 4 considers the many strategies used to control Legionella, including the use of heat, biocides, flow control, aerosol formation prevention, and distal devices, along with their application in several typical built environments. The chapter also describes what is known about the efficacy of different control methods and their potential unintended consequences.
Finally, Chapter 5 reviews the array of laws, regulations, codes, standards, and guidance documents that relate to Legionella management, both in the United States and abroad. It includes suggestions for how these various policy tools can be strengthened to better protect the public from legionellosis.
Each chapter ends with conclusions and recommendations that synthesize more technical and specific statements found within the body of each chapter. The most important conclusions and recommendations are compiled in the report summary.
REFERENCES
Adam, E. A., S. A. Collier, K. E. Fullerton, J. W. Gargano, and M. J. Beach. 2017. Prevalence and direct costs of emergency department visits and hospitalizations for selected diseases that can be transmitted by water, United States. Journal of Water and Health 15(5):673-683.
Adams, D. A., K. Fullerton, R. A. Jajosky, P. Sharp, D. H. Onweh, A. W. Schley, W. J. Anderson, A. Faulkner, K.Kugeler, and the Nationally Notifiable Infectious Conditions Group. 2015. Summary of notifiable infectious diseases and conditions—United States, 2013. Morbidity and Mortality Weekly Report 62(53):1-119.
Adams, D. A., K. R. Thomas, R. A. Jajosky, L. Foster, P. Sharp, D. H. Onweh, A. W. Schley, W. J. Anderson, and the Nationally Notifiable Infectious Conditions Group. 2016. Summary of notifiable infectious diseases and conditions—United States, 2014. Morbidity and Mortality Weekly Report 63(54):1-152.
Adams, D. A., K. R. Thomas, R. A. Jajosky, L. Foster, G. Baroi, P. Sharp, D. H. Onweh, A. W. Schley, and W. J. Anderson. 2017. Summary of notifiable infectious diseases and conditions—United States, 2015. Morbidity and Mortality Weekly Report 64(53):1-143.
American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE). 2016. 2016 ASHRAE Handbook : HVAC Systems & Equipment (S-I Edition). Chapter S40 Cooling towers. Pp 40.2. American Society of Heating, Refrigerating and Air-Conditioning Engineers, Atlanta, GA.
Arancibia, F., C. P. Cortes, M. Valdés, J. Cerda, A. Hernández, L. Soto, and A. Torres. 2014. Importance of Legionella pneumophila in the etiology of severe community-acquired pneumonia in Santiago, Chile. Chest 145(2):290-296.
Ashbolt, N. J. 2015a. Microbial contamination of drinking water and human health from community water systems. Current Environmental Health Reports 2(1):95-106.
Ashbolt, N. J. 2015b. Environmental (saprozoic) pathogens of engineered water systems: Understanding their ecology for risk assessment and management. Pathogens 4(2):390-405.
Beauté, J. 2017. Legionnaires’ disease in Europe, 2011 to 2015. European Legionnaires’ Disease Surveillance Network. Eurosurveillance 22(27):pii=30566. https://doi.org/10.2807/1560-7917.ES.2017.22.27.30566.
Benedict, K. M., H. Reses, Vigar M., D. M. Roth., V. A. Roberts, M. Mattioli, L. A. Cooley, E. S. Hilborn, T. J. Wade, K. E. Fullerton, J. S. Yoder, and V. R. Hill. 2017. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2013–2014. Morbidity and Mortality Weekly Report 66:1216-1221.
Berdal, B. P., C. E. Farshy, and J. C. Feeley. 1979. Detection of Legionella pneumophila antigen in urine by enzyme-linked immuno-specific assay. Journal of Clinical Microbiology 9(5):575-578.
Bozeman, F. M., J. W. Humphries, and J. M. Campbell. 1968. A new group of rickettsia-like agents recovered from guinea pigs. Acta Virologica 12:87-93.
Broome, C. V., and D. W. Fraser. 1979. Epidemiologic aspects of legionellosis. Epidemiologic Reviews 1:1-16.
Buse, H., M. E. Schoen, and N. J. Ashbolt. 2012. Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Research 46:921-933.
Carvalho, F. R. S., F. R. Nastasi, R. C. Gamba, A. S. Foronda, and V. H. Pellizari. 2008. Occurrence and diversity of Legionellaceae in polar lakes of the Antarctic Peninsula. Current Microbiology 57(4):294-300.
Cassini, A., E. Colzani, A. Pini, M. J. Mangen, D. Plass, S. A. McDonalds, G. Maringhini, A. van Lier, J. A. Haagsma, A. H. Havelaar, P. Kramarz, M. W. Kretzschmar, on behalf of the Burden of Communicable Diseases in Europe Consortium. 2018. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): Results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Eurosurveillance 23(16):pii=17-00454. https://doi.org/10.2807/1560-7917.ES.2018.23.16.17-00454.
Castor, M. L., E. A. Wagstrom, R. N. Danila, K. E. Smith, T. S. Naimi, J. M. Besser, K. A. Peacock, B. A. Juni, J. M. Hunt, J. M. Bartkus, S. R. Kirkhorn, and R. Lynfield. 2005. An outbreak of Pontiac fever with respiratory distress among workers performing high-pressure cleaning at a sugar-beet processing plant. Journal of Infectious Diseases 191(9):1530–1537.
Characklis, W. G., and K. C. Marshall. 1990. Biofilms. New York: Wiley.
Chaudhry, R., A. Valavane, K. K. Sreenath, M. Choudhary, T. Sagar, T. Shende, M. Varma-Basil, S. Mohanty, S. K. Kabra, A. B. Dey, and B. Thakur. 2017. Detection of Mycoplasma pneumoniae and Legionella pneumophila in patients having community-acquired pneumonia: a multicentric study from New Delhi, India. American Journal of Tropical Medicine and Hygiene 97(6):1710-1716.
Chedid, M. B. F., D. D. O. Ilha, M. F. Chedid, P. R. Dalcin, M. Buzzetti, P. Jaconi Saraiva, D. Griza, and S. S. Menna Barreto. 2005. Community-acquired pneumonia by Legionella pneumophila serogroups 1–6 in Brazil. Respiratory Medicine 99(8):966-975.
Collier, S. A., L. J. Stockman, L. A. Hicks, L. E. Garrison, F. J. Zhou, and M. J. Beach. 2012. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiology and Infection 140(11):2003-2013.
Correia, A. M., J. S. Ferreira, V. Borges, A. Nunes, B. Gomes, R. Capucho, J. Gonçalvez, D. M. Antunes, S. Almeida, A. Mendes, M. Guerreiro, D. A. Sampaio, L. Viera, J. Machado, M. J. Simões, P. Gonçalves, and J. P. Gomes. 2016. Probable person-to-person transmission of Legionnaires’ disease. New England Journal of Medicine 374(5):497-498.
Cunha, B. A., A. Burillo, and E. Bouza. 2016. Legionnaires’ disease. Lancet 387(10016):376-385.
Declerck, P. 2010. Biofilms: the environmental playground of Legionella pneumophila. Environmental Microbiology 12(3):557-566.
den Boer, J. W., E. P. Yzerman, J. Schellekens, K. D. Lettinga, H. C. Boshuizen, J. E. Van Steenbergen, A. Bosman, S. Van den Hof, H. A. Van Vliet, M. F. Peeters, R. J. Van Ketel, P. Speelman, J. L. Kool, and M. A. Conyn-Van Spaendock. 2002. A large outbreak of Legionnaires’ disease at a flower show, The Netherlands, 1999. Emerging Infectious Diseases 8:37-43.
Dooling, K. L., K.-A. Toews, L. A. Hicks, L. E. Garrison, B. Bachaus, S. Zansky, L. R. Carpenter, B. Schaffner, E. Parker, S. Petit, A. Thomas, S. Thomas, R. Mansmann, C. Morin, B. White, and G. E. Langley. 2015. Active bacterial core surveillance for legionellosis—United States, 2011–2013. Morbidity and Mortality Weekly Report 64(42):1190-1193.
Emory University Center for Public Health Preparedness and Research. 2016. From watersheds to showerheads: A workshop on Legionella research and policy. May 25–26, 2016. Atlanta, GA. http://www.cphpr.emory.edu/research/legionella/workshop/index.html.
Exner, M. 2018. Presentation at the 3rd meeting to the Committee on Legionella Management in Waters Systems. Woods Hole, MA. July 30, 2018.
Falkinham, III, J. O. 2011. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerging Infectious Diseases 17(3):419-24.
Feeley, J. C., G. W. Gorman, R. E. Weaver, D. C. Mackel, and H. W. Smith. 1978. Primary isolation media for Legionnaires’ disease bacterium. Journal of Clinical Microbiology 8:320-325.
Fields, B., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires’ disease: 25 years of investigation. Clinical Microbiology Reviews 15(3):506-526.
Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, and J. E. McDade. 1977. Legionnaires’ disease: Description of an epidemic of pneumonia. New England Journal of Medicine 297(22):1189-1197.
Fraser, D. W., C. Bopp. I. K. Wachsmuth, J. C. Feeley, and T. F. Tsai. 1978. Antibiotic treatment of guinea pigs infected with agent of Legionnaires’ disease. Lancet 1:175-178.
Garrity, G. M., J. A. Bell, and T. Lilburn. 2005. Legionellales ord. nov. Pp. 210-247. In: Bergey’s Manual® of systematic bacteriology: Volume two, the proteobacteria, part B, the gammaproteobacteria. D. J. Brenner et al., (eds.), Boston, MA: Springer.
Glick, T. H., M. B. Gregg, B. Berman, G. Mallison, W. W. Rhodes, Jr., and I. Kassanoff. 1978. Pontiac fever: An epidemic of unknown etiology in a health department. I. Clinical and epidemiologic aspects. American Journal of Epidemiology 107:149-160.
Gregersen, P., K. Grunnet, S. A. Uldum, B. H. Andersen, and H. Madsen. 1999. Pontiac fever at a sewage treatment plant in the food industry. Scandinavian Journal of Work, Environment and Health 25(3):291-295.
Guo, J., T. Liang, C. Hu, R. Lv, X. Yang, Y. Cui, Y. Song, R. Yang, Q. Zhu, and Y. Song. 2015. Sequence types diversity of Legionella pneumophila isolates from environmental water sources in Guangzhou and Jiangmen, China. Infection, Genetics, and Evolution 29:35-41.
Hamilton, K. A., M. T. Hamilton, W. Johnson, P. Jjemba, Z. Bukhari, M. LeChevallier, and C. N. Haas. 2018. Health risks from exposure to Legionella in reclaimed water aerosols: Toilet flushing, spray irrigation, and cooling towers. Water Research 134:261-279.
Hamilton, K. A., W. Ahmed, S. Toze, and C. N. Haas. 2017. Human health risks for Legionella and Mycobacterium avium complex (MAC) from potable and non-potable uses of roof-harvested rainwater. Water Research 119(August):288-303.
Haupt, T. E., R. T. Heffernan, J. J. Kazmierczak, H. Nehls-Lowe, B. Rheineck, C. Powell, K. K. Leonhardt, A. S. Chitnis, and J. P. Davis. 2012. An outbreak of Legionnaires’ disease associated with a decorative water wall fountain in a hospital. Infection Control and Hospital Epidemiology 33(February):185-191.
Hellinga, J. R., R. A. Garduno, J. D. Kormish, J. R. Tanner, D. Khan, K. Buchko, C. Jimenez, M. M. Pinette, and A. K. Brassinga. 2015. Identification of vacuoles containing extraintestinal differentiated forms of Legionella pneumophila in colonized Caenorhabditis elegans soil nematodes. Microbiology Open 4(4):660-681.
Hicks, L., L. E. Garrison, G. E. Nelson, and L. M. Hampton, 2011. Legionellosis—United States, 2000–2009. Morbidity and Mortality Weekly Report 60(32):1083-1086.
Hines, S. A., D. J. Chappie, R. A. Lordo, B. D. Miller, R. J. Janke, H. A. Lindquist, K. R. Fox, H. S. Ernst, and S. C. Taft. 2014. Assessment of relative potential for Legionella species or surrogates inhalation exposure from common water uses. Water Research 56:203-213.
Johnson, W. J., P. K. Jjemba, Z. Bukhari, and M. LeChevallier. 2018. Occurrence of Legionella in non-potable reclaimed water. Journal of the American Water Works Association 110(3):15-27.
Karumathil, D. P., H. B. Yin, A. Kollanoor-Johny, and K. Venkitanarayanan. 2014. Effect of chlorine exposure on the survival and antibiotic gene expression of multidrug resistant Acinetobacter baumannii in water. International Journal of Environmental Research and Public Health 11(2):1844-1854.
Kuiper, M. W., B. A. Wullings, A. D. L. Akkermans, R. R. Beumer, and D. van der Kooij. 2004. Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Applied and Environmental Microbiology 70:6826-6833.
Leoni, E., F. Catalani, S. Marini, and L. Dallolio. 2018. Legionellosis associated with recreational waters: A systematic review of cases and outbreaks in swimming pools, spa pools, and similar environments. International Journal of Environmental Research and Public Health 15(8):1612.
Loenenbach, A. D., C. Beulens, S. M. Euser, J. P. G. van Leuken, B. Bom, W. van der Hoek, A. M. de Roda Husman, W. L. M. Ruijs, A. A. Bartels, A. Rietveld, J. W. den Boer, and P. S. Brandsema. 2018. Two community clusters of Legionnaires’ disease directly linked to a biologic wastewater treatment plant, The Netherlands. Emerging Infectious Diseases 24(10):1914-1918.
Mauchline, W. S., R. Araujo, R. Wait, A. B. Dowsett, P. J. Dennis, and C. W. Keevil. 1992. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. Microbiology 138:2371-2380.
McDade, J. E., D. J. Brenner, and F. M. Bozeman. 1979. Legionnaires’ disease bacterium isolated in 1947. Annals of Internal Medicine 90:659-661.
Mercante, J. W., and J. M. Winchell. 2015. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clinical Microbiology Reviews 28(1):95-133.
NRC (National Research Council). 2006. Drinking water distribution systems: Assessing and reducing risks. Washington, DC: National Academies Press.
Olsen, J. S., T. Aarskaug, I. Thrane, C. Pourcel, E. Ask, G. Johansen, V. Waagen, and J. M. Blatny. 2010. Alternative routes for dissemination of Legionella pneumophila causing three outbreaks in Norway. Environmental Science and Technology 44:8712-8717.
Osterholm, M. T., T. D. Y. Chin, D. O. Osborne. H. B. Dull, A. G. Dean, D. W. Fraser, P. S. Hayes, and William N. Hall. 1983. A 1957 outbreak of Legionnaires’ disease associated with a meat-packing plant. American Journal of Epidemiology 117(1):60-67.
Parker, B. C., M. A. Ford, H. Gruft, and J. O. Falkinham, III. 1983. Epidemiology of infection by nontuberculous mycobacteria. IV. Preferential aerosolization of Mycobacterium intracellularae from natural waters. American Review of Respiratory Disease 128(4):652-656.
Phin, N., F. Parry-Ford, T. Harrison, H. R. Stagg, N. Zhang, K. Kumar, O. Lortholary, A. Zumla, I. Abubakar. 2014. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infectious Diseases 14:1011-1021.
Politi, B. D., D. W. Fraser, G. F. Mallison, J. V. Mohatt, G. K. Morris, C. M. Patton, J. C. Feeley, R. D. Telle, and J. V. Bennett. 1979. A major focus of Legionnaires’ disease in Bloomington, Indiana. Annals of Internal Medicine 90(4):587-591.
Reeves, M. W., L. Pine, S. H. Hutner, J. R. George, and W. K. Harrell. 1981. Metal requirements of Legionella pneumophila. Journal of Clinical Microbiology 13:688-695.
Rhoads, W. J., A. Pruden, and M. A. Edwards. 2016. Survey of green building water systems reveals elevated water age and water quality concerns. Environmental Science: Water Research and Technology 2(1):164-173.
Shands, K. N., J. L. Ho, R. D. Meyer, G. W. Gorman P. H. Edelstein, G. F. Mallison, S. M. Finegold, and D. W. Fraser. 1985. Potable water as a source of Legionnaires’ disease. Journal of the American Medical Association 253:1412-1416.
Shah, P., A. Barskey, A. Binder, C. Edens, S. Lee, J. Smith, S. Schrag, C. Whitney, and L. Cooley. 2018. Legionnaires’ disease surveillance summary report, United States, 2010–2015. Atlanta, GA: CDC.
States, S. J., L. F. Conley, M. Ceraso, T. E. Stephenson, R. S. Wolford, R. M. Wadowsky, A. M. McNamara, and R. B. Yee. 1985. Effects of metals on Legionella pneumophila growth in drinking water plumbing systems. Applied and Environmental Microbiology 50(5):1149-1154.
St-Martin, G., S. Uldum, and K. Mølbak. 2013. Incidence and prognostic factors for Legionnaires’ disease in Denmark, 1993–2006. ISRN Epidemiology Volume 2013, Article ID 847283, 8 pages.
Tatlock, H. 1944. A Rickettsia-like organism recovered from guinea pigs. Proceedings of the Society for Experimental Biology and Medicine 57:95.
Thacker, S. B., J. V. Bennett, T. F. Tsai, D. W. Fraser, J. E. McDade, C. C. Shepard, K. H. Williams, Jr., W. H. Stuart, H. B. Dull, and T. C. Eickhoff. 1978. An outbreak in 1965 of severe respiratory illness caused by Legionnaires’ disease bacterium. Journal of Infectious Diseases 138:512-519.
Tobin, J. O’H., M. S. Dunnill, M. French, P. J. Morris, J. Bear, S. Fisher-Hoch, R. G. Mitchell, and M. F. Muers. 1980. Legionnaires’ disease in a transplant unit: Isolation of the causative agent from shower baths. Lancet 316(8186):118-121.
Tobin, J. O’H., R. A. Swann, and C. L. R. Bartlett. 1981. Isolation of Legionella pneumophila from water systems; methods and preliminary results. British Medical Journal 282:515-517.
von Baum, H., S. Ewig, R. Marre, N. Suttorp, S. Gonschior, T. Welte, and C. Lück for the Competence Network for Community Acquired Pneumonia Study Group. 2008. Community-acquired Legionella pneumonia: New insights from the German Competence Network for Community Acquired Pneumonia. Clinical Infectious Diseases 46:1356-1364.
Winn, W. C. 1988. Legionnaires’ disease: Historical perspective. Clinical Microbiology Reviews 1(1):60-81.
Wolter, N., M. Carrim, C. Cohen, S. Tempia, S. Walaza, P. Sahr, L. de Gouveia, F. Treurnicht, O. Hellferscee, A. L. Cohen, A. J. Benitez, H. Dawood, E. Variava, J. M. Winchell, and A. von Gottberg. 2016. Legionnaires’ disease in South Africa, 2012–2014. Emerging Infectious Diseases 22(1):2012-2014.
Yu, V. L., J. F. Plouffe, M. C. Pastoris, J. E. Stout, M. Schousbo, A. Widmer, J. Summersgill, T. File, C. M. Heath, D. L. Paterson, and A. Chereshsky. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: An international collaborative survey. Journal of Infectious Diseases 186:127-128.
FIGURE 1-1 CITATIONS:
- Tatlock, H. 1944. A Rickettsia-like organism recovered from guinea pigs. Proceedings of the Society for Experimental Biology and Medicine 57:95.
- McDade, J. E., D. J. Brenner, and F. M. Bozeman. 1979. Legionnaires’ disease bacterium isolated in 1947. Annals of Internal Medicine 90:659-661.
- Drozanski, W. 1956. Fatal bacterial infection in soil amoebae. Acta Microbiologica Polonica 5:315-317.
- Osterholm, M. T., T. D. Y. Chin, D. O. Osborne. H. B. Dull, A. G. Dean, D. W. Fraser, P. S. Hayes, and W. N. Hall. 1983. A 1957 outbreak of Legionnaires’ disease associated with a meat packing plant. American Journal of Epidemiology 117(1):60-67.
- Feeley, J. C., G. W. Gorman, R. E. Weaver, D. C. Mackel, and H. W. Smith. 1978. Primary isolation media for Legionnaires’ disease bacterium. Journal of Clinical Microbiology 8:320-325.
- Glick, T. H., M. B. Gregg, B. Berman, G. Mallison, W. W. Rhodes, Jr., and I. Kassanoff. 1978. Pontiac fever. An epidemic of unknown etiology in a health department. I. Clinical and epidemiologic aspects. American Journal of Epidemiology 107:149-160.
- Thacker, S. B., J. V. Bennett, T. F. Tsai, D. W. Fraser, J. E. McDade, C. C. Shepard, K. H. Williams, Jr., W. H. Stuart, H. B. Dull, and T. C. Eickhoff. 1978. An outbreak in 1965 of severe respiratory illness caused by Legionnaires’ disease bacterium. Journal of Infectious Diseases 138:512-519.
- Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, and J. E. McDade. 1977. Legionnaires’ disease: Description of an epidemic of pneumonia. New England Journal of Medicine 297(22):1189-1197.
- Fraser, D. W., C. Bopp. I. K. Wachsmuth, J. C. Feeley, and T. F. Tsai. 1978. Antibiotic treatment of guinea pigs infected with agent of Legionnaires’ disease. Lancet 1:175-178.
- Berdal, B. P., C. E. Farshy, and J. C. Feeley. 1979. Detection of Legionella pneumophila antigen in urine by enzyme-linked immuno-specific assay. Journal of Clinical Microbiology 9(5):575-578.
- Politi, B. D., D. W. Fraser, G. F. Mallison, J. V. Mohatt, G. K. Morris, C. M. Patton, J. C. Feeley, R. D. Telle, and J. V. Bennett. 1979. A major focus of Legionnaires’ disease in Bloomington, Indiana. Annals of Internal Medicine 90(4):587-591.
- Shands, K. N., J. L. Ho, R. D. Meyer, G. W. Gorman P. H. Edelstein, G. F. Mallison, S. M. Finegold, and D. W. Fraser. 1985. Potable water as a source of Legionnaires’ disease. Journal of the American Medical Association 253:1412-1416.
- Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Geroghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A., Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968.
- Cazalet, C., C. Rusniok, H. Brüggermann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bourchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchreiser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nature Genetics 36:1165-1173.
- García-Fulgueiras, A., C. Navarro, D. Fenoll, J. García, P. González-Diego, T. Jiménez-Buñuales, M. Rodriguez, R. Lopez, F. Pacheco, J. Ruiz, M. Segovia, B. Baladrón, and C. Pelaz. 2003. Legionnaires’ disease outbreak in Murica, Spain. Emerging Infectious Diseases 9(8):915-921.
- McKinney, R. M., R. K. Porschen, P. H. Edelstein, M. L. Bissett, P. P. Harris, S. P. Bondell, A. G. Steigerwalt, R. E. Weaver, M. E. Ein, D. S. Lindquist, R. S. Kops, and D. J. Brenner. 1981. Legionella longbeachae species nova, another etiologic agent of human pneumonia. Annals of Internal Medicine 94:739-743.
- Correia, A. M., J. S. Ferreira, V. Borges, A. Nunes, B. Gomes, R. Capucho, J. Gonçalvez, D. M. Antunes, S. Almeida, A. Mendes, M. Guerreiro, D. A. Sampaio, L. Viera, J. Machado, M. J. Simões, P. Gonçalves, and J. P. Gomes. 2016. Probable person-to-person transmission of Legionnaires’ disease. New England Journal of Medicine 374(5):497-498.
- Russo, A., C. M. Gouveia1, P. M. M. Soares, R. M. Cardoso, M. T. Mendes, and R. M. Trigo1. 2018. The unprecedented 2014 Legionnaires’ disease outbreak in Portugal: Atmospheric driving mechanisms. International Journal of Biometeorology 62(7):1167-1179.
- den Boer, J. W., E. P. Yzerman, J. Schellekens, K. D. Lettinga, H. C. Boshuizen, J. E. Van Steenbergen, A. Bosman, S. Van den Hof, H. A. Van Vliet, M. F. Peeters, R. J. Van Ketel, P. Speelman, J. L. Kool, and M. A. Conyn-Van Spaendock. 2002. A large outbreak of Legionnaires’ disease at a flower show, The Netherlands, 1999. Emerging Infectious Diseases 8:37-43.
- Grieg, J. E., J. A. Carnie, G. F. Tallis, B. Zwolakz, W. G. Hart, C. S. Guest, N. J. Ryan, J. A. Leydon, A. G. Tan, and I. R. Gordon. 2004. An outbreak of Legionnaires’ disease at the Melbourne Aquarium, April 2000: Investigation and case-control studies. Medical Journal of Australia 180(11):566-572.
- Lapierre, P., E. Nazarian, Y. Zhu, D. Wroblewski, A. Saylors, T. Passaretti, S. Hughes, A. Tran, Y. Lin, J. Kornblum, S. S. Morrison, J. W. Mercante, R. Fitzhenry, D. Weiss, B. H. Raphael, J. K. Varma, H. A. Zucker, J. L. Rakeman, and K. A. Musser. 2015. Legionnaires’ disease outbreak caused by endemic strain of Legionella pneumophila, New York, New York, USA, 2015. Emerging Infectious Diseases 223(11):1784-1791.
- Nelson, R. 2016. Crisis in Flint: Lead and Legionnaires’ disease. Lancet Infectious Diseases 16:298-299.




















