Page 291
10—
Dietary Fiber
Dietary fiber is a complex material; its composition varies from one food to another. Trowell (1972) first defined dietary fiber as components of the plant cell wall that resist digestion by secretions of the human alimentary tract. These include cellulose, hemicelluloses, pectin, and lignin. Later, he extended the definition to include indigestible plant materials that are not cell-wall components. These materials include gums, such as guar and locust bean gums, algal polysaccharides, such as alginates and carrageenan, and mucilages (Trowell et al., 1976). This expanded definition recognizes that analytical methods failed to distinguish indigestible plant cell-wall components from indigestible storage polysaccharides that exist within plant cells. The term edible fiber has also been suggested and includes, in addition to the above-named components, chitins from fungi and crustaceans, indigestible fiberlike materials such as aminopolysaccharides from animals, and partially synthetic polysaccharides such as methyl cellulose (Trowell et al., 1978). Recently, the Life Sciences Research Office of the Federation of American Societies for Experimental Biology defined dietary fiber as ''the endogenous components of plant materials in the diet which are resistant to digestion by enzymes produced by humans" (LSRO, 1987). This definition excludes such substances as cutin, saponins, phytates, lectins, proteins, waxes, nonenzymatic browning products, resistant starch, silicon, and other inorganic constituents, which are associated with the plant cell wall. A Canadian report on dietary fiber contained a similar definition, but it included some nonnutritive substances (e.g., phytates) associated with the plant cell wall (Health and Welfare Canada, 1985). The term endogenous in the 1987 LSRO definition of dietary fiber excludes indigestible substances (e.g., nonenzymatic browning products) formed during food processing.
Analytically, dietary fiber is defined as nonstarch polysaccharides and lignin from plants. Lignin is a complex polymer of phenylpropane residues; the remaining dietary fiber components are polysaccharides. These polysaccharides resist digestion because they are non-a-linked-glucan-polysaccharides, whereas the human digestive tract appears to secrete only a-glucosidases (Southgate, 1982). Any degradation of dietary fiber in the human gastrointestinal tract results from the action of enzymes secreted by the intestinal microflora.
Various fractionations of dietary fiber are shown in Figure 10-1. The nonstarch polysaccharides consist of cellulose, hemicelluloses, pectin, gums, and mucilages, whereas noncellulosic polysaccharides include all of these except cellulose.
It is difficult to analyze dietary fiber chemically and thus to understand its role in health. Problems in analyzing this complex substance are reviewed by Dintzis (1982), Lanza and Butrum (1986),
Page 292
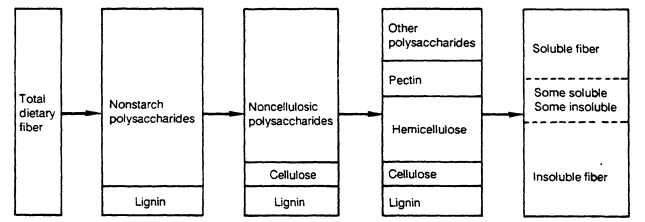
FIGURE 10-1.
Total dietary fiber can be categorized as shown above. For example, total dietary fiber can be divided into
nonstarch polysaccharides and lignin. Nonstarch polysaccharides can be further divided into noncellulosic
polysaccharides and cellulose. Note: Cellulose, lignin, and most hemicelluloses are not soluble in water, whereas
pectins and other polysaccharides (such as gums and mucilages) are water soluble. Adapted from Asp and Johansson (1984).
Selvendran and Du Pont (1984), Southgate et al. (1978), and Theander (1981).
Pectins, gums, and mucilages are soluble in water, as are some hemicelluloses, whereas lignins, cellulose, and most hemicelluloses are insoluble in water. Although the physical and chemical properties of different dietary fiber components, such as viscosity, water-holding capacity, ion-exchange capacity, and binding capacity, have been studied, these properties do not adequately predict the physiological properties of specific dietary fibers and of high-fiber whole foods.
Physiological Effects of Dietary Fiber
Some physiological effects of dietary fiber are systemic, whereas others are localized in the gastrointestinal tract. Diets with a high fiber content are high-volume diets, requiring longer mastication and ingestion time (Heaton, 1980), and subjective assessments indicate that they may increase satiety (Bolton et al., 1981; Duncan et al., 1983; Kay and Stitt, 1978). Although guar gum and pectin, both viscous fibers, have been shown in some studies to delay gastric emptying (Holt et al., 1979; Tadesse, 1982; Wilmshurst and Crawley, 1980), other studies fail to confirm this (Rydning et al., 1985).
Some soluble dietary fiber components, such as oat bran, pectin, and guar gum, stimulate fecal excretion of bile acids. However, wheat bran has no such effect; it promotes a different composition of bile acids than does pectin (Hillman et al., 1986; Pomare and Heaton, 1973; Pomare et al., 1976). Some soluble fibers also lower serum cholesterol (Judd and Truswell, 1985). In short-term studies (a single meal or a few days), soluble fiber fed to healthy subjects enhanced glucose tolerance and increased insulin sensitivity, but the results of longer studies are conflicting (LSRO, 1987).
Dietary fiber affects colonic function and activities of the microflora. High fiber intakes promote increased bacterial mass but do not alter the microflora composition (Baird et al., 1977; Drasar et al., 1976; Finegold and Sutter, 1978). Colonic bacteria attack fermentable fiber components and degrade at least a portion of them to short-chain fatty acids and gases. The role and importance of these short-chain fatty acids in the colon have not been determined.
Insoluble fibers such as wheat bran decrease intestinal transit time and increase stool weight and volume. Increased stool volume observed after high intakes of dietary fiber is due in part to indigestible remnants of plant cell walls and in part to increased bacterial mass, but certain dietary fibers may also result in increased fecal nitrogen excretion. Most studies have not assessed the source of the increased nitrogen excretion. Stephen and Cummings (1980b) concluded that most of it can be attributed to bacterial mass and to mucosal cell debris and intestinal secretions.
Sources of Dietary Fiber
Quantifying total dietary fiber as well as the different fiber components in foods has been extremely difficult, because the complex nature of
Page 293
dietary fiber demands complex analytical techniques. Until recently, food composition data bases quantified only crude fiber, which was obtained by extracting the fiber from plant foods with acid and alkali. This process resulted in the destruction of large and inconsistent portions of dietary fiber components. More recently, R.A. McCance and E.M. Widdowson developed a table containing the estimated composition of dietary fiber in different foods (Paul and Southgate, 1978), but the method used to obtain these data has been criticized because the removal of starch is incomplete (Selvendran and Du Pont, 1980) and the calorimetric methods used to measure sugars are nonspecific (Hudson and Bailey, 1980). The Association of Official Analytical Chemists approved a method for quantifying total dietary fiber (Prosky et al., 1985), but no data on dietary fiber in foods have been published with this method. At present, there are no satisfactory data bases on the individual components of dietary fiber in foods.
Lanza and Butrum (1986) compiled provisional dietary fiber tables based on published values derived from a variety of analytical methods. The magnitude of the error introduced by combining data in this way is unknown, but the variation among laboratories using the same analytical method is known to be large (Theander, 1981). In addition, most values in Lanza and Butrum's tables are based on analysis of only one or a few samples; genetic varieties and changes in fiber due to food processing could not be taken into account (Lanza and Butrum, 1986).
In general, foods with a high fiber content include whole-grain breads and cereals, fruits, vegetables, legumes, and nuts. Fruit skins, seeds, berries, and the bran layers of cereal grains contain higher concentrations of fiber than do the remainder of these foods (Lanza and Butrum, 1986).
Components of dietary fiber vary from food to food, as noted in a comprehensive review by Selvendran (1984). Plant species, stage of maturity, and parts of the plant consumed all influence the composition of dietary fiber. High levels of cellulose are found in root and leafy vegetables, legumes, and some fruits such as pears and apples. Lignin content is highest in fruits, particularly strawberries and peaches, whereas pectin levels are highest in citrus fruits and apples. Cereals and grains contain high levels of the insoluble fibers cellulose and hemicelluloses (Lanza and Butrum, 1986; Selvendran, 1984).
Patterns of Fiber Intake in the United States
The paucity of accurate population-based data on fiber intake, due largely to the lack of data on fiber in foods, hinders interpretation of epidemiologic studies of the relationship of dietary fiber to chronic diseases. In addition, many of these studies used only crude fiber data. Crude fiber in the U.S. food supply declined from 6.1 g/day per capita during 1909-1913 to 4.1 g/day in 1982. Using the dietary fiber data published by Paul and Southgate (1978), Bingham and Cummings (1980) estimated that the availability (not the actual consumption) of total dietary fiber fell from 40 g/day per capita during 1909-1913 to 26.7 g/day in 1980. Most of the decline resulted from decreased use of cereal grains and potatoes.
The Nationwide Food Consumption Survey (NFCS) of the U.S. Department of Agriculture (USDA) contained the first estimates of total fiber intake in its reports of the 1985 Continuing Survey of Food Intakes of Individuals (CSFII) (USDA 1985, 1986a,b, 1987a,b, 1988). Estimates of the amount of dietary fiber in foods were derived chiefly by the methods of Englyst et al. (1982) and, to a lesser extent, Prosky et al. (1985). The mean intake of women 19 to 50 years of age, based on four nonconsecutive days of intake, was 10.9 g/day (7.3 g/1,000 kcal); for children 1 to 5 years of age, mean intake was 9.8 g/day (6.9 g/1,000 kcal) (USDA, 1987a). Low-income women in the same age group averaged, on the one day surveyed, 10.1 g/day (6.7 g/1,000 kcal) in 1985 (USDA, 1986a) and 10.2 g/day (6.9 g/1,000 kcal) in 1986 (USDA, 1987b). Low-income children averaged 8.6 g/day (6.2 g/1,000 kcal) in 1985 (USDA, 1986a) and 9.3 g/day (6.2 g/1,000 kcal) in 1986 (USDA, 1987b). The 1-day fiber intakes of men 19 to 50 years of age surveyed in 1985 averaged 17.5 g/day (7.0 g/1,000 kcal) (USDA, 1986b).
The provisional dietary fiber table of Lanza and Butrum (1986) was used to calculate the fiber intake of adults in the Second National Health and Nutrition Examination Survey (NHANES II) (Lanza et al., 1987). Mean intakes for the total adult population, men as well as women, were 11.1 g/day, compared with 13.3 g/day using the table of Paul and Southgate (1978).. Women in the NHANES II study consumed an average of 6.5 g/ 1,000 kcal, whereas men consumed 5.5 g/1,000 kcal.
The USDA and NHANES II surveys indicate that men have a higher absolute daily intake of
Page 294
dietary fiber than do women, but that women's intakes are higher per 1,000 kcal. Limited data suggest that dietary fiber intake per 1,000 kcal may be higher among the elderly than among young adults and that adolescents may have low intakes (LSRO, 1987).
A more definitive assessment of current fiber intake patterns must await improvement in the food composition data base, now in progress, and more accurate information about dietary practices.
Methodological Problems in Assessing Fiber-Disease Interrelationships
Several caveats must be borne in mind when reviewing studies on dietary fiber and chronic disease etiology. First, as noted above, the term fiber is used to define a complex mixture of dietary substances with differing chemical, physical, and physiological properties. Analyses of studies in terms of total dietary fiber content could be misleading if the effect of one component of fiber masks differing effects of the other components. This problem is compounded by the lack of complete data on the various components of dietary fiber in different foods and the imprecision of current methods for estimating fiber intake in epidemiologic studies.
Second, in some populations consuming Western diets, fiber intake is positively correlated with total caloric intake, which itself is highly correlated with fat intake. There may therefore be a positive correlation between fiber intake and fat intake. When the effect of caloric intake is controlled, however, fiber and fat intakes of individuals are usually negatively correlated (i.e., among people with the same caloric intake, those with a higher fat intake tend to have a lower fiber intake and vice versa). Thus, in assessing associations with fiber intake, it is necessary to consider caloric as well as fat intakes. Nevertheless, statistical analyses of fiber intake that specifically take caloric and fat intake into account simultaneously may be unstable due to multicollinearity.
Third, any fiber effect could be interactive (e.g., high fiber intake could be protective only in people with high fat intake). Such interactions can usually be detected only in much larger studies than those required to detect main effects.
Fourth, epidemiologic studies that rely on evidence from vegetarians (who as a rule consume more fiber than nonvegetarians) should be evaluated carefully, since most types of vegetarians (e.g., complete vegetarians, lacto-ovovegetarians, and others) differ from omnivores in many ways that may confound the association between fiber intake and health. In addition, there is a general lack of consistency among and within some studies regarding the length of time that subjects have followed a vegetarian diet.
Finally, many populations are fairly homogeneous in their fiber intake. Studies in such populations may fail to detect a true effect of fiber.
Evidence Associating Dietary Fiber with Chronic Diseases
Hyperlipidemia and Coronary Heart Disease
Epidemiologic Studies
The effects of dietary fiber on lipid metabolism in humans have been described in several comprehensive reviews (Judd and Truswell, 1985; Kay and Truswell, 1980; Story, 1980; Vahouny, 1982, 1985). Most epidemiologic data on fiber intake and levels of serum lipid, lipoprotein, and apolipoprotein come from comparisons of vegetarian and nonvegetarian populations. In a study of complete vegetarians, lacto-ovovegetarians, and nonvegetarians, Hardinge and colleagues observed that serum cholesterol levels were significantly lower in complete vegetarians (Hardinge and Stare, 1954; Hardinge et al., 1958). Intakes of fiber, presumably crude fiber, among complete vegetarians, lacto-ovovegetarians, and nonvegetarians were 7.9, 5.4, and 2.9 g/1,000 kcal, respectively, for males and 8.6, 5.2, and 3.1, respectively, for females. Subsequent studies (Burslem et al., 1978; Knuiman and West, 1982; Sacks et al., 1975) confirmed the lower serum lipid levels in complete vegetarians and also noted that vegetarians have higher ratios of high-density lipoprotein (HDL) cholesterol to total cholesterol and lower levels of apo-B and apo-AI lipoproteins than either lacto-ovovegetarians or nonvegetarians.
The epidemiologic evidence linking fiber intake to coronary heart disease (CHD) risk is less consistent than the data on serum lipids. Although the rate of CHD in 20 economically advanced countries varied inversely with estimated fiber intake (Liu et al., 1982), adjustment for fat intake removed the association. In the Ireland-Boston Diet-Heart Study—a 20-year cohort study of 1,001 middle-aged men—decreased fiber consumption was associated with increased risk of CHD, but the association was no longer significant when
Page 295
other risk factors were taken into account (Kushi et al., 1985). A 10-year follow-up study of diet and mortality in the Netherlands (Kromhout et al., 1982) showed no significant difference in fiber intake between people dying of CHD (9.5 g/1,000 kcal) and noncases (10.0 g/1,000 kcal). In a subsequent follow-up, Kromhout and de Lezenne Coulander (1984) found a negative association between fiber intake and risk of CHD, but the association disappeared after controlling for total caloric intake. Similarly, in a study of 200 males, Kay et al. (1980) showed that serum cholesterol levels were inversely associated with fiber intake, but this association no longer held when caloric intake was taken into account in the analysis. The significance of such observations is not clear, however, since people with CHD tend to have lower caloric intakes than those without the disease (see Chapter 7) and, thus, will inevitably tend to have lower fiber intakes.
Morris et al. (1977) reported a protective effect of dietary fiber from cereals on risk of CHD independent of caloric intake. Khaw and Barrett-Connor (1987) found that fiber intake was inversely associated with ischemic heart disease mortality in a 12-year follow-up study of 859 men and women aged 50 to 79 years. In this study, intake was measured by 24-hour dietary recall, which, as noted in Chapter 2, has limited applicability in the assessment of the usual dietary intake of individuals in the United States. Relative risks in those with a 24-hour fiber intake of 16 g or more compared with those with an intake less than 16 g were 0.33 for men and 0.37 for women. A 6 g increment in daily fiber intake was associated with a 25% reduction in ischemic heart disease mortality. This effect was independent of other dietary variables, including calories, fats, cholesterol, protein, carbohydrates, alcohol, calcium, and potassium. In England, 10,943 vegetarians were shown to be at lower risk of CHD, but their decreased risk could not be accounted for by increased fiber consumption alone (Burr, 1982).
Clinical and Metabolic Studies
Metabolic studies (Anderson et al., 1984; Challen et al., 1983; Kay et al., 1985; Schweizer et al., 1983) suggest that increasing consumption of fiber-rich foods can reduce serum cholesterol levels. For example, serum cholesterol concentrations have been lowered by diets providing 45 g of dietary fiber per day in fresh fruits, vegetables, and legumes (Grande et al., 1965; Jenkins et al., 1979b; Keys et al., 1960) and by isocaloric substitution of either sugar or bread by a mixture of vegetables, providing 40 g/day, but not fruit, providing 20 g/day (Grande et al., 1974). Unlike vegetables, legumes, and possibly fruits, the addition of cereals to the diet has little effect on serum cholesterol levels.
Not all dietary fibers appear to influence serum lipid levels to the same degree or in the same manner. Soluble fibers such as guar gum, pectin, and oat gum have some hypocholesterolemic effect in hyperlipidemics. Guar supplements tend to lower low-density lipoprotein (LDL) cholesterol but not to influence HDL cholesterol, whereas oat bran has been shown in some studies to reduce triglycerides (LSRO, 1987). In contrast, supplements containing insoluble fibers have been shown to have little influence on serum lipid levels in hyperlipidemic people (Jenkins et al., 1986; LSRO, 1987; Schneeman and Lefevre, 1986).
Animal Studies
Studies on dietary fiber in relation to atherosclerosis in different animal models have been reviewed by Kritchevsky (1986a). Early studies in animals were undertaken to determine why natural-ingredient (chow) diets appear to be less atherogenic than semipurified diets, even when the latter contain no cholesterol (Kritchevsky, 1964). The casein used as the protein component of semipurified diets was subsequently found to be primarily responsible for the hypercholesterolemia and the atherogenic effects of the semipurified diets (Hamilton and Carroll, 1976), but experiments in which the fiber component was varied showed that fiber could also influence serum cholesterol levels and the development of atherosclerosis in animals (Kritchevsky, 1986a; Kritchevsky and Story, 1986).
The effects depend on the animal model used and the type of fiber added to the diet. For example, grain residues and wheat straw reduce the atherogenicity of a semipurified, high-fat, cholesterol-free diet in rabbits (Kritchevsky and Tepper, 1965; Kritchevsky et al., 1977) but have less effect in vervet monkeys than does cellulose (Kritchevsky, 1986a,b). Pectin inhibits atherogenesis in cholesterol-fed chickens (Fisher et al., 1966) but does not appear to affect cholesterolemia in vervet monkeys (Kritchevsky, 1986a,b). In general, the water-soluble, viscous, polysaccharide types of dietary fiber appear to be most effective in lowering plasma cholesterol and LDL cholesterol (Schneeman and Lefevre, 1986). Dietary fiber of this type has been shown to inhibit the intestinal absorption
Page 296
of cholesterol and other lipids in rats (Vahouny and Cassidy, 1986a,b).
Mechanisms of Action
Several mechanisms have been suggested to explain the hypocholesterolemic effect of certain dietary fibers. Since dietary fibers do not appear to be readily absorbable, it might be expected that their effects on serum cholesterol levels and atherosclerosis would be exerted in the gastrointestinal tract. This has led to studies on adsorption of bile acids by dietary fiber in relation to fecal steroid excretion. Story (1986) concluded, however, that the effects of dietary fiber on bile acid excretion were not consistent or large enough to account for observed changes in serum cholesterol levels. They suggested two other possible mechanisms: (1) The site at which lipids are absorbed from the intestine could be altered by dietary fiber, which could affect the composition of chylomicrons and influence cholesterol metabolism. (2) Absorbable products of the bacterial degradation of dietary fiber (e.g., short-chain fatty acids) in the colon may modify some aspect of cholesterol metabolism such as cholesterol synthesis (Story, 1986). Tocotrienols, which are effective inhibitors of cholesterol synthesis, have been found in some plant sources of dietary fiber (Qureshi et al., 1986).
Dietary fiber alters the morphology of the intestinal mucosa in rats in a manner that could influence lipid digestion and absorption (Cassidy et al., 1986; Tasman-Jones, 1986a,b; Vahouny and Cassidy 1986b). Soluble dietary fiber may also influence serum lipid levels through effects on lipoprotein secretion and metabolism. Mechanisms posited include increased catabolism of LDL cholesterol (Chen and Anderson, 1986) and increased serum acetate, which has been shown to inhibit cholesterol synthesis in hepatocytes (Beynen et al., 1982) and, possibly, to increase peripheral LDL receptors and LDL clearance (LSRO, 1987).
Cancer
Large Intestine
The relative lack of cancer of the large intestine in Africa led to the hypothesis that fiber might be protective against a number of chronic diseases common in Western countries (including CHD, diverticulitis, and colorectal cancer) (Burkitt and Trowell, 1975). Most epidemiologic evidence on cancer relates to cancer of the colon, and the correlation between colon cancer rates and fiber consumption has been the subject of several studies. Direct comparisons of fiber intake between two geographic areas with differing colon cancer incidence rates but similar levels of fat intake have been reported for northern and southern India (Malhotra, 1977), for rural Finland and New York (Reddy et al., 1978), and for Denmark and Finland (Jensen et al., 1982). In these studies, the lower risk populations had the higher fiber intake. In contrast, a study in New Zealand showed that Maoris have lower colon cancer rates (and CHD rates) than do whites despite higher fat and lower fiber intakes (Smith et al., 1985).
Early international comparisons did not suggest an inverse association of fiber intake with colon cancer risk. Drasar and Irving (1973) failed to find a correlation between colon cancer incidence in 37 countries and per-capita intake of fiber-containing foods, but they did find a negative association with intake of cereals (Irving and Drasar, 1973). Liu et al. (1979) compared per-capita intake from 1954 to 1965 in 20 industrialized countries with colon cancer mortality from 1967 to 1973. Although fiber intake was inversely correlated with colon cancer mortality, this relationship was no longer significant in a partial correlation analysis controlling for cholesterol intake. This analysis was considered appropriate in light of the low colon cancer rates in Finland where cholesterol intake is high. In a later study of 38 countries, international age-standardized colon cancer rates did not correlate with fiber intake after controlling for intake of fat. However, there was a negative correlation of cereal intake with colon cancer, even after controlling for fat intake (McKeown-Eyssen and Bright-See, 1984). This finding supported three earlier studies showing an inverse association of colon cancer with cereal intake (Armstrong and Doll, 1975; Drasar and Irving, 1973; Knox, 1977).
Intracountry correlation studies in general have produced negative results. For example, Lyon and Sorenson (1978) found little difference in intake of fiber and other dietary constituents between the low-risk population of Utah and the U.S. population as a whole. However, the power of this study was probably low, since no significant differences were found for any of the other dietary items studied. Total fiber intake did not correlate with colon cancer rates among counties in England and Wales, but there was a negative correlation with both vegetable intake (excluding potatoes) and the pentose subfraction of fiber (Bingham et al.,
Page 297
1979). Subsequently, Bingham and coworkers (1985) reanalyzed the results using data that distinguished more specifically among various fiber fractions and showed that colon cancer mortality in these counties was negatively associated with the uronic acid fraction of fiber (derived largely from fruits and vegetables) rather than with the pentose fraction.
Case-control studies of large bowel cancer have been conducted in a variety of geographic locations and populations using several different techniques for sampling cases and controls. Most did not include indices of fiber intake, and their findings were presented in terms of specific foods or food groups. For these studies, it is difficult to conclude that a particular association with colon cancer indicates an effect of dietary fiber per se rather than an effect of other factors associated with the intake of fiber-rich foods.
Four case-control studies did include indices of total fiber intake, and they provide inconsistent findings. In these studies, total fiber intake was estimated from diet history questionnaires administered to populations consuming diets similar to average diets in the United States. One of these, in Canada, showed no association between colorectal cancer and crude fiber intake or total dietary fiber (Jain et al., 1980; Miller et al., 1983). In a recent reanalysis of those data, which included data on intakes of calories, saturated fat, and dietary fiber, Howe et al. (1986) estimated that the relative risk in the upper two tertiles of fiber consumption was very close to 1.0 with narrow confidence intervals. In a case-control study conducted in South Australia, Potter and McMichael (1986) found higher fiber intake by colon cancer cases than by controls, especially among females. In both sexes, a finding of an increased risk associated with high protein and total energy intakes was confined to those consuming a low-fiber diet. In females, adjusting fiber intake for these and other variables attenuated the increase in risk associated with fiber. In Melbourne, Australia, Kune et al. (1987) found high fiber intake to be protective in association with a high vegetable intake. In Utah, Lyon et al. (1987) used an index of crude dietary fiber and found a weak protective effect, especially in females, after adjustment for caloric intake.
The remaing case-control studies did not assess fiber intake per se. In studies conducted in Minnesota (Bjelke, 1978), San Francisco (Dales et al., 1979), and New York State (Graham et al., 1978), the reported frequency of eating foods with a high-fiber content was used rather than a direct index of fiber consumption. All three studies showed that cases consumed less of these foods than did controls. In the New York State study, the protective effect was specifically attributed to vegetables of the Brassica genus (e.g., cabbage, cauliflower, Brussels sprouts).
A study in Norway (Bjelke, 1978), conducted in parallel with the Minnesota study, produced similar results. In a case-control study in Puerto Rico, on the other hand, Martinez et al. (1979) found that large bowel cancer cases consumed greater amounts of fiber-containing foods (as well as other dietary items) than did controls. They did not study the association of fiber-containing foods and colon cancer risk when other dietary factors were controlled.
Three dietary case-control studies of cancer of the large intestine have been conducted in Mediterranean populations. In Greece (Manousos et al., 1983) and in Marseilles, France (Macquart-Moulin et al., 1986), the consumption of certain vegetables was associated with a protective effect. In the Greek study, the protective effect provided by the consumption of cabbage, lettuce, spinach, and beets persisted in a multivariate analysis that adjusted for age, sex, and meat intake (Manousos et al., 1983). In the French study, vegetables with a low fiber content (e.g., cucumbers, zucchini, tomatoes, lettuce, and onion) were protective in a similar multivariate analysis, but, notably, other vegetables with medium or high fiber content were not (Macquart-Moulin et al., 1986). However, the individual contribution of the low-fiber vegetable group and the high-fiber vegetable group to total dietary fiber consumption was not considered in the analysis. In the third study, conducted earlier in Israel, investigators found that a grouping of foods with a crude fiber content of -0.5% was protective for colon cancer, but not for rectal cancer (Modan et al., 1975). The specific food items found to be protective were primarily vegetables.
Like epidemiologic studies, laboratory animal studies on dietary fiber and cancer have focused largely on colon cancer. These studies were reviewed by the National Research Council's Committee on Diet, Nutrition, and Cancer (NRC, 1982) and more recently by Jacobs (1986b, 1987), Kritchevsky (1986b), and Reddy (1986). A variety of compounds carcinogenic to the colon, including 1,2-dimethylhydrazine (DMH), azoxymethane
Page 298
(AOM), 3,2'-dimethyl-4-aminobiphenyl (DMAB), and methylnitrosourea (MNU), have been used in animal models to study the effects of the amount and type of dietary fibers on colon carcinogenesis (Shamsuddin, 1986). The results of these studies are conflicting (Jacobs, 1986b; Kritchevsky, 1986b), possibly reflecting differences in susceptibility across animal species; in the dosages, type, and mode of administration of the carcinogen; in the amount and type of dietary fiber consumed; in the timing of the feeding (i.e., at the stage of initiation or promotion); and in the length of the study.
Several animal studies indicate a protective effect of dietary fiber on colon cancer risk. For example, addition of 15% pectin to a semipurified diet containing 20% fat inhibited AOM-induced, but not MNU-induced, colon carcinogenesis in Fischer 344 rats (Watanabe et al., 1979). The authors hypothesized that pectin interferes with the metabolic activation of AOM in the liver or colon that is necessary for carcinogenesis, but that it has no corresponding effect on the more direct-acting MNU. Addition of 15% wheat bran inhibited both AOM- and MNU-induced colon carcinogenesis. In another study, the same substitution protected against oral and subcutaneous DMH-induced colon cancer (Barbolt and Abraham, 1978). Wheat bran fed with dehydrated citrus fiber at the 15% level with 5% fat also yielded a lower incidence and multiplicity of colon tumors in AOM-challenged Fischer 344 rats (Reddy et al., 1981), whereas wheat bran alone exhibited the same effect in DMAB-challenged animals (Reddy and Mori, 1981). Nigro et al. (1979) reported that diets consisting of 35% beef fat plus 10% wheat bran, alfalfa, or cellulose did not inhibit AOM-induced colon carcinogenesis, whereas diets with 5% fat and 20% or 30% wheat bran or cellulose did. This finding suggests that the promoting effect of fat on colon carcinogenesis can outweigh the protective effect of dietary fiber.
Other animal studies indicate either an enhancing effect or no effect of dietary fiber on colon carcinogenesis (Jacobs, 1987). For example, 20% wheat bran added to the diet of rats did not protect them against DMH-induced colon carcinogenesis in three separate studies (Bauer et al., 1979; Cruse et al., 1978; Jacobs, 1983), nor did the addition of cellulose and guar gum (Bauer et al., 1981; Jacobs and Lupton, 1986).
These findings suggest that type of fiber is important in modulating the effects of a colon carcinogen and that wheat bran has the most consistent inhibiting effect.
Several plausible biologic mechanisms may explain a protective effect of dietary fiber against colorectal cancer (Jacobs, 1986a,b). Fiber could act as a diluent, increasing fecal bulk and thus reducing exposure to a carcinogen (MacLennan et al., 1978) or may reduce such exposure by decreasing transit time in the gastrointestinal tract (Stephen and Cummings, 1980a). In general, high intakes of insoluble fibers (e.g., cereal fibers) tend to increase fecal bulk (Eastwood and Brydon, 1985) and weight and to decrease transit time (Cummings, 1986), whereas water-soluble fibers such as pectin have variable or little effect (LSRO, 1987). Comparisons between rural Finnish and New York populations suggest that the increased bulk resulting from the intake of certain dietary fibers serves to dilute bile acids, which are believed to promote colon carcinogenesis (Reddy et al., 1978). In addition, certain dietary fibers may decrease the excretion of fecal secondary bile acids, thereby reducing colonic exposure to these substances (Reddy 1986).
As noted earlier in this chapter, dietary fiber can also influence intestinal cell morphology and cell proliferation, which may modify the effects of colon carcinogens. For example, animal studies indicate that diets with 15% fiber produce ultrastructural cell surface changes in the small intestine and colon that may modify risk; the strongest effects are produced by alfalfa, pectin, cellulose, and bran (Cassidy et al., 1981). Paradoxically, diets supplemented with wheat bran or cellulose can increase the growth of epithelial cells in the large bowel of the rat—a phenomenon believed to enhance carcinogenesis (Jacobs, 1984). The process may be mediated by fermentation of fiber in the large bowel, which has been reported to produce short-chain fatty acids capable of stimulating intestinal cell proliferation (Jacobs, 1986b).
Other Cancers
The role of dietary fiber in other cancers has not been studied extensively. Two case-control studies suggest that a decreased risk of stomach cancer is associated with high fiber intake. In one of these, conducted in Israel, Modan et al. (1974) did not calculate a specific fiber index but, rather, showed that controls consumed fiber-rich foods more fre-
Page 299
quently than did the cases. In Canada, Risch et al. (1985) calculated a fiber index that showed a strong protective effect of dietary fiber and "fibrous foods," including vegetables, fruits, soybeans, seeds, and nuts.
Two case-control studies suggest an inverse association between dietary fiber and breast cancer. Adlercreutz et al. (1982) reported that excretion of enterolactone and enterodiol—urinary lignans that correlate with fiber intake and are produced by intestinal microflora acting on precursors in fiber-rich foods—was lower in women with breast cancer than in normal controls. Lubin et al. (1986) found that diets highest in animal fats and protein and low in fiber were associated with increased risk of breast cancer and that the risk was higher in women under the age of 50. Information on intake of specific fiber subfractions was not reported in either study.
Intake of high-fiber foods has also been studied in relation to ovarian cancer (Byers et al., 1983) and endometrial cancer (La Vecchia et al., 1986). Both studies found that consumption of fiber-rich foods provided a protective effect, but that the effect did not persist when other factors were considered.
The committee found no data on dietary fiber in relation to other cancers.
Diabetes
Epidemiologic Studies
Although the evidence generally suggests that the risk of diabetes mellitus is inversely associated with diets high in fiber-containing foods, the nature of the association has not been established as causal. For example, comparisons of diabetes prevalence in 11 countries and 2 locations in the United States demonstrated an inverse association with total carbohydrate intake (West and Kalbfleisch, 1971). This finding was confirmed in two subsequent studies (West, 1974a,b). A time-trend analysis of diabetes death rates in England and Wales from 1920 to 1970 showed an inverse association with intake of grains and high-fiber flour. Similarly, groups consuming high-fiber diets in Africa were found to have a lower prevalence of diabetes than groups consuming diets with lower levels of fiber (Trowell, 1960; Walker, 1961; Walker et al., 1970). The epidemiologic evidence is not entirely consistent, however. In a comparison of two populations in Micronesia (King et al., 1984), one at high risk and the other at low risk of noninsulin-dependent diabetes, estimates of dietary fiber intake were of no predictive value in estimating risk of subsequent diabetes. The wide differences in diet, environment, genetic factors, and socioeconomic level among the various groups studied may explain the difference.
Clinical and Metabolic Studies
Although clinical and metabolic studies indicate that some fiber supplements can control glycemic response in diabetics, the relevance of these studies to the prevention of diabetes is unknown. In general, these studies indicate that water-soluble fibers such as guar and pectin are most effective in reducing the postprandial rise in serum glucose after mixed meals or glucose load than are water-insoluble fibers such as wheat, corn bran, soy hulls, and cellulose (Jenkins et al., 1976, 1978b, 1979a; LSRO, 1987; Monnier et al., 1978; Morgan et al., 1979; Poynard et al., 1980). Water-soluble fibers also appear to be more effective in reducing serum insulin response in diabetics Jenkins et al., 1976, 1978a).
In some studies, diets emphasizing fiber-rich foods (rather than fiber supplements) have also appeared to be effective in reducing fasting serum glucose levels, insulin requirements, and urinary excretion of glucose in insulin-dependent diabetics (Kinmonth et al., 1982; H.C.R. Simpson et al., 1981) and noninsulin-dependent diabetics (Barnard et al., 1983; Karlström et al., 1984; H.C.R. Simpson et al., 1981). Other studies have shown no effect (McCulloch et al., 1985; Pacy et al., 1986). However, the beneficial effect of high-fiber diets noted in most studies cannot be attributed solely to their increased fiber content, since they also differ from average diets in other important respects, such as the content of total calories, fats, and cholesterol, which are usually higher, and in the carbohydrate content, which is usually lower.
Animal Studies
Dietary fiber has been studied in several different animal models of diabetes. Berglund et al. (1982) used two strains of mice with a genetic susceptibility to diabetes to study effects of diets prepared from skim milk and breads made from either whole-rye flour or refined-rye flour. The dietary fiber content of these diets was 13.9 and 4.4 g/100 g, respectively. The non-inbred C57BL/KsJ=ob/ob mice, which develop a moderate hyperglycemia with compensatory b-cell hyperplasia, became more hyperglycemic on the low-fiber diet than on the high-fiber diet, but no effect on serum insulin
Page 300
was observed. C57BL/KsJ=db/db mice, which develop severe diabetes resembling the disease in humans, became obese on the high-fiber diet but began to lose weight at 20 to 25 weeks of age and died at a median age of 28 weeks. The mice on the low-fiber diet had more severe hyperglycemia and less pronounced hyperinsulinemia than did those on the high-fiber diet.
In a study of rats made diabetic by treatment with streptozotocin, Yamashita et al. (1980) found that the addition of 5% fiber reduced fasting blood sugar and triglyceride levels and decreased plasma glucagon levels relative to controls. In another study, Yamashita and Yamashita (1980) found that the addition of 10% dietary fiber lowered fasting blood sugar and increased the level of HDL cholesterol in the same rat model. Madar (1983) also used this rat model to show that dietary soybean fiber was more effective than brown rice in reducing plasma glucose, triglyceride, and glucagon levels. He noted that soybean fiber contains pectins, galactomannans, and arabinogalactans, which are highly viscous, whereas rice fiber consists mainly of cellulose and hemicellulose, which have low viscosity.
These studies provide evidence that dietary fiber can help to delay the development of diabetes in diabetes-prone animals. This finding is consistent with the weaker epidemiologic evidence suggesting a positive association between diabetes prevalence and diets high in fiber-depleted starch.
Diverticulosis
Epidemiologic Studies
Diverticulosis of the colon is an acquired pathological defect characterized by small, saccular herniations of the mucosa through the muscular wall of the colon, most often the sigmoid colon. This defect is common in Western industrialized nations and is estimated to occur in 30 to 40% of people aged 50 and over in the United States (Berkow, 1982). The true prevalence of diverticulosis is unknown, since the great majority of cases are asymptomatic.
Ohi et al. (1983) compared fiber intake in the United States and Japan with subsequent prevalence rates of diverticulosis in the populations of these countries. Although these factors correlate well, many other factors, both dietary and nondietary, changed in these countries during the relevant time periods. Segal and coworkers diagnosed several cases of diverticulosis in black South African residents of Johannesburg, normally a low-risk population, who had been consuming diets with high levels of refined carbohydrates and low levels of fiber. Dietary fiber intakes were lower in these cases than in matched controls (Segal and Walker, 1982; Segal et al, 1977).
Clinical Studies
As with diabetes, most clinical studies on diet and diverticular disease focus on treatment of existing cases. Several studies demonstrated a beneficial effect of fiber-rich diets in treating uncomplicated diverticular disease (Plumley and Francis, 1973; Tarpila et al., 1978), but others did not (Devroede et al., 1977; Ornstein et al., 1981). Several investigators suggested that the inconsistent study findings may reflect, in part, differences among individuals in response to supplemental fiber (LSRO, 1987; Painter, 1985).
Other Chronic Diseases
Hypertension
Epidemiologic studies have demonstrated lower mean blood pressures in vegetarians and other groups consuming diets high in fiber than in nonvegetarians and other groups consuming diets lower in fiber (Armstrong et al., 1977; Rouse et al., 1982; Sacks et al., 1974; Trowell, 1981). Similarly, clinical studies indicate a fairly consistent blood pressure-lowering effect of diets with high levels of fiber from various sources in normal as well as hypertensive subjects (Anderson, 1986; Dodson et al., 1984; Lindahl et al., 1984). However, the diets observed in the majority of studies also differ in other respects that could influence blood pressure (e.g., lower in total calories, fats, and animal protein, and atypical in sodium, potassium, chloride, and calcium content). Thus, no firm conclusions can be drawn about the effect of high levels of dietary fiber on blood pressure. No animal data are available.
Gallstones
Epidemiologic evidence linking dietary fiber to risk of gallstones is indirect. For example, Kameda et al. (1984) contrasted the nearly fivefold increase in gallstone incidence observed in Japan from 1950 to 1975 to the increased intakes of total energy (4%), animal protein (129%), and fats (190%) and decreased intake of carbohydrates (32%) observed during the same period. They suggested that the increase in gallstone incidence was attributable
Page 301
to the increased fat intake and decreased fiber intake. Burkitt (1976) similarly attributed the low rates of gallstones in sub-Saharan Africa to the high-fiber diets prevalent in his study populations. Scragg et al. (1984) in a case-control analysis, noted that fiber was protective against gallstones when they controlled for sugar intake.
Clinical studies in healthy people and in subjects with gallstones indicated that supplemental wheat bran (30 to 50 g/day) can lower both the saturation index (Weschler et al., 1984) and the deoxycholic acid content of bile (McDougall et al., 1978; Watts et al., 1978). Normal subjects fed pectin (12 g/day), cellulose (15 g/day), or lignin (12 g/day) for 4 weeks experienced an 11% drop in their lithogenic index on the pectin diet but not on the other diets. The ratio of primary to secondary bile acids fell on the pectin diet, rose on the cellulose diet, and did not change demonstrably on the lignin diet (Hillman et al., 1986).
The addition of pectin, cellulose, or lignin to semipurified diets known to be lithogenic to hamsters inhibited gallstone formation and reduced the lithogenic index (Rotstein et al., 1981). Pectin, but not cellulose, can also promote regression of gallstones (Kritchevsky et al., 1984).
Potential Undesirable Effects of Dietary Fiber
Effects on Mineral Bioavailability
Some concern has been expressed that high-fiber diets may lead to decreased absorption of minerals due to binding by fiber. However, vegetarians consuming high-fiber diets have normal levels of hemoglobin and serum transferrin, as well as normal zinc levels in serum, hair, and urine (Anderson et al., 1981; King et al., 1981), copper levels in serum, and copper and selenium levels in urine (Gibson et al., 1983; Shultz and Leklem, 1983). Similarly, levels of iron, calcium, and magnesium in serum and total iron-binding capacity are reported to be no different in diabetics fed a high-fiber diet than in diabetics consuming an average diet (Anderson et al., 1980).
There have been several studies of the effect of supplemental fiber on mineral bioavailability. People who took two tablespoons of bran daily for 6 months had normal total iron-binding capacity and normal serum levels of iron, magnesium, calcium, phosphorus, and zinc (Rattan et al., 1981). Vaaler et al. (1985) placed insulin-dependent diabetics on ordinary diets including low-fiber bread (15 to 20 g of fiber per day) for 3 months, then gave them supplemental guar gum (29 g/day) for 3 months, and then bran (33 g/day) in the place of guar for another 3 months. These subjects had normal serum concentrations of iron, zinc, selenium, magnesium, calcium, and inorganic phosphate at the end of each 3-month period. Urinary calcium concentrations were somewhat lower after treatment with wheat bran, but urinary concentrations of zinc, magnesium, and inorganic phosphates did not change throughout the study. Addition of whole-wheat bread to meals, however, decreases absorption of nonheme iron in normal subjects (K.M. Simpson et al., 1981). This effect appears to be countered by the addition of protein to the whole-wheat bread (Sandström et al., 1980). When bran, pectin, or cellulose was added to whole-wheat muffins, only bran significantly lowered iron absorption (Cook et al., 1983). Godara et al. (1981) fed female adolescents on low-fiber diets 21 g of cellulose daily for 3 weeks. At the end of this period, fecal excretion of calcium, phosphorus, and iron had increased. Stasse-Wolthuis et al. (1980) compared fecal excretions of calcium and magnesium among four groups fed one of four diets: a low-fiber diet (18 g of fiber per day), a diet containing fruits and vegetables (43 g of fiber per day), a diet containing citrus pectin (28 g of fiber per day), and a diet containing wheat bran (37 g of fiber per day). Subjects fed the wheat bran had an increased fecal excretion of magnesium, but no other differences were noted.
Walker (1985) reviewed mineral deficiencies in populations consuming high-fiber diets in developing countries. He concluded that there is little evidence that high-fiber diets alone induce a mineral deficiency in people who otherwise consume a balanced diet.
Other Potential Effects
Sudden shifts from low-fiber to high-fiber diets, particularly those with increased intakes of wheat bran and guar gum, can produce undesirable gastrointestinal symptoms, including bloating, nausea, increased flatulence, eructation, and vomiting as well as steatorrhea in patients with pancreatic insufficiency (Dutta and Hlasko, 1985; LSRO, 1987); however, these effects seem to be temporary. Studies to investigate the influence of high levels of dietary fiber on the bioavailability of water-soluble and fat-soluble vitamins show no effect, although concern about a possible fiber-associated decrease in the bioavailability of fat-
Page 302
soluble vitamins was originally expressed when dietary fiber was found to affect serum lipid levels (Kasper, 1986; Kelsey, 1982).
Diets containing high levels of unleavened whole-wheat bread have been associated with increased risk of rickets in some populations (Reinhold, 1976), suggesting a deleterious effect on vitamin D metabolism. Infants given wheat bran to treat constipation developed clinical features (e.g., low serum levels of calcium and elevated serum levels of akaline phosphatase) indicative of vitamin D-dependent rickets (Zoppi et al., 1982).
Summary
In general, the evidence for a protective role of dietary fiber per se in CHD, colon and rectal cancers, stomach cancer, female gynecologic cancers, diabetes, diverticulosis, hypertension, and gallstones is inconclusive. Even where the evidence is strongest, it has not been possible to adequately separate the effects of fiber from those of other components of the diet (e.g., total calories, fats, vitamins, minerals, and nonnutritive constituents of fruits and vegetables) and nondietary factors (e.g., socioeconomic status). It is possible that the ranges of fiber intake in most populations studied to date are too small to demonstrate a clear effect. The general lack of data on dietary levels of specific fiber fractions may also have contributed to the inconsistency in study findings.
Total Dietary Fiber
Metabolic and epidemiologic studies indicate that the excretion of fecal mutagens is more prevalent and the concentration of fecal secondary bile acids higher in populations at high risk of colon cancer than in those at low risk. Such studies suggest that dietary fiber, particularly from whole-grain cereals and bread, may be effective in inhibiting production and excretion of fecal mutagens and in decreasing the concentration of fecal secondary bile acids. Clinical studies indicate that dietary fiber can decrease colonic exposure to carcinogens by increasing fecal bulk.
Case-control studies of colon cancer have provided inconsistent findings of a protective effect of dietary fiber per se, but reasonably consistent findings of a protective effect of fiber-rich foods. Such foods may be protective because of factors other than fiber per se—e.g., carotenoids (discussed in Chapter 11) or inducers of microsomal monoxygenase activity, which have been shown to protect against chemical carcinogens. Two studies suggest a protective effect of fiber on gastric cancer. The ability of dietary fiber to reduce risk of other cancers, such as those of the breast, endometrium, and ovaries, has not been adequately documented.
Evidence from epidemiologic and clinical studies suggests that populations consuming high levels of dietary fiber (e.g., vegetarians) have lower blood pressures as well as reduced levels of serum total cholesterol and LDL cholesterol. Clinical studies have shown that high fiber intake decreases insulin requirements and improves glycemic response in diabetics, but the implication of this finding for reduction of diabetes risk is unknown. Descriptive epidemiologic and clinical evidence suggests that high-fiber diets may reduce the risk of diverticulosis. The evidence on dietary fiber and risk of gallstones is inconclusive.
Specific Dietary Fibers
Clinical studies of hyperlipidemics demonstrate that water-soluble fibers, including pectin, guar gum, and oat gum, can markedly reduce serum total cholesterol and LDL cholesterol without affecting serum HDL cholesterol. Water-insoluble fibers have little or no effect in this regard. Animal studies are consistent with these clinical findings. Soluble fiber supplements improve glycemic control and decrease insulin requirements in diabetics, but their potential to prevent diabetes is unknown.
In clinical studies, water-insoluble fibers, including wheat bran and cellulose, have been effective in providing stool bulk and decreasing intestinal transit time. There is no direct evidence from studies in humans to indicate whether or not these fibers affect colon carcinogenesis. However, animal studies suggest that wheat bran in particular is an effective inhibitor of experimentally induced carcinogenesis. Clinical studies show that water-insoluble fibers may relieve symptoms of uncomplicated diverticulosis, but their effect on reducing diverticulosis risk is unknown.
Potential Adverse Effects
There is little evidence that high fiber intake impedes mineral absorption and bioavailability. Although some clinical studies suggest that fiber decreases absorption of iron and zinc, the differences seem too small to pose a major health hazard. The evidence for calcium is further reviewed in Chapter 23.
Page 303
Therefore, until more detailed information on intake of fiber in general and specific fiber types in particular is available, evidence for a protective role of high fiber intake per se for cancer, CHD, or other diseases must be regarded as inconclusive. However, epidemiologic studies are consistent in showing that a diet with large amounts of fiber-containing foods, including vegetables, and relatively low levels of meat and fat products is beneficial with respect to cancer of the colon and possibly atherogenesis. It is not known whether this is due to the high fiber content of such diets or to the presence or absence of some other dietary factor. Therefore, although it is reasonable to recommend a diet containing high levels of fiber-rich foods, there is little evidence to support direct supplementation of the diet with fiber products.
Directions for Research
The committee recommends that the following types of research be undertaken:
· More definitive analytical epidemiologic studies that are designed carefully to include adequate variation of dietary fiber intake in the study population; improved methods for assessing dietary intake in general; improved quality and quantity of data about specific fractions of fiber consumed by the study population; adequate sample size; and collaboration among investigators to adopt a common protocol and method of dietary assessment so that any inconsistencies in results can be related to differences in populations.
· Intervention studies in human populations that could serve to clarify the role of specific fiber components vis-à-vis that of dietary fiber per se.
· Further animal and clinical metabolic studies to define the mechanisms by which dietary fibers protect against chronic diseases. Such definition would serve to identify the appropriate variables on which epidemiologic studies could focus, e.g., the specific fraction of fiber under investigation or the possibility of interaction between fiber subfractions and other food components.
· Studies to examine the long-term effects of increasing the percentage of complex carbohydrates (starches and fibers) in the diet on the risk of and biochemical markers for several diseases, especially stomach and pancreatic cancers, noninsulin-dependent diabetes mellitus, and atherosclerotic cardiovascular diseases. Studies in the elderly should be given a high priority, because the elderly may be prone to more severe adverse effects (e.g., calcium malabsorption).
· Studies to clarify the metabolic role of fiber in colon cancer etiology. As this role becomes known, the new knowledge should be applied in studies of other cancers, CHD, and diabetes.
References
Adlercreutz, H., T. Fotsis, R. Heikkinen, J.T. Dwyer, M. Woods, B.R. Goldin, and S.L. Gorbach. 1982. Excretion of the lignans enterolactone and enterodial and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet 2:1295-1299.
Anderson, B.M., R.S. Gibson, and J.H. Sabry. 1981. The iron and zinc status of long-term vegetarian women. Am. J. Clin. Nutr. 34:1042-1048.
Anderson, J.W. 1986. High-fiber, hypocaloric vs. very-low-calorie diet effects on blood pressure of obese men. Am. J. Clin. Nutr. 43:695.
Anderson, J.W., S.K. Ferguson, D. Karounos, L. O'Malley, B. Sieling, and W.J.L. Chen. 1980. Mineral and vitamin status on high-fiber diets: long-term studies of diabetic patients. Diabetes Care 3:38-40.
Anderson, J.W., L. Story, B. Sieling, W.J.L. Chen, M.S. Petro, and J. Story. 1984. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am. J. Clin. Nutr. 40:1146-1155.
Armstrong, B., and R. Doll. 1975. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int. J. Cancer 15:617-631.
Armstrong, B., A.J. Van Merwyk, and H. Coates. 1977. Blood pressure in Seventh-Day Adventist vegetarians. Am. J. Epidemiol. 105:444-449.
Asp, N.G., and C.G. Johansson. 1984. Dietary fibre analysis. Nutr. Abstr. Rev. Clin. Nutr. 54:735-752.
Baird, I.M., R.L. Walters, P.S. Davies, M.J. Hill, B.S. Drasar, and D.A.T. Southgate. 1977. The effects of two dietary fiber supplements on gastrointestinal transit, stool weight and frequency, and bacterial flora, and fecal bile acids in normal subjects. Metab. Clin. Exp. 26:117-128.
Barbolt, T.A., and R. Abraham. 1978. The effect of bran on dimethylhydrazine-induced colon carcinogenesis in the rat. Proc. Soc. Exp. Biol. Med. 157:656-659.
Barnard, R.J., M.R. Massey, S. Cherny, LT. O'Brien, and N. Pritikin. 1983. Long-term use of a high-complex-carbohydrate, high-fiber, low-fat diet and exercise in the treatment of NIDDM patients. Diabetes Care 6:268-273.
Bauer, H.G., N.G. Asp, R. Öste, A. Dahlqvist, and P.E. Fredlund. 1979. Effect of dietary fiber on the induction of colorectal tumors and fecal b-glucuronidase activity in the rat. Cancer Res. 39:3752-3756.
Bauer, H.G., N.G. Asp, A. Dahlqvist, P.E. Fredlund, M. Nyman, and R. Oste. 1981. Effect of two kinds of pectin and guar gum on 1,2-dimethylhydrazine initiation of colon tumors and on fecal b-glucuronidase activity in the rat. Cancer Res. 41:2518-2523.
Berglund, O., G. Hallmans, C. Nygren, and I.B. Täljedal. 1982. Effects of diets rich and poor in fibres on the development of hereditary diabetes in mice. Acta Endocrinol. 100:556-564.
Page 304
Berkow, R., ed. 1982. P. 794 in The Merck Manual of Diagnosis and Therapy, 14th ed. Merck, Sharpe & Dohme Research Laboratories, Rahway, N.J.
Beynen, A.C., K.F. Buechler, A.J. Van der Molen, and M.J.H. Geelan. 1982. The effects of lactate and acetate on fatty acid and cholesterol biosynthesis by isolated rat hepatocytes. Int. J. Biochem. 14:165-169.
Bingham S., and J.H. Cummings. 1980. Sources and intakes of dietary fiber in man. Pp. 261-284 in G.A. Spiller and R.M. Kay, eds. Medical Aspects of Dietary Fiber. Plenum Medical Book Co., New York.
Bingham, S., D.R.R. Williams, T.J. Cole, and W.P.T. James. 1979. Dietary fibre and regional large-bowel cancer mortality in Britain. Br. J. Cancer 40:456-463.
Bingham, S.A., D.R.R. Williams, and J.H. Cummings. 1985. Dietary fiber consumption in Britain: new estimates and their relation to large bowel cancer mortality. Br. J. Cancer 52:399-402.
Bjelke, E. 1978. Dietary factors and the epidemiology of cancer of the stomach and large bowel. Aktuel. Ernaehrungsmed. Klin. Prax. Suppl. 2:10-17.
Bolton, R.P., K.W. Heaton, and L.F. Burroughs. 1981. The role of dietary fiber in satiety, glucose, and insulin: studies with fruit and fruit juice. Am. J. Clin. Nutr. 34:211-217.
Burkitt, D.P. 1976. Economic development—not all bonus. Nutr. Today 11:6-13.
Burkitt, D.P., and H.C. Trowell. 1975. Refined Carbohydrate Foods and Disease: Some Implications of Dietary Fibre. Academic Press, London. 356 pp.
Burr, M.L. 1982. Vegetarianism, dietary fiber, and mortality. Am. J. Clin. Nutr. 36:873-877.
Burslem, J., G. Schonfeld, M.A. Howald, S.W. Weidman, and J.P. Miller. 1978. Plasma apoprotein and lipoprotein lipid levels in vegetarians. Metabolism 27:711-719.
Byers, T., J. Marshall, S. Graham, C. Mettlin, and M. Swanson. 1983. A case-control study of dietary and nondietary factors in ovarian cancer. J. Natl. Cancer Inst. 71:681-686.
Cassidy, M.M., F.G. Lightfoot, L.E. Grau, J.A. Story, D. Kritchevsky, and G.V. Vahouny. 1981. Effect of chronic intake of dietary fibers on the ultrastructural topography of rat jejunum and colon: a scanning electron microscopy study. Am. J. Clin. Nutr. 34:218-228.
Cassidy, M.M., LR. Fitzpatrick, and G.V. Vahouny. 1986. The effect of fiber in the post weaning diet on nutritional and intestinal morphological indices in the rat. Pp. 229-251 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Challen, A.D., W.J. Branch, and .H. Cummings. 1983. The effect of pectin and wheat bran on platelet function and haemostasis in man. Hum. Nutr. Clin. Nutr. 37:209-217.
Chen, W.J.L, and J.W. Anderson. 1986. Hypocholesterolemic effects of soluble fibers. Pp. 275-286 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Cook, J.D., N.L. Noble, T.A. Morck, S.R. Lynch, and S.J. Petersburg. 1983. Effect of fiber on nonheme iron absorption. Gastroenterology 85:1354-1358.
Cruse, J.P., M.R. Lewin, and C.G. Clark. 1978. Failure of bran to protect against experimental colon cancer in rats. Lancet 2:1278-1280.
Cummings, J.H. 1986. The effect of dietary fiber on fecal weight and composition. Pp. 211-280 in G.A. Spiller, ed. CRC Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Dales, L.G., G.D. Friedman, H.K. Ury, S. Grossman, and S.R. Williams. 1979. A case-control study of relationships of diet and other traits to colorectal cancer in American blacks. Am. J. Epidemiol. 109:132-144.
Devroede, G., J.S. Vobecky, J.M. Vobecky, R. Beaudry, H. Haddad, H. Navert, B. Perey, and J. Poisson. 1977. Medical management of diverticular disease: a random trial. Gastroenterology 72:1157.
Dintzis, F.R. 1982. Dietary fiber analysis—concepts and problems. Pp. 312-332 in D.R. Lineback and G.E. Inglett, eds. Food Carbohydrates: IFT Basic Symposium Series. Avi Publishing Co., Westport, Conn.
Dodson, P.M., P.J. Pacy, P. Bal, A.J. Kubicki, R.F. Fletcher, and K.G. Taylor. 1984. A controlled trial of a high fibre, low fat and low sodium diet for mild hypertension in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 27: 522-526.
Drasar, B.S., and D. Irving. 1973. Environmental factors and cancer of the colon and breast. Br. J. Cancer 27:167-172.
Drasar, B.S., D.J.A. Jenkins, and J.H. Cummings. 1976. The influence of a diet rich in wheat fibre on the human faecal flora. J. Med. Microbiol. 9:423-431.
Duncan, K.H., J.A. Bacon, and R.L. Weinsier. 1983. The effects of high and low energy density diets on satiety, energy intake, and eating time of obese and nonobese subjects. Am. J. Clin. Nutr. 37:763-767.
Dutta, S.K., and J. Hlasko. 1985. Dietary fiber in pancreatic disease: effect of high fiber diet on fat malabsorption in pancreatic insufficiency and in vitro study of the interaction of dietary fiber with pancreatic enzymes. Am. J. Clin. Nutr. 41:517-525.
Eastwood, M., and W.G. Brydon. 1985. Physiological effects of dietary fibre on the alimentary tract. Pp. 105-131 in H. Trowell, D. Burkitt, and K. Heaton, eds. Dietary Fibre, Fibre-Depleted Foods and Disease. Academic Press, New York.
Englyst, H., H.S. Wiggins, and J.H. Cummings. 1982. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 107:307-318.
Finegold, S.M., and V.L. Sutter. 1978. Fecal flora in different populations, with specific reference to diet. Am. J. Clin. Nutr. 31:S116-S122.
Fisher, H., W.G. Siller, and P. Griminger. 1966. The retardation by pectin of cholesterol-induced atherosclerosis in the fowl. J. Atheroscler. Res. 6:292-298.
Gibson, R.S., B.M. Anderson, and J.H. Sabry. 1983. The trace metal status of a group of post-menopausal vegetarians. J. Am. Diet. Assoc. 82:246-250.
Godara, R., A.P. Kaur, and C.M. Bhat. 1981. Effect of cellulose incorporation in a low fiber diet on fecal excretion and serum levels of calcium, phosphorus, and iron in adolescent girls. Am. J. Clin. Nutr. 34:1083-1086.
Graham, S., H. Dayal, M. Swanson, A. Mittelman, and G. Wilkinson. 1978. Diet in the epidemiology of cancer of the colon and rectum. J. Natl. Cancer Inst. 61:709-714.
Grande, F., J.T. Anderson, and A. Keys. 1965. Effect of carbohydrates of leguminous seeds, wheat and potatoes on serum cholesterol concentration in man. J. Nutr. 86:313-317.
Grande, F., J.T. Anderson, and A. Keys. 1974. Sucrose and various carbohydrate-containing foods and serum lipids in man. Am. J. Clin. Nutr. 27:1043-1051.
Hamilton, R.M.G., and K.K. Carroll. 1976. Plasma choles-
Page 305
terol levels in rabbits fed low fat, low cholesterol diets. Effects of dietary proteins, carbohydrates and fibre from different sources. Atherosclerosis 24:47-62.
Hardinge, M.G., and F.J. Stare. 1954. Nutritional studies of vegetarians. 2. Dietary and serum levels of cholesterol. Am. J. Clin. Nutr. 2:83-88.
Hardinge, M.G., A.C. Chambers, H. Crooks, and F.J. Stare. 1958. Nutritional studies in vegetarians. III. Dietary levels of fiber. Am. J. Clin. Nutr. 6:523-525.
Health and Welfare Canada. 1985. Report of the Expert Advisory Committee on Dietary Fibre. Health Protection Branch, Health and Welfare Canada, Ottawa, Canada. 58 pp.
Heaton, K.W. 1980. Food intake regulation and fiber. Pp. 223-238 in G.A. Spiller and R.M. Kay, eds. Medical Aspects of Dietary Fiber. Plenum Medical Book Co., New York.
Hillman, L.C., S.G. Peters, C.A. Fisher, and E.W. Pomare. 1986. Effects of the fibre components pectin, cellulose, and lignin on bile salt metabolism and biliary lipid composition in man. Gut 27:29-36.
Holt, S., R.C. Heading, D.C. Carter, L.F. Prescott, and P. Tothill. 1979. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet 1:636-639.
Howe, G.R., A.B. Miller, and M. Jain. 1986. Re: ''Total energy intake: implications for epidemiologic analyses." Am. J. Epidemiol. 124:157-159.
Hudson, G.J., and B.S. Bailey. 1980. Mutual interference effects in the calorimetric methods used to determine the sugar composition of dietary fibre. Food Chem. 5:201-206.
Irving, D., and B.S. Drasar. 1973. Fibre and cancer of the colon. Br. J. Cancer 28:462-463.
Jacobs, L.R. 1983. Enhancement of rat colon carcinogenesis by wheat bran consumption during the stage of 1,2-dimethylhydrazine administration. Cancer Res. 43:4057-4061.
Jacobs, L.R. 1984. Stimulation of rat colonic crypt cell proliferative activity by wheat bran consumption during the stage of 1,2-dimethylhydrazine administration. Cancer Res. 44:2458-2463.
Jacobs, LR. 1986a. Dietary fiber and gastrointestinal epithelial cell proliferation. Pp. 211-228 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Jacobs, L.R. 1986b. Relationship between dietary fiber and cancer: metabolic, physiologic and cellular mechanisms. Proc. Soc. Exp. Biol. Med. 183:290-311.
Jacobs, L.R. 1987. Dietary fiber and cancer. J. Nutr. 117: 1319-1321.
Jacobs, L.R., and J.R. Lupton. 1986. Relationship between colonic luminal pH, cell proliferation, and colon carcinogenesis in 1,2-dimethylhydrazine treated rats fed high fiber diets. Cancer Res. 46:1727-1734.
Jain, M., G.M. Cook, F.G. Davis, M.G. Grace, G.R. Howe, and A.B. Miller. 1980. A case-control study of diet and colo-rectal cancer. Int. J. Cancer 26:757-768.
Jenkins, D.J.A., D.V. Goff, A.R. Leeds, K.G.M.M. Alberti, T.M.S. Wolever, M.A. Gassull, and T.D.R. Hockaday. 1976. Unabsorbable carbohydrates and diabetes: decreased postprandial hyperglycemia. Lancet 2:172-174.
Jenkins, D.J.A., T.M.S. Wolever, A.R. Leeds, M.A. Gassull, P. Haisman, J. Dilawari, D.V. Goff, G.L. Metz, and K.G.M.M. Alberti. 1978a. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br. Med. J. 1:1392-1394.
Jenkins, D.J.A., T.M.S. Wolever, R. Nineham, R. Taylor, G.L. Metz, S. Bacon, and T.D.R. Hockaday. 1978b. Guar crispbread in the diabetic diet. Br. Med. J. 2:1744-1746.
Jenkins, D.J.A., R.H. Taylor, R. Nineham, D.V. Goff, S.R. Bloom, D. Sarson, and K.G.M.M. Alberti. 1979a. Combined use of guar and acarbose in reduction of postprandial glycaemia. Lancet 2:924-927.
Jenkins, D.J.A., D. Reynolds, A.R. Leeds, A.L. Waller, and J.H. Cummings. 1979b. Hypocholesterolemic action of dietary fiber unrelated to fecal bulking effect. Am. J. Clin. Nutr. 32:2430-2435.
Jenkins, D.J.A., C.G. Rainey-Macdonald, A.L. Jenkins, and G. Benn. 1986. Fiber in the treatment of hyperlipidemia. Pp. 327-344 in G.A. Spiller, ed. CRC Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Jensen, O.M., R. MacLennan, and J. Wahrendorf. 1982. Diet, bowel function, fecal characteristics, and large bowel cancer in Denmark and Finland. Nutr. Cancer 4:5-19.
Judd, P.A., and A.S. Truswell. 1985. Dietary fibre and blood lipids in man. Pp. 23-39 in A.R. Leeds and A. Avenell, eds. Dietary Fibre Perspectives: Reviews and Bibliography. John Libbey and Company Ltd., London.
Kameda, H., F. Ishihara, K. Shibata, and E. Tsukie. 1984. Clinical and nutritional study on gallstone disease in Japan. Jpn. J. Med. 23:109-113.
Karlström, B., B. Vessby, N.G. Asp, M. Boberg, I.B. Gustafsson, H. Lithell, and I. Werner. 1984. Effects of an increased content of cereal fibre in the diet of Type 2 (non-insulin-dependent) diabetic patients. Diabetologia 26: 272-277.
Kasper, H. 1986. Effects of dietary fiber on vitamin metabolism. Pp. 201-208 in G.A. Spiller, ed. CRC Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Kay, R.M., and S. Stitt. 1978. Food form, postprandial glycemia, and satiety. Am. J. Clin. Nutr. 31:738-739.
Kay, R.M., and A.S. Truswell. 1980. Dietary fiber: effects on plasma and biliary lipids in man. Pp. 153-173 in G.A. Spiller and RM. Kay, eds. Medical Aspects of Dietary Fiber. Plenum Medical Book Co., New York.
Kay, R.M., Z.I. Sabry, and A. Csima. 1980. Multivariate analysis of diet and serum lipids in normal men. Am. J. Clin. Nutr. 33:2566-2572.
Kay, R. M., M. Jacobs, M.B. Katan, and B. Lewis. 1985. Relationship between changes in plasma lipoprotein concentrations and fecal steroid excretion in man during consumption of four experimental diets. Atherosclerosis 55:15-23.
Kelsey, J.L. 1982. Effects of fiber on mineral and vitamin bioavailability. Pp. 91-103 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber in Health and Disease. Plenum Press, New York.
Keys, A., J.T. Anderson, and F. Grande. 1960. Diet-type (fats constant) and blood lipids in man. J. Nutr. 70:257-266.
Khaw, K.T., and E. Barrett-Connor. 1987. Dietary fiber and reduced ischemic heart disease mortality rates in men and women: a 12-year prospective study. Am. J. Epidemiol. 126: 1093-1102.
King, H., P. Zimmet, K. Pargeter, L.R. Raper, and V. Collins. 1984. Ethnic differences in susceptibility to noninsulin-dependent diabetes: a comparative study of two urbanized Micronesian populations. Diabetes 33:1002-1007.
King, J.C., T. Stein, and M. Doyle. 1981. Effect of vegetar-
Page 306
ianism on the zinc status of pregnant women. Am. J. Clin. Nutr. 34:1049-1055.
Kinmonth, A.L., R.M. Angus, P.A. Jenkins, M.A. Smith, and J.D. Baum. 1982. Whole foods and increased dietary fibre improve blood glucose control in diabetic children. Arch. Dis. Child. 57:187-194.
Knox, E.G. 1977. Foods and diseases. Br. J. Prev. Soc. Med. 31:71-80.
Knuiman, J.T., and C.E. West. 1982. The concentration of cholesterol in serum and in various serum lipoproteins in macrobiotic, vegetarian and non-vegetarian men and boys. Atherosclerosis 43:71-82.
Kritchevsky, D. 1964. Experimental atherosclerosis in rabbits fed cholesterol-free diets. J. Atheroscler. Res. 4:103-105.
Kritchevsky, D. 1986a. Dietary fiber and atherosclerosis. Pp. 265-274 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Kritchevsky, D. 1986b. Fat, calories and fiber. Pp. 495-515 in C. Ip, D.F. Birt, A.E. Rogers, and C. Mettlin, eds. Dietary Fat and Cancer. Alan R. Liss, New York.
Kritchevsky, D., and J.A. Story. 1986. Influence of dietary fiber on cholesterol metabolism in experimental animals. Pp. 129-142 in G.A. Spiller, ed. Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Kritchevsky, D., and S.A. Tepper. 1965. Factors affecting atherosclerosis in rabbits fed cholesterol-free diets. Life Sci. 4:1467-1471.
Kritchevsky, D., S.A. Tepper, D.E. Williams, and J.A. Story. 1977. Experimental atherosclerosis in rabbits fed cholesterol-free diets. Part 7: Interaction of animal or vegetable protein with fiber. Atherosclerosis 26:397-403.
Kritchevsky, D., S.A. Tepper, and D.M. Klurfeld. 1984. Effect of pectin and cellulose on formation and regression of gallstones in hamsters. Experientia 40:350-351.
Kromhout, D., and C. de Lezenne Coulander. 1984. Diet, prevalence and 10-year mortality from coronary heart disease in 871 middle-aged men: the Zutphen Study. Am. J. Epidemiol. 119:733-741.
Kromhout, D., E.B. Bosschieter, and C. de Lezenne Coulander. 1982. Dietary fibre and 10-year mortality from coronary heart disease, cancer, and all causes: the Zutphen Study. Lancet 2:518-522.
Kune, S., G.A. Kune, and L.F. Watson. 1987. Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer Study. Nutr. Cancer 9:21-42.
Kushi, LH., R.A. Lew, F.J. Stare, C.R. Ellison, M. el Lozy, G. Bourke, L Daly, I. Graham, N. Hickey, and R. Mulcahy. 1985. Diet and 20-year mortality from coronary heart disease: the Ireland-Boston Diet-Heart Study. N. Engl. J. Med. 312:811-818.
Lanza, E., and R.R. Butrum. 1986. A critical review of food fiber analysis and data. J. Am. Diet. Assoc. 86:732-743.
Lanza, E., D.Y. Jones, G. Block, and L. Kessler. 1987. Dietary fiber intake in the U.S. population. Am. J. Clin. Nutr. 46: 790-797.
La Vecchia, C., A. Decarli, M. Fasoli, and A. Gentile. 1986. Nutrition and diet in the etiology of endometrial cancer. Cancer 57:1248-1253.
Lindahl, O., L Lindwall, A. Spangberg, A. Stenram, and P.A. Öckerman. 1984. A vegan regimen with reduced medication in the treatment of hypertension. Br. J. Nutr. 52:11-20.
Liu, K., J. Stamler, D. Moss, D. Garside, V. Persky, and I. Soltero. 1979. Dietary cholesterol, fat, and fibre, and colon-cancer mortality. Lancet 2:782-785.
Liu, K., J. Stamler, M. Trevison, and D. Moss. 1982. Dietary lipids, sugar, fiber, and mortality from coronary heart disease: bivariate analysis of international data. Arteriosclerosis 2:221-227.
LSRO (Life Sciences Research Office). 1987. Physiological Effects and Health Consequences of Dietary Fiber. Federation of American Societies for Experimental Biology, Bethesda, Md. 236 pp.
Lubin, F., Y. Wax, and B. Modan. 1986. Role of fat, animal protein, and dietary fiber in breast cancer etiology: a case-control study. J. Natl. Cancer Inst. 77:605-612.
Lyon, J.L., and A.W. Sorenson. 1978. Colon cancer in a low-risk population. Am. J. Clin. Nutr. 31:S227-S230.
Lyon, J.L., A.W. Mahoney, D.W. West, J.W. Gardner, K.R. Smith, A.W. Sorenson, and W. Stanish. 1987. Energy intake: its relationship to colon cancer risk. J. Natl. Cancer Inst. 78:853-861.
MacLennan, R., O.M. Jensen, J. Mosbech, and H. Vuori. 1978. Diet, transit time, stool weight, and colon cancer in two Scandinavian populations. Am. J. Clin. Nutr. 31: S239-S242.
Macquart-Moulin, G., E. Riboli, J. Cornée, B. Charnay, P. Berthezène, and N. Day. 1986. Case-control study on colorectal cancer and diet in Marseilles. Int. J. Cancer 38: 183-191.
Madar, Z. 1983. Effect of brown rice and soybean dietary fiber on the control of glucose and lipid metabolism in diabetic rats. Am. J. Clin. Nutr. 38:388-393.
Malhotra, S.L. 1977. Dietary factors in a study of cancer colon from cancer registry, with special reference to the role of saliva, milk and fermented milk products and vegetable fibre. Med. Hypotheses 3:122-126.
Manousos, O., N.E. Day, D. Trichopoulos, F. Gerovassilis, A. Tzonou, and A. Polychronopoulou. 1983. Diet and colorectal cancer: a case-control study in Greece. Int. J. Cancer 32:1-5.
Martinez, I., R. Torres, Z. Frias, J.R. Colon, and N. Fernandez. 1979. Factors associated with adenocarcinomas of the large bowel in Puerto Rico. Pp. 45-52 in J.M. Birch, ed. Advances in Medical Oncology, Research and Education, Vol. 3: Epidemiology. Pergamon Press, New York.
McCulloch, D.K., R.D. Mitchell, J. Ambler, and RB. Tattersall. 1985. A prospective comparison of "conventional" and high carbohydrate/high fiber/low fat diets in adults with established type 1 (insulin-dependent) diabetes. Diabetologia 28:208-212.
McDougall, R.M., L. Yakymyshyn, K. Walker, and O.G. Thurston. 1978. Effect of wheat bran on serum lipoproteins and biliary lipids. Can. J. Surg. 21:433-435.
McKeown-Eyssen, G.E, and E. Bright-See. 1984. Dietary factors in colon cancer: international relationships. Nutr. Cancer 6:160-170.
Miller, A.B., G.R. Howe, M. Jain, K.J.P. Craib, and L. Harrison. 1983. Food items and food groups as risk factors in a case-control study of diet and colo-rectal cancer. Int. J. Cancer 32:155-161.
Modan, B., F. Lubin, V. Barell, R.A. Greenberg, M. Modan, and S. Graham. 1974. The role of starches in the etiology of gastric cancer. Cancer 34:2087-2092.
Modan, B., V. Barell, F. Lubin, M. Modan, R.A. Greenberg, and S. Graham. 1975. Low-fiber intake as an etiologic factor in cancer of the colon. J. Natl. Cancer Inst. 55:15-18.
Monnier, L., T.C. Pham, L. Aguirre, A. Orsetti, and J. Mirouze. 1978. Influence of indigestible fibers on glucose tolerance. Diabetes Care 1:83-88.
Page 307
Morgan, L.M., T.J. Goulder, D. Tsiolakis, V. Marks, and K.G.M.M. Alberti. 1979. The effect of unabsorbable carbohydrate on gut hormones: modification of post-prandial GIP secretion by guar. Diabetologia 17:85-89.
Morris, J.N., J.W. Marr, and D.G. Clayton. 1977. Diet and heart: a postscript. Br. Med. J. 2:1307-1314.
Nigro, N.D., A.W. Bull, B.A. Klopfer, M.S. Pak, and R.L. Campbell. 1979. Effect of dietary fiber on azoxymethane-induced intestinal carcinogenesis in rats. J. Natl. Cancer Inst. 62:1097-1102.
NRC (National Research Council). 1982. Diet, Nutrition, and Cancer. Report of the Committee on Diet, Nutrition, and Cancer. Assembly of Life Sciences. National Academy Press, Washington, D.C. 478 pp.
Ohi, G., K. Minowa, T. Oyama, M. Nagahashi, N. Yamazaki, S.-I. Yamamoto, K. Nagasako, K. Hayakawa, K. Kimura, and B. Mori. 1983. Changes in dietary fiber intake among Japanese in the 20th century: a relationship to the prevalence of diverticular disease. Am. J. Clin. Nutr. 38:115-121.
Ornstein, M.H., E.R. Littlewood, I.M. Baird, J. Fowler, W.R.S. North, and A.G. Cox. 1981. Are fibre supplements really necessary in diverticular disease of the colon: a controlled clinical trial. Br. Med. J. 282:1353-1356.
Pacy, P.J., P.M. Dodson, and R.F. Fletcher. 1986. Effect of a high carbohydrate, low sodium and low fat diet in type 2 diabetics with moderate hypertension. Int. J. Obesity 10: 43-52.
Painter, N. 1985. Diverticular disease of the colon. Pp. 145-160 in H. Trowell, D. Burkitt, and K. Heaton, eds. Dietary Fibre, Fibre-Depleted Foods and Disease. Academic Press, New York.
Paul, A.A., and D.A. Southgate. 1978. McCance and Widdowson's The Composition of Foods. Elsevier/North Holland, Amsterdam. 418 pp.
Plumley, P.F., and B. Francis. 1973. Dietary management of diverticular disease. Am. J. Diet. Assoc. 63:527-530.
Pomare, E.W., and K.W. Heaton. 1973. Alteration of bile salt metabolism by dietary fibre (bran). Br. Med. J. 4:262-264.
Pomare, E.W., K.W. Heaton, T.S. Low-Beer, and H.J. Espiner. 1976. The effect of wheat bran upon bile salt metabolism and upon the lipid composition of bile in gallstone patients. Am. J. Dig. Dis. 21:521-536.
Potter, J.C., and A.J. McMichael. 1986. Diet and cancer of the colon and rectum: a case-control study. J. Natl. Cancer Inst. 76:557-569.
Poynard, T., G. Slama, A. Delage, and G. Tchobroutsky. 1980. Pectin efficacy in insulin-treated diabetics assessed by the artificial pancreas. Lancet 1:158.
Prosky, L, N.G. Asp, I. Furda, J.W. DeVries, T.F. Schweizer, and B.F. Harland. 1985. Determination of total dietary fiber in foods and food products: collaborative study. J. Assoc. Off. Anal. Chem. 68:677-679.
Qureshi, A.A., W.C. Burger, D.M. Peterson, and C.E. Elson. 1986. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J. Biol. Chem. 261:10544-10550.
Rattan, J., N. Levin, E. Graff, N. Weizer, and T. Gilat. 1981. A high-fiber diet does not cause mineral and nutrient deficiencies. J. Clin. Gastroenterol. 3:389-393.
Reddy, B.S. 1986. Diet and colon cancer: evidence from human and animal model studies. Pp. 47-65 in B.S. Reddy and L.A. Cohen, eds. Diet, Nutrition, and Cancer—A Critical Evaluation, Vol. 1: Macronutrients and Cancer. CRC Press, Boca Raton, Fla.
Reddy, B.S., and H. Mori. 1981. Effect of dietary wheat bran and dehydrated citrus fiber on 3,2'-dimethyl-4-aminobiphenyl-induced intestinal carcinogenesis in F344 rats. Carcinogenesis 2:21-25.
Reddy, B.S., A.R. Hedges, K. Laakso, and E.L. Wynder. 1978. Metabolic epidemiology of large bowel cancer: fecal bulk and constituents of high-risk North American and low-risk Finnish population. Cancer 42:2832-2838.
Reddy, B.S., H. Mori, and M. Nichois. 1981. Effect of dietary wheat bran and dehydrated citrus fiber on azoxymethane-induced intestinal carcinogenesis in Fischer 344 rats. J. Natl. Cancer Inst. 66:553-557.
Reinhold, J.G. 1976. Rickets in Asian immigrants. Lancet 2: 1132-1133.
Risch, H.A., M. Jain, N.W. Choi, J.G. Fodor, C.J. Pfeiffer, G.R. Howe, L.W. Harrison, K.J.P. Craib, and A.B. Miller. 1985. Dietary factors and the incidence of cancer of the stomach. Am. J. Epidemiol. 122:947-959.
Rotstein, O.D., R.M. Kay, M. Wayman, and S.M. Strasberg. 1981. Prevention of cholesterol gallstones by lignin and lactulose in the hamster. Gastroenterology 81:1098-1103.
Rouse, I.L., B.K. Armstrong, and L.J. Beilin. 1982. Vegetarian diet, lifestyle and blood pressure in two religious populations. Clin. Exp. Pharmacol. Physiol. 9:327-330.
Rydning, A., A. Berstad, T. Berstad, and L. Hertzenberg. 1985. The effect of guar gum and fiber-enriched wheat bran on gastric emptying of a semisolid meal in healthy subjects. Scand. J. Gastroenterol. 20:330-334.
Sacks, F.M., B. Rosner, and E.H. Kass. 1974. Blood pressure in vegetarians. Am. J. Epidemiol. 100:390-398.
Sacks, F.M., W.P. Castelli, A. Donner, and E.H. Kass. 1975. Plasma lipids and lipoproteins in vegetarians and controls. N. Engl. J. Med. 292:1148-1151.
Sandstrom, B., B. Arvidsson, A. Cederblad, and E. Björn-Rasmussen. 1980. Zinc absorption from composite meals. I. The significance of wheat extraction rate, zinc, calcium, and protein content in meals based on breads. Am. J. Clin. Nutr. 33:739-745.
Schneeman, B.O., and M. Lefevre. 1986. Effects of fiber on plasma lipoprotein composition. Pp. 309-321 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Schweizer, T.F., A.R. Bekhechi, B. Koellreutter, S. Reimann, D. Pometta, and B.A. Bron. 1983. Metabolic effects of dietary fiber from dehulled soybeans in humans. Am. J. Clin. Nutr. 38:1-11.
Scragg, R.K.R., A.J. McMichael, and P.A. Baghurst. 1984. Diet, alcohol, and relative weight in gall stone disease: a case-control study. Br. Med. J. 288:1113-1119.
Segal, I., A. Solomon, and J.A. Hunt. 1977. Emergence of diverticular disease in the urban South African black. Gastroenterology 72:215-219.
Segal, I., and A.R.P. Walker. 1982. Diverticular disease in urban Africans in South Africa. Digestion 24:42-46.
Selvendran, R.R. 1984. The plant cell wall as a source of dietary fiber: chemistry and structure. Am. J. Clin. Nutr. 39:320-337.
Selvendran, R.R., and M.S. Du Pont. 1980. Simplified methods for the preparation and analysis of dietary fibre. J. Sci. Food Agric. 31:1173-1182.
Selvendran, R.R., and M.S. Du Pont. 1984. The analysis of dietary fiber. Pp. 1-68 in R.D. King, ed. Food Analysis Techniques, Vol. 3. Applied Science, London.
Shamsuddin, A.K.M. 1986. Carcinoma of the large intestine:
Page 308
animal models and human disease. Hum. Pathol. 17:451- 453.
Shultz, T.D., and J.E. Leklem. 1983. Selenium status of vegetarians, nonvegetarians, and hormone-dependent cancer subjects. Am. J. Clin. Nutr. 37:114-118.
Simpson, H.C.R., R.W. Simpson, S. Lousley, R.D. Carter, M. Geekie, T.D.R. Hockaday, and J.I. Mann. 1981. A high carbohydrate leguminous fibre diet improves all aspects of diabetic control. Lancet 1:1-5.
Simpson, K.M., E.R. Morris, and J.D. Cook. 1981. The inhibitory effect of bran on iron absorption in man. Am. J. Clin. Nutr. 34:1469-1478.
Smith, A.H., N.E. Pearce, and J.G. Joseph. 1985. Major colorectal aetiological hypotheses do not explain mortality trends among Maori and non-Maori New Zealanders. Int. J. Epidemiol. 14:79-85.
Southgate, D.A.T., G.J. Hudson, and H. Englyst. 1978. The analysis of dietary fibre, the choices for the analyst. J. Sci. Food Agric. 29:979-998.
Southgate, D.A.T. 1982. Definitions and terminology of dietary fiber. Pp. 1-7 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber in Health and Disease. Plenum Press, New York.
Stasse-Wolthuis, M., H.F.F. Albers, J.G.C. van Jeveren, J. Wil de Jong, J.G.A.J. Hautvast, R.J.J. Hermus, M.B. Katan, W.G. Brydon, and M.A. Eastwood. 1980. Influence of dietary fiber from vegetables and fruits, bran or citrus pectin on serum lipids, fecal lipids, and colonic function. Am. J. Clin. Nutr. 33:1745-1756.
Stephen, A.M., and J.H. Cummings. 1980a. Mechanism of action of dietary fibre in the human colon. Nature 284:282-284.
Stephen, A.M., and J.H. Cummings. 1980b. The microbial contribution to human faecal mass. J. Med. Microbiol. 13: 45-56.
Story, J.A. 1980. Dietary fiber and lipid metabolism: an update. Pp. 137-152 in G.A. Spiller and R.M. Kay, eds. Medical Aspects of Dietary Fiber. Plenum Medical Book Co., New York.
Story, J.A. 1986. Modification of steroid excretion in response to dietary fiber. Pp. 253-264 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Tadesse, K. 1982. The effect of dietary fibre on gastric secretion and emptying in man. J. Physiol. 332:102P-103P.
Tarpila, S., T.A. Miettinen, and L. Metsäranta. 1978. Effects of bran on serum cholesterol, faecal mass, fat bile acids and neutral sterols, and biliary lipids in patients with diverticular disease of the colon. Gut 19:137-145.
Tasman-Jones, C. 1986a. Effect of dietary fiber and fiber-rich foods on structure of the upper gastrointestinal tract. Pp. 285-286 in G.A. Spiller, ed. Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Tasman-Jones, C. 1986b. Effect of dietary fiber on structure of the colon. Pp. 287-288 in G.A. Spiller, ed. Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Theander, O. 1981. Review of the different analytical methods and remaining problems. Pp. 263-276 in W.P.T. James and O. Theander, eds. The Analysis of Dietary Fiber in Food. Marcel Dekker, New York.
Trowell, H. 1972. Crude fibre, dietary fibre and atherosclerosis. Atherosclerosis 16:138-140.
Trowell, H. 1981. Hypertension, obesity, diabetes mellitus and coronary heart disease. Pp. 3-32 in H.C. Trowell and D.P. Burkitt, eds.. Western Diseases: Their Emergence and Prevention. Harvard University Press, Cambridge, Mass.
Trowell, H., D.A.T. Southgate, T.M.S. Wolever, A.R. Leeds, M.A. Gassull, and D.J.A. Jenkins. 1976. Dietary fibre redefined. Lancet 1:967.
Trowell, H., E. Godding, G. Spiller, and G. Briggs. 1978. Fiber bibliographies and terminology. Am. J. Clin. Nutr. 31:1489-1490.
Trowell, H.C. 1960. Non-infective Disease in Africa. Edward Arnold Publishers Ltd., London. 481 pp.
USDA (U.S. Department of Agriculture). 1985. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Women 19-50 Years and Their Children 1-5 Years, 1 Day, 1985. Report No. 85-1. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 102 pp.
USDA (U.S. Department of Agriculture). 1986a. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Low-Income Women 19-50 Years and Their Children 1-5 Years, 1 Day, 1985. Report No. 85-2. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 186 pp.
USDA (U.S. Department of Agriculture). 1986b. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Men 19-50 Years, 1 Day, 1985. Report No. 85-3. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 94 pp.
USDA (U.S. Department of Agriculture). 1987a. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Women 19-50 Years and Their Children 1-5 Years, 4 Days, 1985. Report No. 85-4. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 182 pp.
USDA (U.S. Department of Agriculture). 1987b. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Low-Income Women 19-50 Years and Their Children 1-5 Years, 1 Day, 1986. Report No. 862. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 166 pp.
USDA (U.S. Department of Agriculture). 1988. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Low-Income Women 19-50 Years and Their Children 1-5 Years, 4 Days, 1985. Report No. 85-5. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 220 pp.
Vaaler, S., J. Aaseth, K.F. Hanssen, K. Dahl-Jörgensen, W. Frølich, B. Ødegaard, and O. Agenaes. 1985. Trace elements in serum and urine of diabetic patients given bread enriched with wheat bran or guar gum. Pp. 446-449 in International Symposium on Trace Element Metabolism in Man and Animals, TEMA-5. Churchill Livingstone, Edinburgh.
Vahouny, G.V. 1982. Dietary fiber, lipid metabolism, and atherosclerosis. Fed. Proc. 41:2801-2806.
Vahouny, G.V. 1985. Dietary fibers: aspects of nutrition, pharmacology, and pathology. Pp. 207-277 in H. Sidransky, ed. Nutritional Pathology: Pathobiochemistry of Dietary Imbalances. Marcel Dekker, New York.
Vahouny, G.V., and M.M. Cassidy. 1986a. Dietary fiber and intestinal adaptation. Pp. 181-209 in G.V. Vahouny and D. Kritchevsky, eds. Dietary Fiber: Basic and Clinical Aspects. Plenum Press, New York.
Vahouny, G.V., and M.M. Cassidy. 1986b. Effect of dietary fiber on intestinal absorption of lipids. Pp. 121-128 in G.A.
Page 309
Spiller, ed. Handbook of Dietary Fiber in Human Nutrition. CRC Press, Boca Raton, Fla.
Walker, A. 1985. Mineral metabolism. Pp. 361-375 in H. Trowell, D. Burkitt, and K. Heaton, eds. Dietary Fibre, Fibre-Depleted Foods and Disease. Academic Press, New York.
Walker, A.R.P. 1961. Crude fibre, bowel motility, and pattern of diet. S. Afr. Med. J. 35:114-115.
Walker, A.R.P., B.F. Walker, and B.D. Richardson. 1970. Glucose and fat tolerances in Bantu children. Lancet 2:51-52.
Watanabe, K., B.S. Reddy, C.Q. Wong, and J.H. Weisburger. 1979. Effect of dietary alfalfa, pectin, and wheat bran on azoxymethane- or methylnitrosourea-induced colon carcinogenesis in F344 rats. J. Natl. Cancer Inst. 63:141-145.
Watts, J.M., P. Jablonski, and J. Toouli. 1978. The effect of added bran to the diet in the saturation of bile in people without gallstones. Am. J. Surg. 135:321-324.
Wechsler, J.G., W. Swobodnik, H. Wenzel, T. Heuchemer, W. Nebelung, V. Hutt, and H. Ditschuneit. 1984. Ballaststoffe vom Typ Weizenkleie senken Lithogenität der Galle. Dtsch. Med. Wochenschr. 109:1284-1288.
West, K.M. 1974a. Diabetes in American Indians and other native populations of the New World. Diabetes 23:841-855.
West, K.M. 1974b. Epidemiologic observations on thirteen populations of Asia and the Western Hemisphere. Pp. 34-43 in S.S. Hillebrand, ed. Is the Risk of Becoming Diabetic Affected by Sugar Consumption? Proceedings of the Eighth International Sugar Research Symposium, March 14, Washington, D.C. International Sugar Research Foundation, Bethesda, Md.
West, K.M., and .M. Kalbfleisch. 1971. Influence of nutritional factors on prevalence of diabetes. Diabetes 20:99-108.
Wilmshurst, P., and J.C.W. Crawley. 1980. The measurement of gastric transit time in obese subjects using 24Na and the effects of energy content and guar gum on gastric emptying and satiety. Br. J. Nutr. 44:1-6.
Yamashita, S., and K. Yamashita. 1980. Effect of high-fiber diet on plasma high density lipoprotein (HDL) cholesterol level in streptozotocin-induced diabetic rats. Endocrinol. Jpn. 27:671-673.
Yamashita, S., K. Yamashita, H. Yasuda, and E. Ogato. 1980. High-fiber diet in the control of diabetes in rats. Endocrinol. Jpn. 27:169-173.
Zoppi, G., L. Gobio-Casali, A. Deganello, R. Astolfi, F. Saccomani, and M. Cecchettin. 1982. Potential complications in the use of wheat bran for constipation in infancy. J. Pediatr. Gastroenterol. Nutr. 1:91-95.




















