2
The Existing Global Governance Landscape for Influenza Vaccines
WORLD HEALTH ORGANIZATION AND THE INFLUENZA “REGIME COMPLEX”
Global health governance structures and frameworks for pandemic preparedness have often been developed reactively. In recent years, major outbreaks and pandemics have resulted in new international agreements and reforms; the International Health Regulations (IHR) revisions were rolled out in 2005 in response to the severe acute respiratory syndrome (SARS) outbreak of 2002–2003, the 2009 H1N1 pandemic led to discussions that ultimately resulted in a new access and benefit sharing (ABS) system for influenza, and the World Health Organization (WHO) research and development (R&D) blueprint was a reaction to the 2013–2014 Ebola epidemic in West Africa. This reactive dynamic does not mean that pandemic preparedness and response (PPR) efforts have lacked strategic grounding, such as linking “health” to the less altruistic (and potentially more lucrative) goal of “security.” However, such strategies were not sufficiently effective to attract the resources required to respond to the COVID-19 pandemic—and a window for “strategic reactivity” may now exist for pandemic preparedness and influenza governance. In David Fidler’s words (NASEM, 2018), the global governance infrastructure for pandemic influenza may be best thought of as a “regime complex: a set of interlinked and overlapping institutions, rules, processes, and practices.” It provides a web of PPR activities at both the functional and strategic levels. Governance mechanisms enable surveillance, virus sharing, scientific research, and vaccine development. Strategically, the “ecosystem” also integrates national security, economic interests, human rights, and ethics into pandemic influenza governance.
Much of the global infrastructure for pandemic preparedness is based on the scaffolds provided by long-standing governance structures for influenza. The first influenza vaccines were developed in 1942, and the newly minted WHO initiated the Global Influenza Program (GIP) in 1947. The Global Influenza Surveillance Network (GISN)—renamed the “Global Influenza Surveillance and Response System” (GISRS) in 2011—was launched under the auspices of GIP in 1952, with the recognition that more needed to be done globally to monitor changes in influenza viruses (Monto, 2017). As Hay and McCauley (2018) argue, the precise modus operandi of GISRS has changed over the years, but it has consistently been a mechanism for monitoring global influenza viral activity, identifying strains for annual vaccines, and forecasting potential pandemic threats.
GISRS is based on a largely “informal autonomous trust-based system” of laboratories and collaborating centers coordinated by WHO. The backbone of this network consists of approximately 143 National Influenza Centres (NICs) spread across 101 GISRS-participating countries that provide continuous surveillance of influenza. The NICs collect, analyze, and classify biological samples to monitor which strains are causing illness, how efficiently these strains are spreading, and how well previous vaccines have worked to combat their targeted viruses. Information is then disseminated through the FluNet database. These smaller centers pass the results from their wide-reaching investigations to one of six WHO Collaborating Centres (WHO CCs) for Reference and Research on Influenza, which provide more in-depth analysis of strains. GISRS then aggregates the data from FluNet and collaborators to predict the prevalence of influenza strains and allow WHO to select 3–4 strains to use in seasonal vaccines (Hay and McCauley, 2018; Ziegler et al., 2018). About 60 percent of countries now participate in WHO-mediated global influenza surveillance, supporting the IHR requirement for member states to notify WHO of all human infections with novel influenza viruses (WHO, 2008). GISRS is widely seen as one of the more successful global disease surveillance mechanisms and is now being leveraged for a pilot program for respiratory syncytial virus (Broor et al., 2020; Carroll et al., 2021).
GOVERNANCE GAPS EXPOSED IN THE 2009 H1N1 “SWINE FLU” PANDEMIC
From 1997 to 2007, a series of avian influenza outbreaks brought international attention to WHO and GISN/GISRS. First, an H5N1 strain with a high case fatality rate emerged in Hong Kong in 1997. Its reappearance in 2003–2004 and spread to other countries raised fear of a highly pathogenic avian influenza pandemic, compounded by the 2002 SARS coronavirus epidemic and the threat of zoonotic H7N7 influenza (Hay
and McCauley, 2018). Avian influenza fears led to a frantic period of vaccine development and global preparations for pandemic influenza, during which issues of equitable access to vaccines and benefits from research on influenza viruses made some stakeholders question the legitimacy of GISN/GISRS, which reached a peak when Indonesia refused to share its H5N1 virus samples with WHO because it believed that it would not receive equitable access to the benefits derived from them (Fidler, 2010). The persistent H5N1 threat called for a wider appreciation of vaccine operations and benefits, as governments began to stockpile vaccines and antivirals, and led to “greater government scrutiny of the fairness of the global system” (Hay and McCauley, 2018).
In March and April 2009, a novel strain of H1N1 spread in Mexico and the United States (WHO, 2012). This ultimately led to the first influenza pandemic of the twenty-first century, which is estimated to have caused 151,000–575,000 deaths (Rockman et al., 2020). Early reports of H1N1 outbreaks activated information-sharing networks among the numerous WHO surveillance units, and the GISRS backbone responded well, in terms of assessing epidemiological information provided by the NICs and characterizing the virus’ antigenic and genetic characteristics at the WHO CCs. The U.S. Centers for Disease Control and Prevention (CDC) officially reported the first cases of H1N1 to WHO on April 18, 2009. On April 29, 2009, WHO convened an emergency committee under the authority of the IHR to make recommendations on whether a pandemic should be declared (Kamradt-Scott et al., 2018). As the cases increased at more than 200 locations around the world, preparations for vaccine production and other responses began. A suitable candidate vaccine virus (CVV) was selected relatively quickly (Ampofo et al., 2012); by late spring, the seasonal vaccine production cycle was nearly at its end and manufacturers were able to begin preparations for dealing with H1N1 (WHO, 2012).
Although it obtained quick surveillance data for H1N1 influenza, WHO did not officially declare a global pandemic until June 11, 2009. Soon thereafter, it launched the WHO Pandemic Influenza A (H1N1) Vaccine Deployment Initiative, which it later deemed to be the “first coordinated global response to an influenza pandemic” (WHO, 2012). Under this initiative, WHO coordinated the support of governments, foundations, and manufacturers to facilitate access to pandemic influenza vaccines in low- and middle-income countries (LMICs). Eventually, millions of vaccine doses, syringes, safety boxes, and other items were donated, and substantial financial and logistical support was pledged.
Despite this novel deployment coordination structure, the H1N1 experience is generally considered to offer a cautionary tale for the challenges of upscaling production of global influenza vaccines and deploying them during a pandemic. Box 2-1 summarizes major gaps in terms of international
coordination for producing and distributing vaccines. Production was not triggered quickly because the switch from seasonal to pandemic vaccine production was poorly coordinated and did not occur until after many seasonal vaccines were already delivered. High-income countries (HICs),
including the United States, set up procurement contracts with manufacturers and began receiving vaccines as early as October 2009. But the majority of vaccines were mostly rolled out after the peak of the pandemic in the fall; by then, demand was low, many doses were wasted, and many low-income
countries received less than planned. The egg-based vaccine also showed generally poor efficacy, which exacerbated issues with public confidence and markets.
Fidler (2010) offered a particularly biting analysis of equity during the 2009–2010 pandemic: “in terms of vaccines for 2009-H1N1, donations from manufacturers and developed countries were not the product of real negotiations, given that WHO and developing countries had little leverage to influence developed countries other than rhetoric about equity, justice, and solidarity.” In the end, wealthy countries
only agreed to make donations after (1) they learned, unexpectedly, that a one-dose regimen would immunize adults, which doubled the amount of vaccine available; and (2) data from the northern and southern hemispheres revealed that the 2009 H1N1 virus was behaving as a mild virus and not a killer strain, which reduced the threat the virus posed.
These countries ensured that they had sufficient vaccine to cover their populations when pledging donations, and some—including the United States—postponed donations when they were not politically expedient.
Apart from its role with vaccine production and distribution, WHO faced criticism over the timing of its declaration of a pandemic (a necessary step to initiate the “switch”) and its decision to remove pandemic influenza guidance from its website. Three independent panels found no evidence that WHO had engaged in inappropriate conduct but also recommended significant changes for its responses to health emergencies (Cohen and Carter, 2010; Flynn, 2010; Kamradt-Scott et al., 2018; WHO, 2011).
EXPANDING AND ENHANCING INFLUENZA SURVEILLANCE SINCE 2009
The governance and financing challenges during the 2009 H1N1 pandemic stimulated negotiations that led to the launch of the Pandemic Influenza Preparedness (PIP) Framework in 2011. This framework is a formal, but nonbinding, agreement between WHO member states to improve PPR, with a focus on equity and benefit sharing. Under its governance, when countries share influenza viruses with pandemic potential (IVPP) with GISRS, they are entitled to access specific benefits, including timely access to vaccines, derived from these viruses in a pandemic. The PIP Framework agreement only covers IVPP strains. Pharmaceutical companies who use virus data from GISRS for products (such as vaccines or antivirals) provide in-kind donations or discounted product doses to WHO for deployment during a pandemic and also partner contributions, which are used to bolster capabilities for PPR (such as surveillance) for low-income countries
participating in GISRS. Chapter 3 includes more details about areas of success and persisting gaps in the PIP Framework.
Governance structures such as the PIP Framework and the Nagoya Protocol on Access to Genetic Resources to the Fair and Equitable Sharing of Benefits Arising from Their Utilization of the Convention on Biological Diversity (discussed further in Chapter 3) have yet to be tested in an influenza pandemic. The world can now produce more vaccines than in 2009 but has not yet developed governance or coordination mechanisms to solve serious issues of vaccine access and equity, including the “switch,” poor market-based incentives for producing pandemic vaccines, and deployment and delivery.
Like access and benefit sharing, governance structures for the surveillance and monitoring of zoonotic influenza have evolved significantly since 2009. In 2004, the OIE/FAO Joint Network of Expertise on Animal Influenza (OFFLU) was founded during the peak of the H5N1 (avian influenza) crisis. WHO joined forces with the World Organisation for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO) in 2006, with the launch of the Global Early Warning System for Major Animal Diseases, including Zoonosis (GLEWS). This became an early One Health mechanism, linking together the three organizations for event-based disease surveillance. The 2009 H1N1 pandemic provided momentum for OFFLU, and in 2010, the three organizations published a Tripartite Concept Note on “Sharing responsibilities and coordinating global activities to address health risks at the animal-human-ecosystems interface” (FAO-OIE-WHO, 2010). They named avian influenza as a major priority for this alliance along with rabies and antimicrobial resistance (Dauphin, 2015; FAO-OIE-WHO, 2013). Through OFFLU, representatives of the veterinary and animal health sector have taken part in biannual WHO consultations on influenza virus surveillance data since 2011 that analyze surveillance data and provide recommendations on viral strains to use for influenza vaccines in the upcoming season. In this way, OFFLU participates in GISRS (routine influenza surveillance) and provides data collected from OIE/FAO Reference Centers and national animal health laboratories to WHO. Creating pre-pandemic CVVs for human vaccines relies heavily on this zoonotic virus data (Dauphin, 2015; Mackenzie et al., 2014).
In recent years, momentum has been building for extending the GISRS and GLEWS event-based surveillance or creating “GLEWS+” and “GISRS+.” GLEWS+ is designed as a cross-sectoral mechanism for conducting joint risk assessments for influenza and other zoonotic pathogens (FAO-OIE-WHO, 2013). The GISRS+ proposal is currently in its infancy at WHO. In WHO discussions during June 2021, it was suggested as a mechanism to build on and expand GISRS surveillance capabilities based on capacities developed and gaps identified during COVID-19 (see Figure 2-1). GISRS+
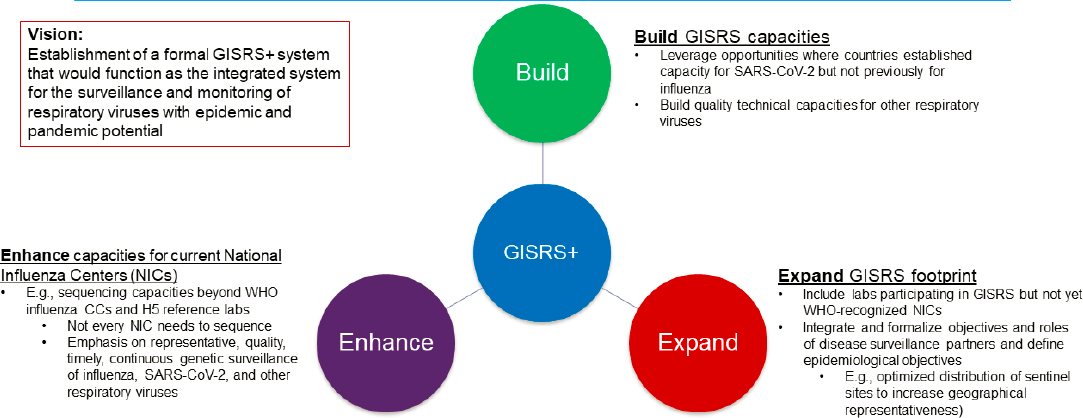
NOTE: CC = WHO Collaborating Centre; NIC = WHO National Influenza Centre; ORV = other respiratory virus.
SOURCES: Moen, 2021; WHO, 2021.
would function as the integrated system for surveillance and monitoring of respiratory viruses with epidemic and pandemic potential (WHO, 2021).
The GISRS+ proposal underscores the fact that GISRS structures have been leveraged during the COVID-19 response and can be expanded for other respiratory viruses. The laboratory network underpinning GISRS would expand, as would capacity building and training opportunities. Deciding which structures to extend (such as creating additional WHO CCs and how they will relate to GISRS) or redesign for other respiratory viruses (such as in the PIP Framework) will require a formal coordination mechanism. WHO is considering mechanisms required to support GISRS+ as a formal health governance structure.
A SNAPSHOT OF CURRENT INFLUENZA VACCINE GOVERNANCE STRUCTURES AND FRAMEWORKS
The WHO GISRS and PIP Framework form a major piece of the multilateral ecosystem for influenza surveillance and control. However, the wider “regime complex” or global ecosystem for influenza pandemic vaccines is much more complex and extends far beyond WHO’s normative power. Box 2-2 provides a nonexhaustive overview of some of the major organizations and programs involved in global and regional influenza policy and governance (NASEM, 2018; Schroeder, 2018; WHO, 2019) based on whether they involve multilateral, bilateral, or regional coordination; international regulations; public–private partnerships (PPPs); industry partnerships; and civil society organization (CSO) partnerships. The structures highlighted demonstrate the often-blurred boundaries around seasonal and pandemic influenza vaccines and vaccination.
The list of organizations and programs in Box 2-1 reflects the 2019 Conference Report “Shaping meeting to explore the value of a coordinated work plan for epidemic and pandemic influenza vaccine preparedness,” which called for a systematic effort to map the wide range of national, regional, and global actors that make up the influenza vaccine ecosystem (Ruscio et al., 2020). While this undertaking is beyond the scope of this short consensus study, it is an important next step. Our abridged analysis of major influenza governance structures reinforces that meeting’s finding that strengthening influenza vaccine PPR must be “guided by an alliance of international stakeholders, to include, among others, governmental and nongovernmental organization representation, civil society representatives, vaccine manufacturers, international organizations, and health security and influenza experts” (Ruscio et al., 2020, p. 3179).
THE GLOBAL INFLUENZA STRATEGY AND THE GOVERNANCE PATH FORWARD
Many efforts have been undertaken to distill lessons from the 2009 H1N1 pandemic for developing and rolling out vaccines, and there is, and will be, even more impetus to learn lessons from the COVID-19 pandemic. Given the centrality of WHO’s role in coordinating pandemic responses, one of the most significant post-H1N1 developments was the WHO Global Influenza Strategy 2019–2030, which was issued in 2019 (WHO, 2019). In Annex 1 of the document, WHO discusses the achievements since the release of its first Strategy document in 2002. Annex 2 enumerates the areas wherein WHO sees ongoing challenges. The most relevant of these challenges, in terms of global vaccine PPR, are the following:
- Understanding influenza disease and economic burden: Understanding the morbidity, mortality, and economic burden of influenza enables policy makers to prioritize influenza, make evidence-based decisions, and develop effective immunization and treatment programs. WHO states that most estimates of disease and economic impact have come from HICs, and additional studies in LMICs are needed.
- Undertaking consistent epidemiological and virological surveillance: During the decade following the 2009 pandemic, GISRS was strengthened and improved. The number of member states sharing influenza viruses increased to 130 in 2017; member states sharing laboratory and epidemiological data through FluNet and FluID also increased. However, 31 percent and 58 percent of member states did not routinely share data on these respective platforms during 2016–2017. In addition, some countries still cannot detect novel influenza viruses, which is a core capacity under the IHR.
- Improving vaccine technologies and undertaking early and larger clinical trials that are more globally distributed: Vaccines remain the most effective means of preventing infection and potentially reducing clinical severity. The reliance on embryonated eggs for production is still predominant, but, as described in Chapter 1, it is time intensive. Furthermore, some influenza viruses are increasingly unfit to grow and tend to undergo antigenic changes with passage in eggs, underscoring the need to diversify current production capabilities and technologies. The holy grail is a universal influenza vaccine that would offer broader protection against multiple influenza strains. However, as Fauci underscored in 2018 (NASEM, 2018), it will require long-term investments in advanced manufacturing techniques, such as cell-based production and new platform technologies. Pre-
- pandemic clinical trials in adults and children may also inform the vaccination regimen required to induce a sufficient immune response for a novel influenza subtype, particularly next-generation viruses. This would reduce the response time for vaccine distribution if a similar subtype causes a future pandemic (Rockman et al., 2020).
- Building effective seasonal vaccination programs: Because influenza viruses are highly prone to mutating, seasonal vaccines are strain based, and vaccination must be against the predominant circulating strains each year. WHO states that the need for annual vaccination, coupled with varying and often low vaccine efficacy, continues to contribute to influenza vaccine hesitancy. Both vaccine distribution and use also reflect major disparities. Recent data show that 47 percent of the global population (residents of countries in the Eastern Mediterranean, South-East Asian and African WHO regions) received only 5 percent of annually distributed vaccines. Countries and the global community must understand and address barriers that affect influenza vaccine distribution, uptake, import, and regulation to strengthen seasonal programs and improve the market for them if pandemic capacity is to be kept “warm” by seasonal vaccination.
- Performing national pandemic planning: WHO developed pandemic influenza risk management guidance to encourage countries to develop national pandemic preparedness plans. In early 2018, WHO also published a checklist to help countries develop or update their plans. As of September 2018, however, few countries had updated their plans, and 101 countries did not have plans or had none publicly available. Without plans, countries would be hampered in terms of deploying vaccines during a pandemic (WHO, 2017).
In 2021, the Center for Infectious Disease Research and Policy (CIDRAP) took a step toward highlighting precisely how new technologies may be developed and harnessed for influenza. As discussed further in Chapter 4, its influenza vaccines R&D roadmap describes programs, policies, and financing that may accelerate progress for universal or broadly protective influenza vaccines. CIDRAP again underscores the importance of multi-sector and multi-actor collaboration for influenza governance; it is funded by the Wellcome Trust and advised by a steering group, composed of representatives of WHO, the Sabin Vaccine Institute, The Rockefeller Foundation, the Wellcome Trust, the Bill & Melinda Gates Foundation, and the Global Funders Consortium for Universal Influenza Vaccine Development (CIDRAP, 2021a).
The newest evolution of influenza governance may ultimately be through a pandemic treaty or instrument. The general concept of an international treaty on pandemic preparedness was put forward by the president of
the European Commission in December 2020 and has been endorsed by the European Union, the WHO director-general, and 26 heads of state, although the United States has expressed a preference for a nonbinding agreement (Viñuales et al., 2021). In May 2021, the World Health Assembly reached a consensus to hold a special session in November, during which an international treaty or instrument on pandemic preparedness will be debated (CIDRAP, 2021b; Gostin et al., 2021). This proposal follows the general pattern of reactivity; it is a product of COVID-19 reflections that individual organizations and governments cannot adequately prepare for pandemic threats alone. This argument was taken up by the Independent Review Panel for Pandemic Preparedness and Response (IPPPR). In its May 2021 report, it recommended stronger leadership and better coordination for pandemic preparedness through a more independent WHO, creation of a Global Health Threats Council, and a pandemic treaty.
The pandemic treaty may best be viewed as a recognition that a future global health crisis response system needs to go beyond embracing the IHR and WHO as the global health coordination agency. Much work needs to be done on whether it would be modeled along the lines of the Paris Climate Change Treaty or the Geneva Conventions and what it would require under the auspices of a Global Health Threats Council and institutions such as the G20. More work will be required to determine its core functions and boundaries. For instance, some have argued that it should focus on “deep prevention” to reduce the risk of pathogen spillover from humans to animals (drawing on inspiration from the global governance of nuclear, environmental, and financing system risks) (Viñuales et al., 2021). Others have put forward concrete end points, such as establishing a global agency, similar to WHO, that can coordinate governments, launch large-scale operations, enforce international rules, assess health systems, and provide objective technical advice to countries (Moon and Kickbusch, 2021).
The pandemic treaty debate goes beyond the boundaries of this study on influenza governance. What we are interested in is how to best to ensure that the successful governance structures and frameworks used for influenza inform proposals being advanced by the plethora of groups and studies and that the specific requirements of pandemic influenza are given due weight as a highly hazardous pathogen. Our argument is that the global influenza system may well be the most well established and functioning among those extant systems for pandemic preparedness (e.g., GISRS for surveillance, the PIP Framework for ABS, GAP for vaccine manufacturing capacity building, and IFPMA IVS to provide a link to market generation). Some of these global arrangements and agreements can be made much more visible and prominent as examples that can either effectively withstand geopolitical tensions and sovereignty control or effectively operate sufficiently under the radar at R&D, technical, production, and trade levels.
Each of the next three chapters considers three interrelated questions. What is working for influenza vaccination, from a governance and financing perspective, and should stay specific to influenza? What is working for influenza and should be expanded or scaled up to improve integrated PPR for pathogens with pandemic potential? What is missing from the influenza vaccine governance landscape, and what partnerships or coordination structures could fill these gaps? Each chapter includes key findings and conclusions, which both respond to challenges laid out in the Global Influenza Strategy and form the basis for subsequent recommendations.
KEY FINDINGS AND CONCLUSIONS
- Global coordination for vaccines and vaccination will not be successful without including both public (national governments) and private actors—including civil society and the pharmaceutical and biotechnology industry.
- One of the key challenges for developing broader surveillance systems is how to cover multiple pathogens spanning the human and animal realms. Surveillance represents a prime example of how ministries and organizations, particularly those in the animal and health sectors, can develop misaligned objectives that contribute to silos based on the competition for influence, power, and funding.
- Programs supporting platform technology R&D and industry partnerships to scale up vaccines and address supply chain chokeholds are often performed in a semi-isolated context. This is true for influenza and other respiratory pathogens with pandemic potential. There is a need to move away from historically siloed systems toward a single architecture for the global coordination of PPR, in the spirit of the GISRS+ proposal.
- WHO and other international organizations cannot direct national ministries to take certain steps. They can, however, offer technical and managerial guidance, suggest policy solutions and mechanisms to facilitate multilateral agreements at the country level, and provide close follow-up.
- WHO is well placed to provide normative guidance, technical support for integrated surveillance, and regulatory support for vaccine licensure, for the coordination of global, regional, and national programs for PPR that recognize the importance of operating across multiple pandemic threats.
- WHO is less well positioned to support activities that require deep engagement with the private sector and industry, including vaccine manufacturing, supply chains, and deployment across multiple pathogens with pandemic potential.
REFERENCES
Ampofo, W. K., N. Baylor, S. Cobey, N. J. Cox, S. Daves, S. Edwards, N. Ferguson, G. Grohmann, A. Hay, J. Katz, K. Kullabutr, L. Lambert, R. Levandowski, A. C. Mishra, A. Monto, M. Siqueira, M. Tashiro, A. L. Waddell, N. Wairagkar, J. Wood, M. Zambon, and W. Zhang. 2012. Improving influenza vaccine virus selection: Report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14–16 June 2010. Influenza and Other Respiratory Viruses 6(2):142–152, e141–e145.
Bresee, J. S., K. E. Lafond, M. McCarron, E. Azziz-Baumgartner, S. Y. Chu, M. Ebama, A. R. Hinman, A. Xeuatvongsa, S. Bino, D. Richardson, R. M. Porter, A. Moen, M. McKinlay, G. Sahakyan, S. Wangchuk, P. Ruowen, Z. Yongchao, C. Linlin, C. Daouda, O. Tarkhan-Mouravi, P. Gould, P. Muthoka, G. O. Emukule, S. S. Chaves, M.-A. Widdowson, D. Otorbaeva, V. Khanthamaly, K. Stavridis, V. Mikic, N. Furtuna, D. Capmari, B. Alexander, E. Dueger, M. Kamolzoda, J. Mott, A. Bin Salah, M. Mazur, A. Maria Ropero Alvarez, S. J. Olsen, S. Mirza, C. Sofia Arriola, J. Seward, S. Kluglein, A. F. Bolster, N. Minh Hang, J. W. McFarland, N. Ha Thu, and T. Thi Minh Nguyen. 2019. The partnership for influenza vaccine introduction (PIVI): Supporting influenza vaccine program development in low- and middle-income countries through public–private partnerships. Vaccine 37(35):5089–5095.
Broor, S., H. Campbell, S. Hirve, S. Hague, S. Jackson, A. Moen, H. Nair, R. Palekar, S. Rajatonirina, P. G. Smith, M. Venter, N. Wairagkar, M. Zambon, T. Ziegler, and W. Zhang. 2020. Leveraging the global influenza surveillance and response system for global respiratory syncytial virus surveillance-opportunities and challenges. Influenza and Other Respiratory Viruses 14(6):622–629.
Carroll, D., S. Morzaria, S. Briand, C. K. Johnson, D. Morens, K. Sumption, O. Tomori, and S. Wacharphaueasadee. 2021. Preventing the next pandemic: The power of a global viral surveillance network. BMJ 372:n485.
CEPI (Coalition for Epidemic Preparedness Innovations). 2021. CEPI launches plan to tackle risk of future pandemics and epidemics. March 10, 2021. https://cepi.net/news_cepi/cepi-launches-plan-to-tackle-risk-of-future-pandemics-and-epidemics (accessed July 7, 2021).
CIDRAP (Center for Infectious Disease Research and Policy). 2021a. Influenza vaccines research and development (R&D) roadmap. Minneapolis, MN: CIDRAP.
CIDRAP. 2021b. WHA to hold pandemic treaty talks in November. https://www.cidrap.umn.edu/news-perspective/2021/05/wha-hold-pandemic-treaty-talks-november (accessed October 21, 2021).
Cohen, D., and P. Carter. 2010. Conflicts of interest. WHO and the pandemic influenza “conspiracies.” BMJ 340:c2912.
Dauphin, G. 2015. WHO/FAO/OIE tripartite coordination for the control and prevention of zoonotic influenza viruses: Example of OFFLU, global network of veterinary expertise. Bulletin de l’Académie Vétérinaire de France 168(3):224–232.
FAO-OIE-WHO (Food and Agriculture Organization of the United Nations-World Organisation for Animal Health-World Health Organization). 2010. The FAO-OIE-WHO collaboration: Sharing responsibilities and coordinating global activities to address health risks at the animal-human-ecosystems interfaces. A tripartite concept note. https://www.who.int/foodsafety/zoonoses/final_concept_note_Hanoi.pdf (accessed December 17, 2021).
FAO-OIE-WHO. 2013. GLEWS+: The joint FAO-OIE-WHO global early warning system for health threats and emerging risks at the human–animal–ecosystems interface. http://www.glews.net/?page_id=1041 (accessed December 17, 2021).
Fidler, D. P. 2010. Negotiating equitable access to influenza vaccines: Global health diplomacy and the controversies surrounding avian influenza H5N1 and pandemic influenza H1N1. PLoS Medicine 7(5):e1000247.
Flynn, P. 2010. The handling of the H1N1 pandemic: More transparency needed. https://assembly.coe.int/CommitteeDocs/2010/20100329_MemorandumPandemie_E.pdf (accessed October 21, 2021).
GISAID. 2020. History. https://www.gisaid.org/about-us/history (accessed October 23, 2021).
Gostin, L. O, S. F. Halabi, and K. A. Klock. 2021. An international agreement on pandemic prevention and preparedness. JAMA 326(13):1257–1258.
Hampton, L. 2011. Why delivering COVID-19 vaccines might be just as hard as developing them: From liability laws to production delays, the 2009 H1N1 swine influenza vaccine rollout offers a cautionary tale for today. https://www.gavi.org/vaccineswork/why-delivering-covid-19-vaccines-might-be-just-hard-developing-them (accessed July 6, 2021).
Hay, A. J., and J. W. McCauley. 2018. The WHO global influenza surveillance and response system (GISRS)—a future perspective. Influenza and Other Respiratory Viruses 12(5):551–557.
Hayman, B., and S. Pagliusi. 2020. Emerging vaccine manufacturers are innovating for the next decade. Vaccine X 5:100066.
IPPPR (Independent Review Panel for Pandemic Preparedness and Response). 2021. COVID-19: Make it the last pandemic. https://theindependentpanel.org/wp-content/uploads/2021/05/COVID-19-Make-it-the-Last-Pandemic_final.pdf (accessed October 21, 2021).
Kamradt-Scott, A., C. Dolea, C. Poncé, G. Rodier, M. Lamunu, P. Drury, and S. Ioos. 2018. WHO tracking mechanism for IHR additional health measures. Lancet 392(10161):2250–2251.
Knox, R. 2009. Manufacturing problems with swine influenza vaccine. https://www.npr.org/sections/health-shots/2009/07/manufacturing_problems_with_sw.html?t=1605551277283 (accessed July 6, 2021).
MacDonald, N., E. Mohsni, Y. Al-Mazrou, J. Kim Andrus, N. Arora, S. Elden, M.-Y. Madrid, R. Martin, A. Mahmoud Mustafa, H. Rees, D. Salisbury, Q. Zhao, I. Jones, C. A. Steffen, J. Hombach, K. L. O’Brien, and A. Cravioto. 2020. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine 38(33):5364–5371.
Mackenzie, J. S., M. McKinnon, and M. Jeggo. 2014. One health: From concept to practice. Confronting Emerging Zoonoses 163–189.
Moen, A. 2021 Looking forward: GISRS+. Paper presented at Influenza Preparedness and Response Health Emergencies Programme, June 22, 2021.
Monto, A. S. 2017. Moving toward improved influenza vaccines. The Journal of Infectious Diseases 215(4):500–502.
Moon, S., and I. Kickbusch. 2021. A pandemic treaty for a fragmented global polity. The Lancet 6(6):E355–E356.
Moyer, C. S. 2010. H1N1 vaccine: what physician can do with leftover doses. https://amednews.com/article/20100719/profession/307199943/6 (accessed December 17, 2021).
Pagliusi, S., M. Dennehy, and A. Homma. 2020. Two decades of vaccine innovations for global public good: Report of the Developing Countries’ Vaccine Manufacturers Network 20th meeting, 21–23 October 2019, Rio de Janeiro, Brazil. Vaccine 38(36):5851–5860.
Rockman, S., K. Laurie, and I. Barr. 2020. Pandemic influenza vaccines: What did we learn from the 2009 pandemic and are we better prepared now? Vaccines 8(2):211.
Ruscio, B., A. Bolster, J. Bresee, A. Abelin, P. Boutet, H. Christiansen, P. Etholm, S. Desai, B. Gellin, J. Golding, M. Jit, L. Kerr, M. McKinlay, S. Kluglein, F. Lobos, S. Mathewson, M. Mazur, S. Pagliusi, P. Penttinen, D. Richardson, A. M. Ropero Alvarez, J. R. Scovitch, J. E. Seedorff, L. Shaxson, J. S. Tam, B. Taylor, N. Wairagkar, J. Watson, and A. Xeuatvongsa. 2020. Shaping meeting to explore the value of a coordinated work plan for epidemic and pandemic influenza vaccine preparedness. Vaccine 38(16):3179–3183.
Schroeder, K. 2018. Global challenges in seasonal influenza vaccine supply, use, and policy. Intersect: The Stanford Journal of Science, Technology, and Society 12(1):1–23.
Shu, Y., and J. McCauley. 2017. GISAID: Global initiative on sharing all influenza data—from vision to reality. Euro Surveillance 22(13):30494.
Viñuales, J., S. Moon, G. L. Moli, and G.-L. Burci. 2021. A global pandemic treaty should aim for deep prevention. The Lancet 397(10287):1791–1792.
WHO (World Health Organization). 2008. International Health Regulations. Geneva, Switzerland: World Health Organization.
WHO. 2011. Comparative analysis of national pandemic influenza preparedness plans. Geneva, Switzerland: World Health Organization.
WHO. 2012. Report of the WHO pandemic influenza A (H1N1) vaccine deployment initiative. Geneva, Switzerland: World Health Organization.
WHO. 2017. Pandemic influenza risk management: A WHO guide to inform and harmonize national and international pandemic preparedness and response. Geneva, Switzerland: World Health Organization. http://apps.who.int/iris/bitstream/handle/10665/259893/WHO-WHE-IHM-GIP-2017.1-eng.pdf?sequence=1 (accessed January 4, 2019).
WHO. 2019. Global influenza strategy 2019–2030. Geneva, Switzerland: World Health Organization. https://www.who.int/publications/i/item/9789241515320 (accessed October 21, 2021).
WHO. 2021 (unpublished). Enhancing the global influenza surveillance and response system for respiratory viruses with epidemic and pandemic potential. Draft Concept Note.
Ziegler, T., A. Mamahit, and N. J. Cox. 2018. 65 years of influenza surveillance by a World Health Organization–coordinated global network. Influenza and Other Respiratory Viruses 12(5):558–565.




















