1
Introduction
Formaldehyde, a one-carbon molecule, is a flammable, colorless gas that has a distinct, strong odor. It is endogenously produced in humans through one-carbon metabolism and is also widely present in the environment. It is one of the higher-production chemicals by volume and is used in many manufactured goods: wood products (e.g., cabinets, furniture, plywood, particleboard, laminate flooring, etc.), permanent press fabrics, and household products (e.g., glues, paints, caulks, pesticides, cosmetics, detergents, etc.). It is also formed by combustion sources and is present in cigarette smoke and in emissions from electronic cigarettes, as well as in emissions from gas stoves and open fireplaces.
This report reviews an assessment of the human health risks of formaldehyde that has been carried out by the Integrated Risk Information System (IRIS) Program of the U.S. Environmental Protection Agency (EPA) (EPA, 2022a). According to its website (EPA, 2022b), the IRIS Program was established in 1985 to:
provide an internal database of human health assessments for chemicals found in the environment. The goal of the IRIS Program was to foster consistency in the evaluation of chemical toxicity across the Agency.
For the selected chemicals, the IRIS Program carries out hazard assessments and provides toxicity values. The program is housed within EPA’s Office of Research and Development (ORD). The program’s website states (EPA, 2022b, 2023):
EPA’s mission is to protect human health and the environment. EPA’s IRIS Program supports this mission by identifying and characterizing the health hazards of chemicals found in the environment.
EPA’s first evaluation on the health effects of formaldehyde was under its Office of Pesticides and Toxic Substances (OPTS, 1987). The IRIS Program first reviewed the evidence on formaldehyde and proposed a cancer unit risk estimate (URE) in 1989 and a noncancer reference dose (RfD) in 1990 (NCEA, 1989). The RfD of 0.2 mg/kg/day is based on reduced weight gain and histopathology from a two-year oral bioassay in rats (Til et al., 1989). For carcinogenicity, the weight-of-evidence characterization was B1-probable human carcinogen, based on limited evidence in humans and sufficient evidence in animals, along with supporting mechanistic data (in vitro genotoxicity data and formaldehyde’s structural relationships to other carcinogenic aldehydes, such as acetaldehyde). An inhalation URE of 1.3E-5 per ug/m3 was based on squamous cell carcinomas in male F344 rats (Kerns et al., 1983). The carcinogenicity of formaldehyde was subsequently evaluated by the International Agency for Research on Cancer (IARC), the National Toxicology Program’s Report on Carcinogens (NTP ROC), and in 2014 by a National Research Council (NRC) ad hoc committee (see Figure 1-1) (NRC 2014b).
In 1981 and affirmed the following year, IARC classified formaldehyde as “possibly carcinogenic to humans” (Group 2B), based on recently released findings of nasal cancer in animal studies and short-term studies showing genotoxicity (IARC, 1982a,b). With the growing evidence of nasal cancer from occupational studies, in 1987 and 1995 IARC reclassified formaldehyde as “probably carcinogenic to humans” (Group 2A) based on “limited” evidence of cancer in humans
and “sufficient” evidence in experimental animals (IARC, 1987, 1995). In 2004 (published 2006), IARC classified formaldehyde as “carcinogenic to humans” (Group 1) based on sufficient evidence for nasopharyngeal carcinoma (IARC, 2006). In 2009 (published in 2012), the IARC classification of formaldehyde in Group 1 was reaffirmed, and formaldehyde was also determined to cause leukemia (IARC, 2012).
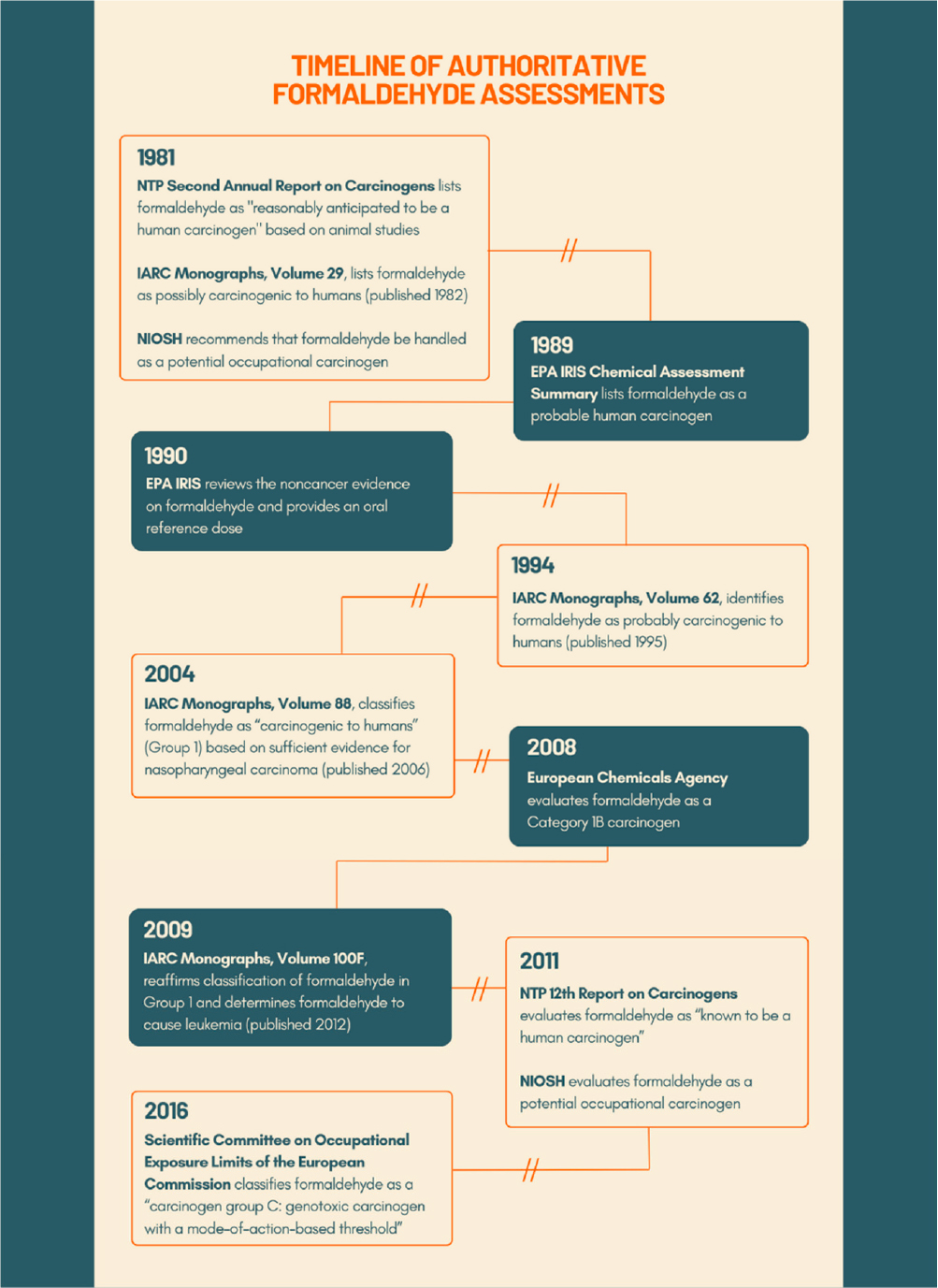
A similar pattern of higher levels of hazard identification classification for formaldehyde can be seen in determinations of another authoritative program in the area of carcinogenicity assessment—the NTP ROC. In 1981, formaldehyde was designated in the ROC as a “reasonably anticipated human carcinogen” (NTP, 1981). More recently, in 2011, the NTP ROC evaluated formaldehyde as “known to be a human carcinogen” (NTP, 2011), a finding that was reaffirmed by an ad hoc NRC committee in 2014 (NRC, 2014b).
The timeline of EPA’s development of the Draft IRIS Toxicological Review of Formaldehyde: Inhalation (hereafter referred to as the 2010 or 2022 Draft Assessment) in the context of reports from the NRC (2011, 2014a) and the National Academies of Sciences, Engineering, and Medicine (in 2018 and 2022) is shown in Figure 1-2.
In 2010, EPA updated its formaldehyde Draft Assessment, which underwent review by an ad hoc NRC committee. The resulting report was released in 2011 (NRC, 2011). Thereafter, EPA began working on a revised Draft Assessment in 2012, convened workshops in 2014, and completed a revised Draft Assessment in 2017. In 2018, work was suspended on the Draft Assessment, according to the IRIS website (EPA, 2022b). The Draft Assessment was updated beginning in 2021 before being released in April 2022. The 2022 Draft Assessment consists of three documents: the Main Assessment (789 pages), accompanying Appendices (1059 pages), and an Assessment Overview (192 pages).
The 2011 NRC review committee identified numerous specific and general problems with EPA’s 2010 Draft Assessment. To summarize, the committee found that the 2010 Draft Assessment lacked clarity, and the assessment methods were not well documented, leading to issues with transparency in how the conclusions of the assessment were drawn. The committee noted that poor documentation of methods was a finding of other NASEM committees that had reviewed IRIS assessments. The 2010 Draft Assessment was characterized as not prepared in a coherent, consistent fashion, with clear linkages to an underlying framework. The committee also concluded that the Draft Assessment did not contain sufficient documentation of methods and criteria for identifying evidence from studies, for evaluating studies, for assessing the weight of evidence, for selecting studies for derivation of toxicity and risk estimates, and for characterizing uncertainty and variability. Specifically, the 2011 NRC report contained the following general recommendations:
- First, rigorous editing is needed to reduce the volume of the text substantially and address the redundancies and inconsistencies; reducing the text could greatly enhance the clarity of the document.
- Second, Chapter 1 of the draft assessment needs to discuss more fully the methods of the assessment. The committee is recommending not the addition of long descriptions of EPA guidelines but rather clear concise statements of criteria used to exclude, include, and advance studies for derivation of the RfCs and unit risk estimates.
- Third, standardized evidence tables that provide the methods and results of each study are needed for all health outcomes; if appropriate tables were used, long descriptions of the studies could be moved to an appendix or deleted.
- Fourth, all critical studies need to be thoroughly evaluated for strengths and weaknesses by using uniform approaches; the findings of these evaluations could be summarized in tables to ensure transparency.
- Fifth, the rationales for selection of studies that are used to calculate RfCs and unit risks need to be articulated clearly.
- Sixth, the weight-of-evidence descriptions need to indicate the various determinants of “weight.” The reader needs to be able to understand what elements (such as consistency) were emphasized in synthesizing the evidence.
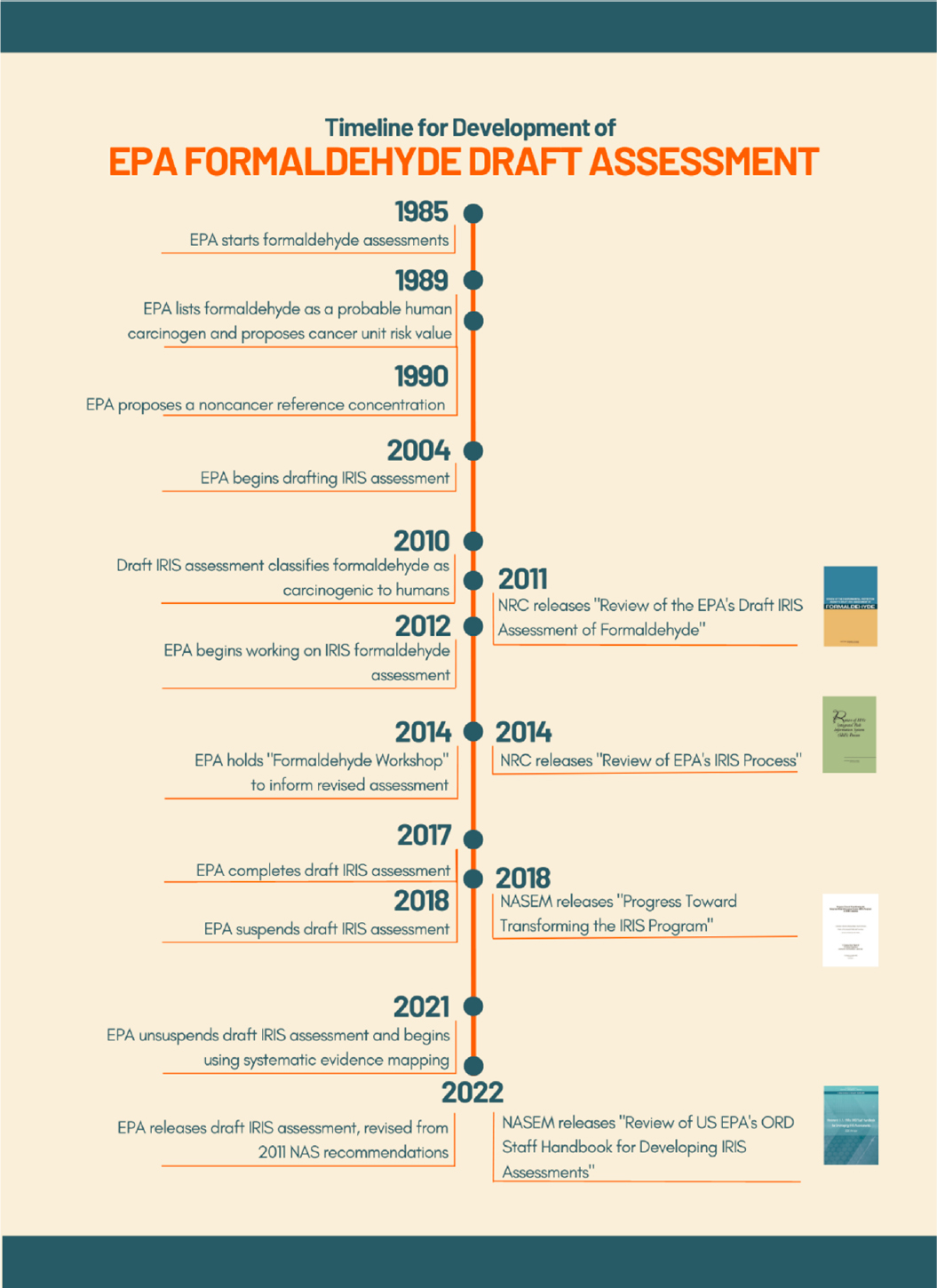
The report’s last chapter, titled “A Roadmap for Revision,” offered suggestions for changing the IRIS process to bring it closer to the state of practice for systematic review and evidence integration.
Several subsequent ad hoc NRC (in 2014) and NASEM (in 2018 and 2022) committees provided a series of recommendations in their reports (NRC, 2014a; NASEM, 2018, 2022) that encouraged the IRIS Program to adopt systematic review approaches, to create a staff handbook with general guidance on the methods for the IRIS assessments, and to develop an a priori protocol for each major IRIS assessment. The 2014 NRC committee defined systematic review as encompassing problem formulation, protocol development, evidence identification, evidence evaluation, and evidence integration to inform hazard identification and dose response. The committee provided specific recommendations on each step of the process.
The 2014 committee noted the substantial improvements made in the IRIS process and offered recommendations for building on that progress, including the creation of a handbook to “provide a single detailed guidance document for all those involved in the development of IRIS assessments.” The 2018 NASEM committee also noted the substantial progress made in the IRIS process and presented findings around adherence to the 2014 recommendations. The 2018 committee observed that guidance for conducting newly planned IRIS assessments is contained in protocols that may overlap with the handbook’s description of standard operating procedures. EPA noted that, although not provided to the 2018 committee, a handbook was under development. The version of the handbook released in 2020 was reviewed in a 2022 NASEM report, which highlighted opportunities to improve the handbook’s scientific rigor and clarity. The report included a recommendation that a time-stamped, read-only final version of the protocol be released before further IRIS assessments were conducted. The 2022 committee recommended clarifying that the protocol would constitute a complete account of planned methods. EPA released an updated version of the handbook in December 2022 which included the recommendations from the NASEM committee.
THE COMMITTEE, ITS TASK, AND ITS APPROACH
EPA requested that NASEM convene a committee to review the 2022 Draft Assessment (EPA, 2022a), which was released for the committee’s evaluation in April 2022. Reflecting its task (Box 1-1), the committee included expertise in public health risk assessment, systematic review methods, biostatistics, environmental epidemiology, toxicology, carcinogenesis (leukemogenesis), reproductive effects, developmental effects, neurotoxicology, respiratory effects (including asthma), biological modeling, exposure assessment, and dose-response analysis (see Appendix A for biographical information on the committee members).
The committee’s charge was to review the 2022 Draft Assessment prepared by EPA, and not to conduct its own formaldehyde assessment. The committee also was not charged with commenting on other interpretations of scientific information relevant to the hazards and risks of formaldehyde, or with reviewing alternative opinions of EPA’s assessment. Any other topics not falling within the committee’s charge were excluded from the committee’s purview.
To address its task, the committee held nine meetings, including three open sessions with public comment periods. Appendix B provides the agendas for the open sessions and a list of the more than 40 public commenters who provided oral input, as well as web links to the presentations, the recordings, and the documents provided by EPA that were reviewed by the committee.
The first open session was convened on October 12, 2022, and included a presentation and question-and-answer session with Professor Lisa Bero, chair of the 2022 NASEM ad hoc committee that reviewed the 2020 draft of the IRIS handbook; a presentation and question-and-answer session with EPA staff on the 2022 Draft Assessment; and a public comment period. The second
open session was convened on December 22, 2022, to provide additional opportunity for public comment. The third open session, held on January 30, 2023, provided an opportunity for the committee to ask questions of EPA staff, as well as to hold a public comment period. In addition to requesting answers to its questions in writing from EPA, the committee had requested an opportunity for EPA to provide any additional clarifications on the answers it had prepared for the committee, which EPA did following the session. Written materials from EPA were made publicly available via the meeting website (see links in Appendix B). In addition to the opportunities provided for oral remarks during the three public meetings, stakeholders were encouraged to submit written comments or other materials relevant to the committee’s charge at any time during the course of the study. Written input provided by members of the public is available upon request via the study’s public access file.
In line with its statement of task, the committee considered that addressing any Tier 1 recommendations would be important to improve critical scientific concepts, issues, or narrative in the 2022 Draft Assessment. Tier 2 and Tier 3 recommendations could also trigger additional work on the Draft Assessment, including document editing to better clarify and support the assessment’s conclusions.
To address its statement of task, the committee organized its review of EPA’s hazard identification and dose-response analyses of noncancer and cancer outcomes around EPA’s overview of its approach to developing the 2022 Draft Assessment (Figure 1-3). This framework follows the most recent version of the IRIS handbook, as reviewed by the 2022 ad hoc NASEM committee; it also parallels the framework proposed for the IRIS process in the 2014 NRC report.
As a comparison for the methods of the 2022 Draft Assessment and their documentation, the committee relied on general principles for conducting a systematic review and for ensuring transparency. To better understand the state of practice applied in preparing the Draft Assessment, the committee sought the protocols for EPA’s reviews of noncancer, cancer, and mechanistic evidence so they could be evaluated against accepted systematic review methods that existed at the time the Draft Assessment was prepared. The committee also relied on EPA’s responses to the questions it had posed.
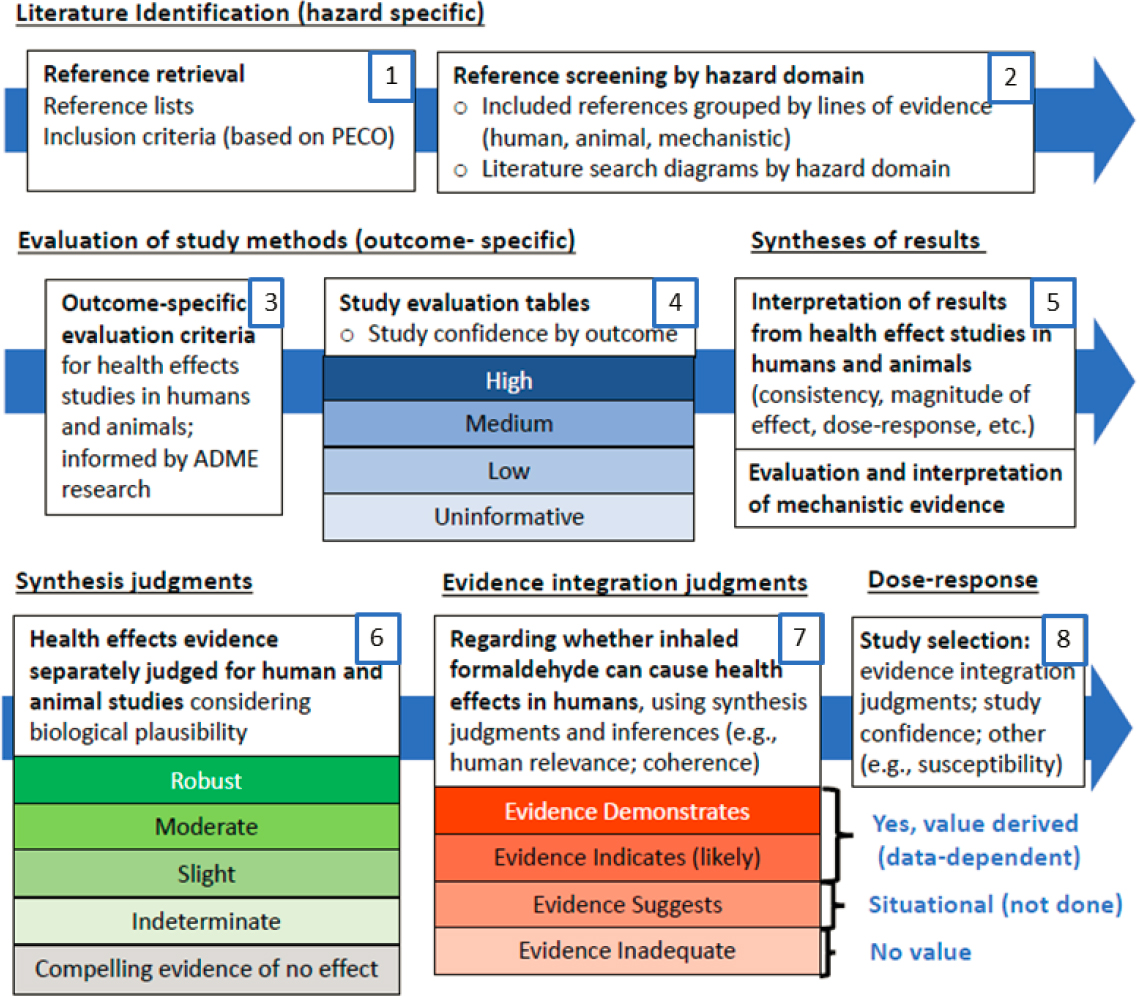
NOTE: Modified from EPA’s presentation to the committee on October 12, 2022.
The committee provided critiques and suggestions on EPA’s methods for each step in the assessment (documentation of methods, evidence identification, study evaluation, evidence synthesis, evidence integration, and dose-response assessment). For each step, the committee considered the alignment of the 2022 Draft Assessment methods with the contemporaneous state of practice and prior advice to EPA from the National Academies. Transparency in EPA’s systematic review methods implies that the committee should be able to replicate each step based on the information included in the assessment documents or in publicly available supplemental materials. Accordingly, the committee used a case study approach to provide a detailed evaluation of the transparency and replicability of the 2022 Draft Assessment methods, relying on the documentation provided by EPA in the 2022 Draft Assessment and in the written responses to the committee’s questions EPA’s response to the committee’s questions1.
___________________
1 See https://www.nationalacademies.org/documents/embed/link/LF2255DA3DD1C41C0A42D3BEF0989ACAECE3053A6A9B/file/D3A22C29743668E583CD8759F633481333EE3E7ECF54?noSaveAs=1, Tables 2 and 3 (accessed July 23, 2023).
The committee also reviewed the hazard and dose-response conclusions for noncancer outcomes (covering sensory irritation, pulmonary function, respiratory pathology, allergy and asthma, reproductive and developmental toxicity, and neurotoxicity) and for cancer outcomes. This aspect of the committee’s review addressed each step of EPA’s assessment methods for each outcome as used to develop evidence integration judgments and derive risk estimates for formaldehyde. In line with its overall charge, the committee focused its review on whether the 2022 Draft Assessment adequately and transparently evaluated the available studies and data, and used appropriate methods in reaching hazard identification conclusions and dose-response analyses that are supported by the scientific evidence. In its review, the committee also considered the recommendations of prior NRC and NASEM committees, including the 2014 NRC committee that reviewed the formaldehyde assessment of the National Toxicology Program (NTP) 12th Report on Carcinogens. In accordance with its statement of task, the committee did not conduct an independent assessment of formaldehyde’s hazards and risks.
ORGANIZATION OF THE REPORT
This report is organized into five chapters and five appendices. Chapter 2 addresses the assessment development methods and organization of the 2022 Draft Assessment. It covers the state of practice, drawing on relevant reports from the NRC (in 2011 and 2014) and NASEM (in 2018 and 2022), addresses the responsiveness of the 2022 Draft Assessment to the recommendations provided by the NRC (2011), and provides illustrative examples of the transparency and replicability of EPA’s assessment using a case study approach. Chapter 3 reviews EPA’s analysis of toxicokinetics. Chapters 4 and 5 review hazard and dose-response for noncancer and cancer outcomes, respectively. For each outcome considered in Chapters 4 and 5, the adequacy of the following aspects is addressed: the literature identification; study evaluation criteria; synthesis and judgments, including any mode-of-action considerations; overall hazard conclusions; and dose-response evaluation. Appendix A contains biographical information on the committee members. Appendix B includes the meeting agendas for the open sessions and a list of the more than 40 public commenters who provided oral input, as well as web links to the presentations and recordings, as well as the documents provided by EPA that the committee reviewed. Appendices C and D contain the committee’s case studies. Appendix E gives examples of issues identified by the committee that should be addressed as EPA revises and finalizes the Draft Assessment.
REFERENCES
EPA (U.S. Environmental Protection Agency). 2022a. IRIS Toxicological Review of Formaldehyde-Inhalation, External Review Draft. Washington, DC. https://iris.epa.gov/Document/&deid=248150 (accessed September 18, 2023).
EPA. 2022b. IRIS Program. Washington, DC. https://www.epa.gov/iris (accessed July 9, 2023).
EPA. 2023. Basic information about the Integrated Risk Information System: History of IRIS. Washington, DC. https://www.epa.gov/iris/basic-information-about-integrated-risk-information-system#history (accessed May 11, 2023).
IARC (International Agency for Research on Cancer). 1982a. IARC monographs supplement 4: Chemicals, industrial processes and industries associated with cancer in humans. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. Lyon, France: World Health Organization.
IARC. 1982b. Some industrial chemicals and dyestuffs. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, volume 29. Lyon, France: World Health Organization.
IARC. 1987. IARC monographs supplement 7: Overall evaluations of carcinogenicity: An updating of IARC monographs volumes 1–42. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. Lyon, France: World Health Organization.
IARC. 1995. Wood dust and formaldehyde. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, volume 62. Lyon, France: World Health Organization.
IARC. 2006. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, volume 88. Lyon, France: World Health Organization.
IARC. 2012. Chemical agents and related occupations. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, volume 100F. Lyon, France: World Health Organization.
Kerns, W. D., K. L. Pavkov, D. J. Donofrio, E. J. Gralla, and J. A. Swenberg. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Research 43(9):4382–4392.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2018. Progress toward transforming the Integrated Risk Information System (IRIS) program. Washington, DC: The National Academies Press.
NASEM. 2022. Review of U.S. EPA’s ORD staff handbook for developing IRIS assessments: 2020 version. Washington, DC: The National Academies Press.
NCEA (National Center for Environmental Assessment). 1989. Formaldehyde; CASRN 50-00-0. Washington, DC: The Environmental Protection Agency. https://iris.epa.gov/static/pdfs/0419_summary.pdf (accessed July 9, 2023).
NRC (National Research Council). 2011. Review of the Environmental Protection Agency’s draft IRIS assessment of formaldehyde. Washington, DC: National Academies Press.
NRC. 2014a. Review of EPA’s Integrated Risk Information System (IRIS) process. Washington, DC: The National Academies Press.
NRC. 2014b. Review of the formaldehyde assessment in the National Toxicology Program 12th Report on carcinogens. Washington, DC: The National Academies Press.
NTP (National Toxicology Program). 1981. Second annual report on carcinogens. Washington, DC: Department of Health and Human Services, Public Health Service.
NTP. 2011. 12th report on carcinogens. Washington, DC: Department of Health and Human Services, Public Health Service.
OPTS (Office of Pesticides and Toxic Substances). 1987. Assessment of health risks to garment workers and certain home residents from exposure to formaldehyde. Washington, DC: The Environmental Protection Agency.
Til, H. P., R. A. Woutersen, V. J. Feron, V. H. Hollanders, H. E. Falke, and J. J. Clary. 1989. Two-year drinking-water study of formaldehyde in rats. Food and Chemical Toxicology 27(2):77–87.









