Page 347
13—
Minerals
Mineral salts are responsible for structural functions involving the skeleton and soft tissues and for regulatory functions including neuromuscular transmission, blood clotting, oxygen transport, and enzymatic activity. Calcium, phosphorus, and magnesium are required in relatively large amounts and are designated as macrominerals. These are discussed in this chapter. Minerals needed in smaller amounts are called trace elements; these are discussed in Chapter 14.
Calcium is the most abundant mineral in the human body, making up 1.5 to 2% of the total body weight. Approximately 1,200 g of calcium are present in the body of an adult human; more than 99% of that amount is found in bones. All living animals possess powerful mechanisms both to conserve calcium and to maintain constant cellular and extracellular concentrations (Arnaud, 1978, 1988; Exton, 1986). These functions are so vital to survival that during severe dietary deficiency or abnormal losses of calcium from the body, they can demineralize bone to prevent even minor degrees of hypocalcemia (i.e., low plasma calcium). Thus, bone acts as a vital physiological tissue providing a readily available source of calcium for maintenance of normal plasma calcium levels, 50% of which is ionized and physiologically active (Arnaud, 1988).
People need more calcium in their diets when they are forming bone, when intestinal absorption of calcium is impaired, and when there are inordinate losses of calcium to the environment (e.g., through increased renal excretion or lactation). If there is insufficient dietary calcium during bone formation, linear growth will be impeded and peak bone mass may not be achieved. If it is insufficient when intestinal absorption is impaired or when there are inordinate losses, the serum concentration of calcium ion (Ca2+) can be maintained at normal levels only at the expense of bone calcium (Arnaud, 1988).
Phosphorus, along with calcium, is essential for calcification of bones (85% of body phosphorus is located in the skeleton). The remainder of body phosphorus is needed in soft tissues as a cofactor in myriad enzyme systems essential in the metabolism of carbohydrates, lipids, and proteins. In the form of high-energy phosphate compounds, phosphorus contributes to the metabolic potential. The phosphate ion also plays an important role in acid/base balance.
Of total body magnesium, 60 to 65% is found in bone and 27% is located in muscles (Shils, 1988). Magnesium is second only to potassium as the most predominant cation within cells and is essential both for the functions of many enzyme systems and for neuromuscular transmission.
Historical trends in the amounts of various minerals present in the food supply have been reported by the U.S. Department of Agriculture
Page 348
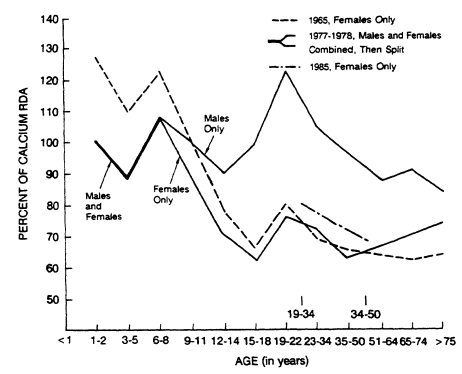
FIGURE 13-1
Mean calcium intakes expressed as percentages of the 1980 RDA (NRC, 1980) for 1965
(females only), 1977 1978 (males and females combined until 8 years of age, then separately for age 9 years and up), and 1985 (females only). Data for 1965 and 1977-1978 are based on one
24-hour recall (USDA, 1984); those for 1985 are based on 4 nonconsecutive days of intake (USDA, 1987).
(USDA) since 1909 (see Table 3-3). These data do not represent actual consumption, however, since they fail to document how much food was wasted. Per-capita calcium availability in the food supply increased 23% from 750 mg/day during 1909-1913 to 920 mg/day in 1985 (see Table 3-3). The change resulted primarily from an increased supply of dairy products during this period. The per-capita availability of phosphorus in the food supply has remained fairly steady at 1,500 mg/day since 1909-1913, and that of magnesium has declined from 380 mg/day during 1909-1913 to 320 mg/day in 1985 (see Table 3-3). The decline resulted primarily from the decreased use of grains and flour and increased practice of low-extraction milling.
Information on current intakes of calcium, phosphorus, and magnesium has been collected in national surveys, including the 1977-1978 Nationwide Food Consumption Survey (USDA, 1984), the second National Health and Nutrition Examination Survey (Carroll et al., 1983), the Continuing Survey of Food Intakes of Individuals (USDA, 1986, 1987), and the Total Diet Study (Pennington et al., 1986) (see Chapter 3). In USDA surveys, calcium intakes have been reported in terms of the 1980 Recommended Dietary Allowance (RDA), which is highest (1,200 mg) at ages 11 to 18 years and is only 800 mg for ages 1 to 10 and 18 and above (NRC, 1980). Mean intakes below the RDA do not necessarily mean that individuals in the group are malnourished. Nutrient requirements differ from individual to individual, and the RDAs are set at high enough levels to cover the requirements of practically all healthy people in the population. Furthermore, these nationwide surveys do not reflect the usual or habitual intakes of individuals. It is inappropriate, therefore, to conclude that failure to meet the RDA indicates that an individual has an inadequate calcium intake, although the risk that some people will have inadequate intakes increases as the mean intake falls further below the RDA. Percentages of the RDA are reported here only to indicate relative intakes on the days surveyed.
Mean intakes of calcium are lower for females than for males and lower for blacks than for whites. USDA surveys indicate that females ages 9 to 19 had somewhat higher intakes in 1965 than during 1977-1978, but that older women (51 to 75 years of age) had higher intakes during 1977-1978 than in 1965 (Figure 13-1) (USDA, 1984). Mean intakes for women 19 to 50 years old were higher in 1985 than in the previous surveys (USDA, 1987).
The 1985 survey indicated that 22% of women ages 19 to 50 consumed the RDA or more, 24% consumed between 70 and 99%, 26% consumed between 50 and 69%, and 29% consumed less than 50% of the RDA. The mean intake for black women
Page 349
was 55% of the RDA compared with 77% for white women. Calcium intakes were lower among men and women in the older age group (35 to 50 years old) and among those living in poverty. Among children 1 to 5 years of age, 45% consumed 100% or more of the calcium RDA, while 39% consumed 70 to 99%. The 1977-1978 survey based on a 1-day intake indicated that males ages 9 to 18 maintained an average calcium intake at 90% of the RDA or above, whereas females in the same age range had progressively lower calcium intakes. Females ages 9 to 11 years, 12 to 14 years, and 15 to 18 years consumed 89, 72, and 64% of the calcium RDA, respectively. In 1985, men and women ages 19 to 50 reported mean calcium intakes of 360 and 397 mg/1,000 kcal, or 115 and 74% of the RDA, respectively. This reflects the lower caloric intake of women.
Major food sources of calcium include milk and milk products. Although leafy greens such as turnip, collard, and mustard greens are good sources, they are not consumed in large amounts by the U.S. population as a whole. The Joint Nutrition Monitoring Evaluation Committee (JNMEC) concluded that calcium deserves public health monitoring priority because of the low dietary intakes by women and the possible association of low intakes with osteoporosis in elderly women (DHHS/ USDA, 1986).
Mean daily intakes of phosphorus for men and women 19 to 50 years of age were 1,536 mg and 966 mg, respectively, in 1985, compared with an RDA of 800 mg for this age group. Children ages 1 to 5 years consumed a mean of 992 mg/day (USDA, 1986, 1987). Major food sources of phosphorus in the U.S. diet include milk and milk products, meats, poultry, fish, and grain products. Some forms of dietary phosphorus—such as phytic acid, which is found in cereals and seeds—are not well absorbed. However, dietary deficiency of phosphorus is unlikely because of its wide distribution in foods. JNMEC judged that phosphorus intake by the U.S. population is generally adequate, requiring less monitoring than certain other nutrients (DHHS/USDA, 1986).
Mean intakes of magnesium in 1985 were 193 mg/day for children 1 to 5 years of age (115% of 1980 RDA) (USDA, 1987). For adults ages 19 to 50, mean intakes were 207 mg/day for women (67% of the 1980 RDA) (USDA, 1987) and 329 mg/day for men (94% of the 1980 RDA) (USDA, 1986). Major food sources of magnesium include grain products, vegetables, dairy products, meat, poultry, and fish. JNMEC found no association of magnesium intake with any chronic disease (DHHS/USDA, 1986). However, since significant portions of the population have magnesium intakes below recommended levels, they recommend further investigation of the role of magnesium nutritional status in disease and health.
Evidence Associating Minerals with Chronic Diseases
Calcium
Osteoporosis
Osteoporosis is a disease characterized by an absolute decrease in bone mass that results in an increased susceptibility to fracture, especially of the wrist, spine, and hip. It is common in postmenopausal women and in the elderly of both sexes and constitutes an important public health problem (Kelsey, 1984, 1987) (see Chapters 5 and 23).
Relative Importance of Bone Cell Activities and Mineral Balance as Determinants of Bone Mass
Bone is a metabolically active tissue that is turning over constantly. This process is regulated by cellular activities that resorb (osteoclastic) and form (osteoblastic) bone. In normal adult bone, resorption is precisely balanced by formation. Furthermore, these activities are coupled so that when one increases or decreases, the other shifts in degree and direction so that little or no net change in the amount of bone ensues. The driving forces for changing net bone mass are intrinsic to the cellular processes that govern bone resorption and formation. Thus, functional uncoupling of these cellular processes is required to either increase or decrease bone mass. Calcium balance generally reflects the degree to which coupling of bone formation and resorption is in balance. Negative calcium balances indicate that bone resorption exceeds formation; positive balances indicate the opposite.
In contrast to cellular processes in bone, calcium, phosphorus, and magnesium play a more passive role in any mass changes that occur in bone. They must be present at physiological concentrations in extracellular fluids for bone mineralization (formation) to occur normally. Dietary minerals contribute to this physiological state by helping to replace minerals that have been lost by obligatory processes (in urine, feces, and sweat).
Page 350
Peak Bone Mass As A Factor In Modifying Osteoporosis Risk
The level of bone mass achieved at skeletal maturity (peak or maximal bone mass) is a major factor modifying the risk for osteoporosis. The more bone mass available before age-related bone loss occurs, the less likely it will decrease to a level at which fracture will occur (Heaney, 1986; Marcus, 1982; Parfitt, 1983). Normally, longitudinal bone growth is completed sometime during the second decade of life. It is axiomatic that positive calcium balance is needed for this to occur normally, and it is easy to calculate that the required average daily body retention of calcium during this 20-year period is approximately 110 mg/day for females and 140 mg/day for males. During the adolescent growth spurt, the required calcium retention is two to three times higher than these average values (Garn, 1970; Nordin et al., 1979). To achieve such retention, the RDA for calcium has been set at 1,200 mg/day for people 10 to 18 years of age (NRC, 1980). If obligatory calcium losses in urine, feces, and sweat are not greater than average, the calcium RDAs are adequate provided that 50% of the calcium ingested is absorbed. A lower percentage of absorption or calcium intakes less than 1,200 mg/day without compensatory increases in the absorption rate would not provide adequate quantities of calcium to achieve peak bone growth. It is not known if teenagers have such levels of calcium absorption nor is it known whether absorption rates in teenagers can increase in response to reduced calcium intakes.
Opinion is mixed as to the age at which peak bone mass is achieved. The only data concerning this issue were collected in cross-sectional studies. These studies suggest that the metacarpal cortical area (Garn, 1970), phalangeal density (Albanese et al., 1975), combined cortical thickness (Matkovic et al., 1979), and bone mineral content of the spine (Krolner and Pors Nielsen, 1982) do not reach maximum levels until sometime during the middle of the third or the early part of the fourth decade of life. Such data suggest that peak bone mass may not be achieved until 5 to 10 years after longitudinal bone growth has ceased. During this period, cortical porosity, which increases during the adolescent growth spurt, is probably filled in and bone cortices become thicker. The quantity of bone mass that can be added is unclear; it has been variously estimated to range from 5 to 10% (Parfitt, 1983). The optimum calcium retention needed to achieve this apparent increment in bone mass is not yet known but probably ranges from 40 to 60 mg/day (Garn, 1970; Nordin et al., 1979; Parfitt, 1983). This association of bone mass with calcium intake is suggested by the results of a Yugoslavian study in which there was a 5 to 10% higher metacarpal bone mass in the inhabitants of a "high-calcium" district starting at age 30 years and extending at least to age 75 years when the investigation terminated (Matkovic et al., 1979).
It is a logical extension of the above that the quantity of dietary calcium required to achieve peak bone mass would be greater than that required to replace obligatory losses through urine, feces, and sweat (approximately 200 to 300 mg/ day). Thus, the period during which positive calcium balance needs to be maintained to achieve peak bone mass should probably be extended beyond the period of longitudinal bone growth to perhaps ages 25 to 30 years (Heaney, 1986; Marcus, 1982; Parfitt, 1983).
Bone Loss As A Factor Modifying Osteoporosis Risk
Another major factor modifying osteoporosis risk is the rate at which bone is lost as life progresses. After peak bone mass is achieved, bone mass appears to be maintained without much change until 40 to 45 years of age. Subsequently, bone is lost at a rate of 0.2 to 0.5% per year in men and women until the eighth or ninth decade of life. In women, bone loss accelerates to 2 to 5% per year immediately before and for approximately 10 years after menopause (Heaney, 1986) and then returns to its former rate—0.2 to 0.5% per year.
Decreased Calcium Absorption As A Factor In Osteoporosis Risk
Intestinal calcium absorption and the ability to adapt to low-calcium diets are impaired in many postmenopausal women (Heaney, 1985, 1986; Heaney et al., 1977) and elderly people of both sexes (Alevizaki et al., 1973; Avioli et al., 1965; Bullamore et al., 1970; Gallagher et al., 1979; Ireland and Fordtran, 1973; Nordin et al., 1976). The pathogenesis of these abnormalities is controversial, but evidence suggests that they may be due either to a functional decrease in the ability of the kidney to produce the major biologically active metabolite of vitamin D—1,25-dihydroxy vitamin D [1,25(OH)2D3] (Gallagher et al., 1979; Riggs et al., 1981)—or to absolute decreases in renal 1,25(OH)2D3 production due to renal diseases such as that occurring in old age (Tsai et al., 1984) (discussed in Chapter 11). The findings that levels of serum immunoreactive parathyroid hormone (Gallagher et al., 1980; Insogna et al., 1981; Marcus et al., 1984;
Page 351
Orwoll and Meier, 1986) and bioactive parathyroid hormone (Forero et al., 1987) increase with age imply that these defects in calcium absorption result in sufficient degrees of hypocalcemia to induce chronic hyperparathyroidism (secondary hyperparathyroidism). It is well established that hyperparathyroidism increases the bone remodeling rate and that a high rate of remodeling leads to accelerated bone loss whenever intrinsic imbalance favors the process of resorption over formation (Parfitt, 1980; Sakhaee et al., 1984).
Thus, it appears that the ability of the intestine to support calcium homeostasis progressively declines with age and that elderly people are increasingly forced to rely on their own bones rather than on the external environment as a source of calcium for maintaining normal extracellular free calcium (Arnaud et al., 1981). The degree to which this occurs depends on the severity of the described defects in calcium absorption, the level and bioavailability of dietary calcium, and whether specific therapeutic means are taken to correct defects in calcium absorption. The quantitative contribution, if any, of this homeostatic mechanism to the decrease in bone mass and the increase in incidence of fractures in the elderly is not known and is the subject of intensive investigation.
Problems In Estimating Bone Mass And Calcium Intake
In most epidemiologic studies of the relationship of dietary calcium to bone mass, investigators have used radiograms (measurements of cortical bone width, area, or calculated volume from x-ray images of metacarpal bones). Such measurements are easy to obtain in the field. Moreover, their precision is similar to the more elegant single- or dual-photon absorptiometry and quantitative computed tomography techniques (Cohn, 1981; Mazess, 1983); however, they are less sensitive and specific. In addition, these cortical bone measurements do not accurately reflect bone mass in the trabecular or spongy bone compartment where rapid turnover occurs. Thus, population-based data obtained with cortical width or area measurements may not detect subtle changes that other, more sensitive and specific techniques might easily detect. Such changes that are detected reflect, at best, those that have occurred in cortical bone and not in trabecular bone. Trabecular bone makes up at least 50% of the bone in the spine (Nottestad et al., 1987) and is affected to the greatest extent early in menopause (Riggs and Melton, 1986).
The methods used to assess dietary calcium intake in these studies have varied from "the best available estimates" (Nordin, 1966) to 7-day replicate dietary records and 47-category interviews (Garn, 1970), to chemical analyses of foodstuffs (Matkovic et al., 1979). Most authors have been aware of the inherent inaccuracies of dietary recall data, and because of the even greater inaccuracies of estimates of calcium intake over a lifetime, most have relied on estimates of current calcium intake. Thus, even the most careful approaches to providing accurate calcium intake data can be faulted, and their interpretation must be approached with caution, especially in relation to estimates of bone mass based on a measurement technique with inherent flaws.
Calcium Intake And Bone Mass
Published reports have shown either no relationship or only a modestly positive relationship between dietary calcium and cortical bone mass. Garn et al. (1969) found the same rate of loss of metacarpal cortical mass in more than 5,800 subjects from seven countries, despite wide variations in calcium intake between groups. In fact, low calcium intakes by some ethnic groups were associated with bone mass values higher than in groups with high calcium intakes over a lifetime. On the other hand, in a 10-state nutrition survey, Garn et al. (1981) found a statistically significant increase in metacarpal cortical area in people in the highest, as compared to the lowest, percentile of calcium intake. In a similar analysis, using data from the first Health and Nutrition Examination Survey (HANES I), investigators observed a significant positive correlation between calcium intake and metacarpal cortical width for all 2,250 subjects (Carroll et al., 1983; DHEW, 1979). When the 960 white women in the study were excluded, the significance of the correlation disappeared.
Matkovic et al. (1979) investigated metacarpal bone mass and the incidence of hip fracture in two regions of Yugoslavia whose inhabitants ingested greatly different quantities of calcium (500 mg/day compared to 1,100 mg/day largely through dairy products). The inhabitants of the high-calcium district ingested more calories, fats, and protein and less carbohydrates than the low-calcium district. However, the regions were similar in their agrarian economy, and except for a significantly longer lower limb length in the high-calcium district, ages, weights, and other anthropomorphic indices were identical. The inhabitants of the high-calcium district had a 50% lower incidence of
Page 352
hip fractures and a significant increase in metacarpal cortical bone volume as compared with the inhabitants of the low-calcium district. Because the differences in bone mass as a function of age were constant, it is more likely that high lifelong calcium intakes in this population increased peak cortical bone mass than that it prevented bone loss. In contrast to the decreased incidence in hip fractures observed in the high-calcium district, the incidence of fractures of the distal forearm (the distal 3 cm of the radius or ulna) was the same in the two regions. This is of interest because the fracture sites at the hip generally are composed mainly of cortical bone, whereas those at the wrist are mainly of trabecular bone. The results of a correlation study reported by Anderson and Tylavsky (1984) are highly relevant in this regard. Those investigators related current and lifelong calcium intake to bone mineral content (measured by single-photon absorptiometry) at the distal radius (mixture of cortical and trabecular bone) and at the midshaft of the radius (largely cortical bone) in residents of four North Carolina communities. They found a positive correlation of bone mineral content with calcium intake at the midshaft site but no correlation at the distal site.
Several clinical studies have been conducted to examine the relationship between calcium intake and bone mass. Using radiograms, Smith and Frame (1965), Smith and Rizek (1966), and Garn (1970) found no association between current calcium intake and current bone mass. Similarly, Lavel-Jeanet et al. (1984) and Pacifici et al. (1985) observed no correlation of calcium intake with vertebral density as measured by quantitative computed tomography. Most recently, Riggs et al. (1987) found no relationship between the calcium intakes (range, 260 to 2,003 mg/day; mean, 922 mg/day) of 106 normal women ages 23 to 84 years and the rates of change in bone mineral density at the midradius (determined by single-photon absorptiometry) and the lumbar spine (determined by dual-photon absorptiometry) over a mean period of 4.1 years.
In contrast to the negative observations made by Lavel-Jeanet et al. (1984) and by Pacifici et al. (1985) with quantitative computed tomography of the spine, Kanders et al. (1984), using dual-photon absorptiometry, found that the BMC of L2 through L4 vertebrae in young women with a high calcium intake was higher than that in women with a low intake. In a longitudinal study of 76 healthy postmenopausal women, Dawson-Hughes et al. (1987) found, using dual-photon absorptiometry, that women with calcium intakes less than 405 mg/day lost spinal bone density at a significantly greater rate than those with an intake of greater than 777 mg/day (p <.026).
Calcium Intake and Osteoporosis
Nordin (1966) reported the results of an intercountry comparison of calcium intake and osteoporotic fractures. Despite inconsistency in the methods used to report calcium intakes in the 12 countries surveyed, it was possible to demonstrate an inverse rank-order relationship between frequency of osteoporotic vertebral fracture as determined by spine x-ray and calcium intakes. Japanese women, whose calcium intake averaged 400 mg/day, had the highest frequency of fracture, whereas women in Finland had the highest intake (1,300 mg/day) and the lowest fracture frequency. This relationship did not hold for some countries. For example, in The Gambia and Jamaica, calcium intakes were low but osteoporotic fractures were rare. As reported by Matkovic et al. (1979), the hip fracture incidence in the Yugoslav district with a high calcium intake was 50% lower than in the low calcium district. But no difference was detected in the incidence of fractures around the wrist.
Most clinical studies show lower calcium intakes by osteoporotic patients than by age-matched controls (Hurxthal and Vose, 1969; Lutwak and Whedon, 1963; Nordin, 1961; Riggs et al., 1967; Vinther-Paulsen, 1953). Dietary calcium was lower than 800 mg/day in patients and controls in all these investigations. In another study, intakes were greater than 800 mg/day in patients and controls, and no differences in calcium intake between the two groups were observed (Nordin et al., 1979). The results of that study support the view of Heaney (1986) that low dietary calcium may play a permissive rather than a causative role in the development of osteoporosis and that this role can be demonstrated best when dietary calcium is below a "saturation" level.
Effect Of Calcium Supplementation On Bone Mass
The long-term effects of calcium supplementation on bone mass are not yet established. The results of short-term investigations (2 years or less) are mixed. In general, they show a slowing of bone loss measured at sites composed mostly of cortical bone but not at sites composed of trabecular bone. All studies in which estrogen treatment was used as a companion protocol have shown that calcium supplementation is inferior to estrogen in slowing
Page 353
cortical bone loss and that estrogen prevents trabecular bone loss completely. Some of these studies were randomized (Lamke et al., 1978; Recker and Heaney, 1985; Recker et al., 1977; Riis et al., 1987; Smith et al., 1981), but only two were blinded (Riis et al., 1987; Smith et al., 1981). In the study by Smith et al. (1981), 40% of the subjects were lost to follow-up.
The results of Recker et al. (1977) reflect those of the others. These investigators showed that after 2 years, a 1.04-g supplement of calcium given as the carbonate salt to 22 women between 55 and 65 years of age resulted in a 0.22% decrease in metacarpal cortical bone area as compared with a 1.18% decrease in 20 placebo-treated age-matched women (p <.05). By contrast, there was no difference in bone mineral content of the distal radius (mixture of trabecular and cortical bone). The reduction of metacarpal cortical bone loss with calcium supplementation was less than the reduction resulting from estrogen treatment of 18 age-matched women, which completely prevented bone loss at the distal radius (Recker et al., 1977).
In a similar but nonrandomized study, Horsman et al. (1977) administered 800 mg of elemental calcium as the gluconate salt to 24 postmenopausal women over a 2-year period and found a significant decrease in bone loss from the ulna (cortical bone) compared to 18 placebo-treated control subjects. However, calcium treatment caused little if any diminution of the bone loss observed at the distal radius or in metacarpal cortices. Similarly, Nilas et al. (1984) found no change in bone mineral content at the distal radius when three groups of women with calcium intakes varying from below 550 mg/day to more than 1,150 mg/day were given a 500-mg elemental calcium supplement daily. In contrast, a randomized and blinded investigation in postmenopausal women by the same group (Riis et al., 1987) showed that daily administration of 2,000 mg of elemental calcium as the carbonate salt for 2 years slowed bone loss at the proximal forearm and slowed calcium loss from the total skeleton, whereas the loss of bone from sites composed predominantly of trabecular bone was no different from that of placebo-treated control subjects. As in previous studies, bone mineral content remained constant at all measurement sites in subjects receiving estrogen.
In a nonrandomized study, Ettinger et al. (1987) observed that calcium supplementation up to 1,500 mg/day as the carbonate salt had no effect on bone mineral content in the spine as assessed by quantitative computed tomography, distal radius, or metacarpal cortical bone mass in 44 postmenopausal women as compared with 25 age-matched women who elected not to receive treatment. By contrast, in 15 women who elected to take low-dose conjugated estrogen (0.3 mg/day) combined with 1,500 mg of calcium per day, there was complete protection against bone loss. This latter observation is of considerable theoretical and practical interest, because this same group of investigators previously demonstrated that conjugated estrogen at the same low dose, given without calcium, failed to prevent vertebral bone loss (Cann et al., 1980). Thus, it is possible that dietary calcium plays a sex hormone-dependent permissive role in the maintenance of bone mass.
Riggs et al. (1976) showed that the increased bone resorption surfaces observed in biopsies of the iliac crest bone from osteoporotic patients are partially restored by combined calcium and vitamin D supplementation. This effect was associated with a decrease in serum immunoreactive parathyroid hormone (iPTH) within the normal range—an event the authors justifiably speculated was responsible for the decrease in resorption surfaces. The results of several other investigations, not involving bone histomorphometry, are consistent with this apparent antiresorption effect of calcium supplementation. Recker et al. (1977) showed that bone resorption, as assessed by kinetic analysis of plasma 45Ca decay curves, was decreased by supplementation of postmenopausal women with calcium carbonate. Horowitz et al. (1984) reported that oral calcium suppresses hydroxyproline excretion, a well-established index of bone resorption, in osteoporotic postmenopausal women.
Effect of Calcium Supplementation on Fracture
The evidence relating calcium supplementation to fracture prevalence is scanty. The only study of substance comes from the Mayo Clinic, where Riggs et al. (1982) conducted a nonrandomized but prospective assessment of the effect of various treatments of postmenopausal females with generalized osteopenia on the occurrence of future vertebral fractures. In that study, eight subjects received calcium carbonate (1,500 to 2,500 mg/ day) and 19 received calcium plus vitamin D (50,000 IU once or twice a week). Both groups had 50% fewer vertebral fractures than did 27 placebo-treated and 18 untreated patients.
Safety of Calcium Supplementation
Calcium supplementation is safe in the absence of condi-
Page 354
tions that cause hypercalcemia or nephrolithiasis (Heath and Callaway, 1985). Thus, in normal individuals, calcium intakes ranging from 1,000 to 2,500 mg/day do not result in hypercalcemia (FDA, 1979) and extremely high intakes (>2,500 mg/day) are required to produce hypercalciuria (>300 mg within 24 hours) (Knapp, 1947). Elemental calcium intakes in excess of 3 to 4 g/day should be avoided because they will cause hypercalcemia in most subjects (Ivanovich et al., 1967). Constipation can be a limiting side effect of calcium supplementation in many people and is particularly bothersome in the elderly. Calcium carbonate is currently the favored and cheapest form of supplemental calcium. Other anionic forms (e.g., calcium gluconate, calcium lactate) are equally effective but are generally more expensive.
There is no completely satisfactory animal model of age-related or postmenopausal osteoporosis. Nevertheless, the animal studies on dietary calcium and bone mass conducted thus far have produced results consistent with those from human studies. However, almost all reports concern young growing or aged animals and thus differ from investigations in humans (Leichsenring et al., 1951; Malm, 1953; Zemel and Linkswiler, 1981), which in general focus on young or middle-aged adults.
Nordin (1960) reviewed the extensive literature describing the many species in which bone mass decreases as a result of calcium deficiency. in all these studies, it is clear that the bone disease produced by calcium deficiency resembles osteoporosis in humans. Low-calcium diets cause a loss of trabecular bone in adult cats (Bauer et al., 1929) and a generalized thinning of bone in dogs Gaffe et al., 1932). After feeding adult cats a low-calcium diet for 5 months, Jowsey and Gershon-Cohen (1964) found that the animals had decreased skeletal weight, decreased density of bone as determined radiographically, and microradiographic evidence of increased bone resorption. These changes were partially reversed by feeding the animals a diet containing increased calcium. Many investigators have demonstrated that low-calcium diets lead to increased bone resorption typical of hyperparathyroidism and a generalized decrease in bone mass in rats and mice (Bell et al., 1941; de Winter and Steendijk, 1975; Gershon-Cohen et al., 1962; Harrison and Fraser, 1960; Ornoy et al., 1974; Rasmussen, 1977; Salomon, 1972; Salomon and Volpin, 1970; Shah et al., 1967; Sissons et al., 1985).
Of interest in relation to the possible influence of calcium deficiency on fracture is the study by Ferretti et al. (1985), who showed that femora from rats maintained on a low-calcium diet for 5 months had reduced inertial parameters and load resistance in comparison to femora from chronically thyroparathyroidectomized (thyroxinetreated) animals or animals fed a high-calcium diet. Griffiths et al. (1975) showed that rhesus monkeys on a low-calcium diet for several years developed radiological and histological changes in their skeletons that were consistent with hyperparathyroidism and osteoporosis.
Estrogen
The lack and diminished levels of estrogen are risk factors for osteoporosis. Estrogen replacement therapy reduces the loss of bone mass associated with oophorectomy and markedly reduces risk of hip and vertebral fracture (see Chapter 23). It is not clear whether the addition of calcium supplements to hormone-replacement therapy results in added benefit.
Phosphorus
Although data from animal studies suggest that high levels of dietary phosphorus increase bone loss, detailed studies in humans show little to no effect of high phosphorus intake on calcium balance (see section on Phosphorus, below).
Protein
Studies over the past half century indicate that high intakes of purified isolated protein increase the renal excretion of calcium (see Chapter 8). However, epidemiologic studies have shown no adverse effect of high dietary protein on either rate of hip fracture (Matkovic et al., 1979) or metacarpal cortical bone mass (Garn et al., 1981). As discussed in Chapters 8 and 23, the calciuric effect of protein is considerably reduced when increased protein intake is accompanied by high phosphorus intake—a common occurrence, since most foods in the United States with a high protein content also contain high levels of phosphorus.
Fiber
Dietary fiber has been reported to chelate calcium and other minerals in the gastrointestinal tract (Dobbs and Baird, 1977; Ismail-Beigi et al., 1977; McCance and Widdowson, 1942). This observation led to concern that high-fiber diets may increase risk of bone loss and osteoporotic fracture. However, there is little evidence that high-fiber diets alone induce calcium deficiency in
Page 355
people who otherwise consume a balanced diet (see Chapters 10 and 23).
Drugs
Although some drugs (e.g., thiazide diuretics) increase renal tubular reabsorption of calcium, they do not appear to influence calcium balance or changes in bone mass (Sakhaee et al., 1985). Phosphate-binding antacids such as the nonprescription aluminum hydroxide gels, if taken chronically even at low doses, can cause phosphate depletion and an accompanying increase in bone resorption and urinary calcium excretion (Maierhofer et al., 1984; Spencer and Lender, 1979; Spencer et al., 1982). It is not clear, however, whether the phosphate-binding type of antacid is related to age-related bone loss, particularly in calcium-deficient people.
Hypertension
Contraction of smooth muscle depends on the interaction among the contractile proteins—actin and myosin—and is the end result of a cascade of reactions initiated by a rise in cytosolic free calcium concentrations (Johansson and Somlyo, 1980). This observation led to the hypothesis that dietary calcium influences blood pressure and possibly risk for hypertension.
Within the past decade, considerable new evidence from human studies has suggested a role for dietary calcium in blood pressure regulation. However, views and theories are still in conflict, in part because of the wide range in study findings. For example, in an analysis of data from HANES I, McCarron et al. (1984) concluded that reduced calcium intake was the best predictor of increased blood pressure among all variables analyzed. Similar conclusions were reached in studies conducted in California (Ackley et al., 1983), Puerto Rico (Garcia-Palmieri et al., 1984), and the Netherlands (Kok et al., 1986).
Other investigators have reached different conclusions. For example, Feinleib et al. (1984) reanalyzed the HANES I data studied by McCarron et al. (1984), controlling for age and weight of subjects, and found no significant association between calcium intake and blood pressure. Harlan et al. (1984) found systolic blood pressure and calcium intake to be negatively correlated in women but positively correlated in men. Gruchaw et al. (1985) concluded that dietary calcium was not a significant predictor of blood pressure. In a large prospective study of omnivorous Japanese men in Hawaii, Reed et al. (1985) found inverse associations between intakes of calcium, potassium, protein, and milk (determined from 24-hour dietary recalls) and both systolic and diastolic blood pressure levels, although it was not possible to determine whether any of these dietary components had an independent effect on blood pressure.
The inconsistency among epidemiologic findings may be, in part, a result of the high degree of collinearity among other dietary factors associated with blood pressure (e.g., potassium and protein) and the limitations in the methods of assessing calcium intake in noninstitutionalized populations (Kaplan and Meese, 1986; Lau and Eby, 1985).
Clinical Studies
Acute elevations of serum calcium by intravenous infusions of calcium sharply raises blood pressure (Weidmann et al., 1972). Chronic hypercalcemia due to primary hyperparathyroidism is frequently accompanied by hypertension (Rosenthal and Roy, 1972), which is often reversible after the hyperparathyroidism is cured by removing abnormal parathyroid tissue (Blum et al., 1977). Serum calcium within the normal range has also been shown to correlate with high blood pressure (Bianchetti et al., 1983; Kesteloot, 1984a).
Hypertensive patients have been shown to have mild hypercalciuria (Morris et al., 1983; Strazzullo et al., 1986) and lower levels of serum ionized and ultrafiltrable calcium than normotensive patients, even in the absence of differences in total serum calcium (Folsom et al., 1986). Postnov and Orlov (1985) reported that the cells of hypertensive patients bind calcium less avidly than normotensives, and Erne et al. (1984) found increased calcium levels in platelets from hypertensive patients. Resnick et al. (1986) reported alterations in the serum concentrations of the calcium-regulating hormones (i.e., parathyroid hormone, calcitonin, and calcitriol) in hypertensive patients that are associated with differences in the renin-aldosterone system. Although all these reported changes indicate that calcium metabolism is probably perturbed in primary hypertension, it is not clear whether they are the cause or the result of the hypertension, and taken together, they do not support any single coherent theory of disordered blood pressure regulation.
Most intervention studies of calcium supplementation demonstrate a mild short-term reduction in blood pressure in certain normotensive and hypertensive subjects (Belizan et al., 1983;
Page 356
Grobbee and Hofman, 1986; McCarron and Morris, 1985; Resnick et al., 1984a; Singer et al., 1985). In some patients with hypertension and high levels of plasma renin, blood pressure may actually rise in response to calcium supplementation (Resnick et al., 1984b). No clinical trial of adequate size and design to test the hypothesis that increasing dietary calcium reduces hypertension risk has yet been reported.
Animal Studies
Most animal studies on the relationship of calcium metabolism and hypertension have compared the spontaneously hypertensive rat (SHR) to its normotensive control—the Wistar-Kyoto rat (WKR) (Young et al., 1988). The results are confusing and controversial. Most investigators (Bindels et al., 1987; Lau et al., 1984b; McCarron et al., 1981; Stem et al., 1984; Wright and Rankin, 1982) reported that serum concentrations of ionized calcium [Ca2+] are lower in the SHR than in the WKR. Some (Lau et al., 1984b; McCarron et al., 1981), but not all (Bindels et al., 1987; Hsu et al., 1986), agree that urinary calcium excretion is increased in SHRs. Although difficult to measure, serum iPTH has generally been reported to be slightly increased in the serum of SHRs (Bindels et al., 1987; McCarron et al., 1981; Stem et al., 1984), whereas serum 1,25-dihydroxycholecalciferol has been found to be increased (Bindels et al., 1987; Lau et al., 1986), decreased (Kurtz et al., 1986; Lucas et al., 1986; Merke et al., 1987; Schedl et al., 1986; Young et al., 1986), or unchanged (Kawashima, 1986; Schedl et al., 1984, 1986; Stem et al., 1984), depending to some extent upon the age and sex of the SHRs studied. Measurements of intestinal calcium absorption by a variety of techniques have been inconsistent (Bindels et al., 1987; Gafter et al., 1986; Hsu et al., 1986; Lau et al., 1984b, 1986; Lucas et al., 1986; McCarron et al., 1985, 1986; Roullet et al., 1986; Schedl et al., 1984; Stem et al., 1984; Toraason and Wright, 1981). It is thus difficult to ascribe the hypercalciuria in the SHR to intestinal hyperabsorption of calcium. Interestingly, studies that have measured bone calcium content (Izawa et al., 1985; Lucas et al., 1986) show it to be decreased in older (22 to 52 weeks) SHRs. These data do not help determine if the recorded abnormalities in calcium metabolism observed in the SHR are the cause, the result, or merely associated with its hypertension. However, they do suggest a pathogenic sequence for the changes in mineral and bone metabolism in SHRs. Such a sequence would include hypercalciuria due to a renal leak of calcium, leading to a decrease in serum calcium, secondary hyperparathyroidism, and finally, bone demineralization. As discussed above in the section on Clinical Studies, some of these same abnormalities have been observed in hypertensive humans. Thus, whether or not they are ultimately proved to be related etiologically to hypertension, they should be investigated independently in the SHR as a potential animal model of a clinical disorder of mineral and bone metabolism that might coexist with, or be caused by, certain hypertensive states.
Dietary calcium supplementation lowers blood pressure in SHRs (Ayachi, 1979; Kageyama et al., 1986; Lau et al., 1984a; McCarron et al., 1981, 1985). These observations suggest a possible etiologic link between the abnormalities of calcium metabolism and the hypertension found in SHRs; however, they fall well short of the evidence needed to prove a causative relationship.
Cancer
The relationship of calcium intake to risk of colon cancer has been examined in a number of epidemiologic studies. In one 19-year cohort study of 1,954 people in the United States, the calcium and vitamin D intake of people with colorectal cancer was significantly lower than in those without the disease (Garland et al., 1985). Mean calcium intake was 290 mg/1,000 kcal for colorectal cancer subjects and 328 mg/1,000 kcal for controls.
The results of case-control studies are inconsistent. G.R. Howe (National Cancer Institute of Canada, personal communication, 1989) found no association between dietary calcium and colorectal cancer in a reanalysis of an earlier study by Jain et al. (1980), who examined the role of a number of nutrients in relation to colorectal cancer risk. However, a protective effect of calcium with increasing intake was suggested in a case-control study conducted in Marseilles, France (Macquart-Moulin et al., 1986). The relative risk for the highest quartile of consumption compared to the lowest was 0.7. The association just failed to achieve statistical significance and was not further considered in a multivariate model. In addition, no association between calcium intake and risk of colorectal cancer was found in case-control studies conducted in Belgium (Tuyns et al., 1987a) and in Melbourne, Australia (Kune et al., 1987). In the Melbourne study, however, there was a suggestion
Page 357
of a protective effect in females when calcium intake was considered as a univariate.
Intercountry comparisons of calcium availability and colorectal cancer mortality rates also do not support a protective role for calcium intake. One comparison of 38 countries (G. McKeown-Eyssen, University of Toronto, and E. Bright-See, Ludwig Institute for Cancer Research, personal communication, 1989) gave a correlation of .51 between estimates of per-capita calcium availability and colorectal cancer mortality that was reduced to -.03 when the investigators controlled for fat intake. Studies of colon cancer incidence in rural Finland, other parts of Scandinavia, and New York that show a protective effect of dietary fiber (IARC, 1977; Jensen et al., 1982; Reddy et al., 1978) could also be explained by differences in calcium intake since the main contributor to dietary fat intake in the low-risk areas was milk products. However, there were no direct measurements of calcium intake in those study areas.
In a pilot study of the effect of calcium supplementation on proliferation of colonic cells in patients considered to be at increased risk for colon cancer, Lipkin and Newmark (1985) found that the daily administration of 1.2 g of elemental calcium as calcium carbonate led to a reduction of colonic crypt labeling with tritiated thymidine, in vitro, that approximated the pattern seen in a low-risk control population.
Tuyns et al. (1987b) found a weak protective effect of dietary calcium against risk of esophageal cancer. However, the finding was not statistically significant and was substantially weaker than the protective effect found for vitamin C.
Dietary calcium has been found to have a significant effect on the colonic epithelium of laboratory animals under several different experimental conditions. Calcium reduces the loss of superficial epithelial cells or the compensatory proliferation of basal crypt cells that occurs in animals exposed to bile and fatty acids or excess dietary fats. This effect has been seen in animals into which bile and fatty acids have been instilled intrarectally (Wargovich et al., 1983), in animals whose colons were perfused with bile acids (Rafter et al., 1986), in animals whose diets were supplemented with cholic acid (Bird et al., 1986), in animals given oral boluses of fat (Bird, 1986), and in animals fed high-fat diets (Caderni et al., 1988). Two studies that did not show an effect of calcium in reducing the number of colonic tumors also showed no cancer-promoting effect of high dietary fat (Bull et al., 1987).
Phosphorus
All living organisms require phosphorus to maintain their structure and function. In biologic fluids, it exists as phosphate ion. A major element in hydroxyapatite, phosphorus is a key inorganic constituent of bone. In cells, it is an important part of many life-sustaining compounds, such as phospholipids, phosphoproteins, and nucleic acids; the hormonal second messengers, cyclic adenosine monophosphate, cyclic guanine monophosphate, and inositol polyphosphates; and 2,3-diphosphoglycerate, which is the regulator of oxygen release by hemoglobin. Phosphorus is also the repository of metabolic energy in the form of the high-energy phosphate bond, an allosteric regulator of many enzymes, and an active participant in many physiological buffer systems. Serum concentrations of phosphate serve as one of the regulators of the rate of renal production of 1,25(OH)2D3.
Hypophosphatemia
Hypophosphatemia is a serious complication of many medical disorders (e.g., acute alcoholism, during the withdrawal phase); however, the food supply is so replete with phosphorus that the condition occurs only under the most adverse nutritional conditions. One exception is found in people who chronically ingest phosphate-binding antacids (see the discussion on Interactions in the section on Calcium). The major clinical manifestation of chronic moderate hypophosphatemia is a defective bone mineralization resembling osteomalacia. Severe hypophosphatemia may cause a life-threatening syndrome that includes blood cell, muscular, hepatic, and central and peripheral nervous system dysfunctions.
Excessive Dietary Phosphorus
Spencer et al. (1978) showed that an increase in phosphorus intake from 800 mg/day (the RDA) to 2,000 mg/day in adult males failed to affect calcium balance regardless of the calcium intake, which ranged from 200 to 2,000 mg/day. Similarly, Heaney and Recker (1982) reported that varying phosphorus intake had no effect on overall calcium balance in perimenopausal women. Both groups observed that urinary calcium excretion varied inversely with dietary phosphorus, implying that fecal calcium excretion must have varied directly with dietary phosphorus because there was no
Page 358
change in calcium balance. It thus appears that changes in phosphorus intake by normal adult humans have important effects on calcium metabolism (i.e., decreased intestinal calcium absorption and decreased renal excretion of calcium) but that these effects probably cancel one another so that calcium balance is not affected.
The mechanism by which increased dietary phosphorus might decrease intestinal absorption of calcium has been investigated by Portale et al. (1986). Those investigators showed that increasing dietary phosphorus from a low intake of <500 mg/day to 3,000 mg/day decreased the production rate of 1,25(OH)2D3 so that its serum concentration fell from a level 80% greater than normal to the low-normal range. This observation strongly suggests that the ability to adapt to decreases or increases in dietary phosphorus depends on the ability of the kidney to respond by increasing or decreasing its production of 1,25(OH)2D3, respectively.
There is, therefore, a question whether or not increases in dietary phosphorus might adversely influence calcium economy in people whose kidneys have a limited capacity to produce 1,25(OH)2D3 or in those who need to be in positive calcium balance, such as pregnant and lactating women. Portale et al. (1984) reported that normal dietary phosphorus levels were sufficient to suppress plasma concentrations of 1,25(OH)2D3 in children with moderate renal insufficiency. No studies of the influence of dietary phosphorus on calcium and bone metabolism have been reported in other populations that may be unduly sensitive to increments in dietary phosphorus above the RDA [e.g., the young who are building bone or some elderly people who have a decreased ability to absorb or conserve calcium (Sakhaee et al., 1984)], who have a decreased ability to absorb or conserve calcium (Sakhaee et al., 1984), even though concern has been expressed (Bell et al., 1977; Lutwak, 1975) that high phosphorus intakes may contribute to age-related bone loss in humans.
There is considerable evidence in animals that diets containing phosphorus in relatively larger quantities than calcium cause hyperparathyroidism and bone loss (Draper and Bell, 1979; Draper et al., 1972; Krishnarao and Draper, 1972; Krook, 1968; Miller, 1969; Saville and Krook, 1969). Almost all these reports concern young (growing) or aged animals and differ from other investigations of the influence of high-phosphate diets on calcium metabolism in young or middle-aged adult humans (Bell et al., 1977; Leichsenring et al., 1951; Malm, 1953; Zemel and Linkswiler, 1981).
Magnesium
Magnesium is the fourth most common positively charged ion in the body and is the second most abundant intracellular cation (next to potassium). It plays important roles in osmotic pressure maintenance, enzyme activation, muscular activity, energy metabolism, stabilization of nerve function, and maintenance of bone structure. The average adult body contains about 25 g of magnesium, approximately 50 to 60% of which is found in bone.
Hypomagnesemia
Hypomagnesemia results either from decreased intestinal absorption of magnesium or from increased renal excretion. The disease occurs only rarely as an isolated dietary deficiency. It is more often associated with severe general nutritional deficiency, intestinal malabsorption syndromes, excessive vomiting and diarrhea, genetic defects in the kidney, uncontrolled diabetes, and prolonged diuretic therapy. Severe hypomagnesemia (serum levels <1.0 mg/dl) can produce cardiac arrythmias, coronary spasm, hypocalcemia, low blood potassium, changes in mental status, seizures, anorexia, and weakness (Miller, 1985).
Hypertension
Magnesium is a potent inhibitor of vascular smooth-muscle contraction. It decreases peripheral vascular resistance and is a vasodilator that may play a role in the regulation of blood pressure. There are no data linking magnesium intake to the prevalence of hypertension. In case-control studies, serum magnesium levels have variously been reported as both higher and lower in hypertensive, compared to normotensive, people. Sangal and Beevers (1982) found an inverse association between serum magnesium and blood pressure in 73 Danish men and women whose mean age was 60 years. Similar findings have been reported by Albert et al. (1958) and Petersen et al. (1977). Kesteloot et al. (1984b) observed an inverse relationship between urinary magnesium and diastolic blood pressure levels in a subsample of the Belgian population. Resnick et al. (1984a) reported a close inverse correlation between erythrocyte magnesium concentration and both systolic and diastolic blood pressure.
Page 359
The few intercountry comparisons of magnesium intake and blood pressure show no association. Thulin et al. (1980) reported that magnesium intake was similar in normotensive and hypertensive Scandinavian women. Likewise, no relationship between magnesium excretion and blood pressure was seen in a Korean population (Kesteloot, 1984a) or in the NHANES II population in the United States (Harlan et al., 1984).
Data from studies in animals show a consistent inverse effect of magnesium intake on blood pressure. An increase in blood pressure and constriction of arteriolar, capillary, and postcapillary blood vessels has been observed in magnesium-deficient rats (Altura et al., 1984). Berthelot and Esposito (1983) noted a more rapid increase in the blood pressure level and heart rate of SHRs fed a magnesium-deficient diet compared with those fed a diet containing a normal amount of magnesium. SHRs fed a magnesium-supplemented diet (1.05%) had a blunted rise in blood pressure and a significantly lower mean blood pressure level as compared with controls after 22 weeks of feeding.
Wallach and Verch (1986) reported that numerous organs from SHRs, including the heart, lungs, kidneys, and bone, had 6 to 10% reductions in their magnesium content as hypertension became manifest. An inverse relationship between arterial blood pressure and tissue magnesium was also noted, but the authors could not determine whether the reduced tissue magnesium was a cause of or a response to the developing hypertension.
Resnick et al. (1986) reported that the higher the intracellular free magnesium in male Wistar rats, the lower the blood pressure. The authors suggest that there is a uniform and tightly coupled relationship between levels of intracellular free magnesium and blood pressure, regardless of pathological subtypes of hypertension or dietary conditions.
Cardiovascular Diseases
Populations in areas with hard water (i.e., water with high levels of minerals, including magnesium) have lower rates of cardiovascular diseases than those in areas with soft water (Neri and Johansen, 1978; Schroeder, 1960). This phenomenon, as well as the relationship between magnesium nutrition and cardiac rhythmicity in ischemic heart disease, has been reviewed extensively by Seelig (1974). She concluded that the role of magnesium in maintaining the normal rhythmicity of the heart during ischemic insult may explain the decrease in sudden cardiac death rates in areas with hard water as compared with rates in soft-water areas.
Summary
Minerals that are required in relatively large amounts are called macrominerals to distinguish them from trace elements—minerals needed in smaller amounts. Calcium, phosphorus, and magnesium are macrominerals. Low intakes of calcium, which occur commonly, have been associated with age-related osteoporosis. A dietary deficiency of phosphorus is unlikely, due to its wide distribution in foods. The mean population intake of magnesium, although slightly below the RDA, probably does not represent a health hazard.
Maximum bone mass is achieved by approximately 25 to 30 years of age. It is maintained until 35 to 45 years of age and then declines. Decreased skeletal mass is the most important risk factor for fracture of bones and is a significant public health problem in the United States. One of the problems in assessing the relationship of calcium intake to bone mass is the inherent inaccuracy of dietary recall. In addition, metabolic balance studies, although conducted extensively to determine nutritional requirements for calcium, also have important limitations that prevent accurate determination of the amount of dietary calcium needed to achieve balance.
It is important to achieve peak bone mass because the more mass that is available before age-related loss begins, the less likely it will decrease to a level at which fracture will occur. More dietary calcium is required to achieve peak bone mass than to replace obligatory losses of this ion in urine, feces, and sweat. Thus, people under 25 years of age probably need to ingest sufficient calcium to maintain a positive balance. This quantity will vary from person to person, depending on individual efficiencies of intestinal calcium absorption, but 1,200 mg/day probably provides a margin of safety for almost all normal people ages 11 to 25 years.
Once maximum bone mass is achieved, it is maintained without much change for 10 to 20 years. Calcium intake need not be greater than 800 mg/day during this period, because bone building has been completed and intestinal absorption of calcium is normal. However, men and women lose bone at a constant rate of 0.2 to 0.5% per year, starting at ages 40 to 45. For approximately 10
Page 360
years immediately before, during, and after menopause, women lose bone more rapidly than men (2 to 5% per year). This rapid rate of bone loss in menopausal women returns to the slower rate shared by the sexes after this 10-year period.
Intestinal calcium absorption and the ability to adapt to low-calcium diets are impaired in many postmenopausal women and elderly people of both sexes. The pathogenesis of these abnormalities is controversial, but evidence suggests that they may be due either to a decreased ability of the kidney to produce the major biologically active metabolite of vitamin D, 1,25(OH)2D3, such as after menopause, or to absolute decreases in the production of this metabolite due to renal disease, as in old age. The finding that serum levels of immunoreactive and bioactive parathyroid hormone increase with age implies that defects in calcium absorption are functionally important in that they result in sufficient degrees of hypocalcemia to produce chronic secondary hyperparathyroidism—a condition generally associated with bone demineralization. It appears, therefore, that the ability of the intestine to support calcium homeostasis progressively declines with age and that elderly people are increasingly forced to rely on their own bones rather than on the external environment as a source of calcium for maintaining normal extracellular fluid calcium. The degree to which this homeostatic response is needed depends on the severity of the described defects in calcium absorption, the level and bioavailability of dietary calcium, and whether specific therapeutic means are taken to correct defects in calcium absorption.
There is no direct evidence that the impaired intestinal calcium absorption observed during menopause and aging can be overcome by increased calcium intake. Moreover, the evidence that calcium supplementation prevents the trabecular bone loss associated with the menopause is, at best, weak. Thus, calcium supplementation should not be substituted for sex hormone replacement, which prevents postmenopausal bone loss in most women and appears to restore intestinal calcium absorption toward normal. Women taking estrogen replacement should continue to ingest 800 mg of calcium (the RDA). Those menopausal and postmenopausal women at risk for osteoporosis who are unable or refuse to take estrogen may require at least 1,200 mg of calcium per day. Such intakes could delay cortical bone loss and prevent chronic secondary hyperparathyroidism.
The association between decreased calcium intake and hypertension is suggestive but inconclusive. The epidemiologic and animal evidence relating calcium to colorectal cancer risk is also inconclusive. High-phosphorus diets may decrease calcium bioavailability, but they also reduce urinary calcium excretion and their influence on bone mass and the risk of osteoporosis is unknown. There are no known adverse effects of magnesium in the amounts currently consumed in the United States, although animal studies show a consistent inverse association between magnesium intake and blood pressure.
Directions for Research
· The age at which peak bone mass is achieved and the influence of calcium supplementation on peak bone mass need to be determined by longitudinal measurements.
· Additional studies should be conducted to determine the dietary requirement for calcium during and immediately before menopause in different groups of women (e.g., whites, blacks, and Asians). If requirements are known to be increased, investigations can then proceed to determine if therapeutic lowering of the requirement by increasing the fraction of calcium absorbed from the diet will influence the rate at which bone is lost in these patients.
· Long-term studies are needed to determine the effect of calcium supplementation on rate of bone loss in the elderly (65 years and older) in whom intestinal absorption of calcium is decreased.
· Dietary phosphorus, protein, and fiber each have potentially deleterious effects on calcium economy. Their individual and joint effects on calcium balance need to be determined in people such as the elderly who have decreased ability to produce 1,25-dihydroxycholecalciferol and in those such as adolescents who have a need to be in positive calcium balance.
· Continued research is needed to develop noninvasive, quantitative, analytical techniques that can accurately predict individuals at risk for osteoporotic fracture.
· Randomized, prospective, long-term studies in humans should be conducted to determine the influences of calcium supplementation on blood pressure.
· The association between magnesium intake and both blood pressure and cardiovascular diseases in humans needs to be clarified.
Page 361
References
Ackley, S., E. Barrett-Connor, and L. Suarez. 1983. Dairy products, calcium, and blood pressure. Am. J. Clin. Nutr. 38:457-461.
Albanese, A.A., A.H. Edelson, E.J. Lorenze, Jr., M.L. Woodhull, and E.H. Wein. 1975. Problems of bone health in elderly. Ten-year study. N.Y. State J. Med. 75:326-336.
Albert, D.G., Y. Morita, and L.T. Iseri. 1958. Serum magnesium and plasma sodium levels in essential vascular hypertension. Circulation 17:761-764.
Alevizaki, C.C., D.G. Ikkos, and P. Singhelakis. 1973. Progressive decrease of true intestinal calcium absorption with age in normal man. J. Nucl. Med. 14:760-762.
Altura, B.M., B.T. Altura, A. Gebrewold, H. Ising, and T. Gunther. 1984. Magnesium deficiency and hypertension: correlation between magnesium-deficient diets and microcirculatory changes in situ. Science 223:1315-1317.
Anderson, J.J.B., and F.A. Tylavsky. 1984. Diet and osteopenia in elderly caucasian women. Pp. 299-304 in C. Christiansen, C.D. Arnaud, B.E.C. Nordin, A.M. Parfitt, W.A. Peck, and B.L. Riggs, eds. Osteoporosis: Proceedings of the Copenhagen International Symposium on Osteoporosis. Department of Clinical Chemistry, Glostrup Hospital, Copenhagen.
Arnaud, C.D. 1978. Calcium homeostasis: regulatory elements and their integration. Fed. Proc. 37:2557-2560.
Arnaud, C.D. 1988. Mineral and bone homeostasis. Pp. 1469-1479 in J.B. Wyngaarden, L.H. Smith, Jr., and F. Plum, eds. Cecil Textbook of Medicine, 18th ed. W.B. Saunders, Philadelphia.
Arnaud, C.D., J.C. Gallagher, C.M. Jerpbak, and B.L. Riggs. 1981. On the role of parathyroid hormone in the osteoporosis of aging. Pp. 215-225 in H.F. DeLuca, H.M. Frost, W.S.S. Jee, C.C. Johnston, Jr., and A.M. Parfitt, eds. Osteoporosis: Recent Advances in Pathogenesis and Treatment. University Park Press, Baltimore.
Avioli, L.V., J.E. McDonald, and S.W. Lee. 1965. The influence of age on the intestinal absorption of 47-Ca absorption in post-menopausal osteoporosis. J. Clin. Invest. 44:1960-1967.
Ayachi, S. 1979. Increased dietary calcium lowers blood pressure in the spontaneously hypertensive rat. Metabolism 28:1234-1238.
Bauer W., J.C. Aub, and F. Albright. 1929. Studies of calcium and phosphorus metabolism. V. A study of the bone trabeculae as a readily available reserve supply of calcium. J. Exp. Med. 49:145-161.
Belizan, J.M., J. Villar, O. Pineda, A.E. Gonzalez, E. Sainz, G. Garrera, and R. Sibrian. 1983. Reduction of blood pressure with calcium supplementation in young adults. J. Am. Med. Assoc. 249:1161-1165.
Bell, G.H., D.P. Cuthbertson, and J. Orr. 1941. Strength and size of bone in relation to calcium intake. J. Physiol. 100: 299-317.
Bell, R.R., H.H. Draper, D.Y. Tzeng, H.K. Shin, and G.R. Schmidt. 1977. Physiological responses of human adults to foods containing phosphate additives. J. Nutr. 107:42-50.
Berthelot, A., and J. Esposito. 1983. Effects of dietary magnesium on the development of hypertension in the spontaneously hypertensive rat. J. Am. Coll. Nutr. 2:343-353.
Bianchetti, M.G., C. Beretta-Piccoli, P. Weidamn, L. Link, K. Boehringer, C. Ferrier, and J.J. Morton. 1983. Calcium and blood pressure regulation in normal hypertensive subjects. Hypertension 5:1157-1165.
Bindels, R.J., L.A. van den Broek, and M.J. Jougen, W.H. Hackeng, C.W. Lowik, and C.H. van Os. 1987. Increased plasma calcitonin levels in young spontaneously hypertensive rats: role in disturbed phosphate homeostasis. Pflugers Arch. 408:395-400.
Bird, R.P. 1986. Effect of dietary components on the pathobiology of colonic epithelium: possible relationship with colon tumorigenesis. Lipids 21:289-291.
Bird, R.P., R. Schneider, D. Stamp, and W.R. Bruce. 1986. Effect of dietary calcium and cholic acid on the proliferation indices of murine colonic epithelium. Carcinogenesis 7: 1657-1661.
Blum, M., M. Kirsten, and M.H. Worth, Jr. 1977. Reversible hypertension. Caused by the hypercalcemia of hyperparathyroidism, vitamin D toxicity, and calcium infusion. J. Am. Med. Assoc. 237:262-263.
Bull, A., R.P. Bird, W.R. Bruce, N. Nigro, and A. Medline. 1987. Effect of calcium on azoxymethane induced intestinal tumors in rats. Gastroenterology 92:1332.
Bullamore, J.R., R. Wilkinson, J.C. Gallagher, B.E. Nordin, and D.H. Marshall. 1970. Effect of age on calcium absorption. Lancet 2:535-537.
Caderni, G., E.W. Stuart, and W.R. Bruce. 1988. Dietary factors affecting the proliferation of epithelial cells in the mouse colon. Nutr. Cancer 11:147-153.
Cann, C.E., H.K. Genant, B. Ettinger, and G.S. Gordan. 1980. Spinal mineral loss in oophorectomized women. Determined by quantitative computed tomography. J. Am. Med. Assoc. 244:2056-2059.
Carroll, M.D., S. Abraham, and C.M. Dresser. 1983. Dietary Intake Source Data: United States, 1976-1980. Vital and Health Statistics, Series 11, No. 231. DHHS Publ. No. (PHS) 83-1681. National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 483 pp.
Cohn, S.H., ed. 1981. Non-Invasive Measurements of Bone Mass and Their Clinical Application. CRC Press, Boca Raton, Fla. 229 pp.
Dawson-Hughes, B., P. Jacques, and C. Shipp. 1987. Dietary calcium intake and bone loss from the spine in healthy postmenopausal women. Am. J. Nutr. 46:685-687.
de Winter, F.R., and R. Steendijk. 1975. The effect of a low-calcium diet in lactating rats; observations on the rapid development and repair of osteoporosis. Calcif. Tissue Res. 17:303-316.
DHEW (U.S. Department of Health, Education, and Welfare). 1979. Dietary Intake Source Data: United States, 1971-74. Vital and Health Statistics, DHEW Publ. No. (PHS) 79-1221. National Center for Health Statistics, Public Health Service, U.S. Department of Health, Education, and Welfare, Hyattsville, Md. 421 pp.
DHHS/USDA (Department of Health and Human Services/ U.S. Department of Agriculture). 1986. Nutrition Monitoring in the United States-A Progress Report from the Joint Nutrition Monitoring Evaluation Committee. DHHS Publ. No. (PHS) 86-1255. National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 356 pp.
Dobbs, R.J., and I.M. Baird. 1977. Effect of whole-meal and white bread on iron absorption in normal people. Br. Med. J. 1:1641-1642.
Draper, H.H., and R.R. Bell. 1979. Nutrition and osteoporosis. Pp. 79-106 in H.H. Draper, ed. Advances in Nutri-
Page 362
tional Research, Vol. 2. Plenum Press, New York.
Draper, H.H., T.L. Sie, and J.G. Bergan. 1972. Osteoporosis in aging rats induced by high phosphorus diets. J. Nutr. 102: 1133-1141.
Erne, P., P. Bolli, E. Bürgisser, and F.R. Bühler. 1984. Correlation of platelet calcium with blood pressure. Effect of antihypertensive therapy. N. Engl. J. Med. 310:1084-1088.
Ettinger, B., H.K. Genant, and C.E. Cann. 1987. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann. Int. Med. 106:40-45.
Exton, J.H. 1986. Mechanisms involved in calcium-mobilizing agonist responses. Adv. Cyclic Nucleotide Res. 20:211-262.
FDA (Food and Drug Administration). 1979. Vitamin and mineral drug products for over-the-counter human use. Establishment of a monograph; notice of proposed rulemaking-1. Calcium. Fed. Reg. 44:16175-16178.
Feinleib, M., C. Lenfant, and S.A. Miller. 1984. Hypertension and calcium. Science 226:384-389.
Ferretti, J.L., R.D. Tessaro, E.O. Audisio, and C.D. Galassi. 1985. Long-term effects of high or low Ca intakes and lack of parathyroid function on rat femur biomechanics. Calcif. Tissue Int. 37:608-612.
Folsom, A.R., C.L. Smith, R.J. Prineas, and R.H. Grimm, Jr. 1986. Serum calcium fractions in essential hypertensive and matched normotensive subjects. Hypertension 8:11-15.
Forero, M.S., R.F. Klein, R.A. Nissenson, K. Nelson, H. Heath III, C.D. Arnaud, and B.L. Riggs. 1987. Effect of age on circulating immunoreactive and bioactive parathyroid hormone levels in women. J. Bone Min. Res. 2:363-366.
Gafter, U., S. Kathpalia, D. Zikos, and K. Lau. 1986. Ca fluxes across the duodenum and colon of spontaneously hypertensive rats: effect of 1,25(OH)2D3. Am. J. Physiol. 251:F278-F282.
Gallagher, J.C., B.L Riggs, J. Eisman, A. Hamstra, S.B. Arnaud, and H.F. Deluca. 1979. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J. Clin. Invest. 64:729-736.
Gallagher, J.C., B.L. Riggs, C.M. Jerpbak, and C.D. Arnaud. 1980. The effect of age on serum immunoreactive parathyroid hormone in normal and osteoporotic women. J. Lab. Clin. Med. 95:373-385.
Garcia-Palmieri, M.R., R. Costas, Jr., M. Cruz-Vidal, P.D. Sorlie, J. Tillotson, and R.J. Havlik. 1984. Milk consumption, calcium intake, and decreased hypertension in Puerto Rico. Puerto Rico Heart Health Program Study. Hypertension 6:322-328.
Garland, C., R.B. Shekelle, E. Barrett-Connor, M.H. Criqui, A.H. Rossof, and O. Paul. 1985. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1 307-309.
Garn, S.M. 1970. The Earlier Gain and Later Loss of Cortical Bone, in Nutritional Perspective. C.C. Thomas, Springfield, Ill. 146 pp.
Garn, S.M., C.G. Rohmann, B. Wagner, G.H. Davila, and W. Ascoli. 1969. Population similarities in the onset and rate of adult endosteal bone loss. Clin. Orthop. 65:51-60.
Garn, S.M., M.A. Solomon, and J. Friedl. 1981. Calcium intake and bone quality in the elderly. Ecol. Food Nutr. 10: 131-133.
Gershon-Cohen, J., J.F. McClendon, J. Jowsey, and W.C. Foster. 1962. Osteoporosis produced and cured in rats by low- and high-calcium diets. Radiology 78:251-252.
Griffiths, H.J., R.D. Hunt, R.E. Zimmerman, H. Fineberg, and J. Cuttino. 1975. The role of calcium and fluoride in osteoporosis in rhesus monkeys. Invest. Radiol. 10:263-268.
Grobbee, D.E., and A. Hofman. 1986. Effect of calcium supplementation on diastolic blood pressure in young people with mild hypertension. Lancet 2:703-707.
Gruchaw, H.W., K.A. Sobocinski, and J.J. Barboriak. 1985. Alcohol, nutrient intake, and hypertension in U.S. adults. J. Am. Med. Assoc. 253:1567-1570.
Harlan, W.R., A.L. Hull, R.L. Schmouder, J.R. Landis, F.E. Thompson, and F.A. Larkin. 1984. Blood pressure and nutrition in adults. The National Health and Nutrition Examination Survey. Am. J. Epidemiol. 120:17-28.
Harrison, M., and R. Fraser. 1960. Bone structure and metabolism in calcium-deficient rats. J. Endocrinol. 21:197-205.
Heaney, R.P. 1985. The role of calcium in osteoporosis. J. Nutr. Sci. Vitaminol. 31:S21-S26.
Heaney, R.P. 1986. Calcium, bone health and osteoporosis. Pp. 255-301 in W.A. Peck, ed. Bone and Mineral Research, Annual 4: A Yearly Survey of Developments in the Field of Bone and Mineral Metabolism. Elsevier, New York.
Heaney, R.P., and R.R. Recker. 1982. Effects of nitrogen, phosphorus, and caffeine on calcium balance in women. J. Lab. Clin. Med. 99:46-55.
Heaney, R.P., R.R. Recker, and P.D. Saville. 1977. Calcium balance and calcium requirements in middle-aged women. Am. J. Clin. Nutr. 30:1603-1611.
Heath, H., III. and C.W. Callaway. 1985. Calcium tablets for hypertension? Ann. Intern. Med. 103:946-947.
Horowitz, M., A.C. Need, J.C. Philcox, and B.E. Nordin. 1984. Effect of calcium supplementation on urinary hydroxyproline in osteoporotic postmenopausal women. Am. J. Clin. Nutr. 39:857-859.
Horsman, A., J.C. Gallagher, M. Simpson, and B.E. Nordin. 1977. Prospective trial of oestrogen and calcium in postmenopausal women. Br. Med. J. 2:789-792.
Hsu, C.H., P.S. Chen, D.E. Smith, and C.S. Yang. 1986. Pathogenesis of hypercalciuria in spontaneously hypertensive rats. Miner. Electrolyte Metab. 12:130-141.
Hurxthal, L.M., and G.P. Vose. 1969. The relationship of dietary calcium intake to radiographic bone density in normal and osteoporotic persons. Calcif. Tissue Res. 4:245256.
IARC (International Agency for Research on Cancer). 1977. Intestinal Microecology Group. Dietary fibre, transit-time, faecal bacteria, steroids and colon cancer in two Scandinavian populations. Lancet 2:207-211.
Insogna, K.L, A.M. Lewis, B.A. Lipinski, C. Bryant, and D.T. Baran. 1981. Effect of age on serum immunoreactive parathyroid hormone and its biological effects. J. Clin. Endocrinol. Metab. 53:1072-1075.
Ireland, P., and J.S. Fordtran. 1973. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J. Clin. Invest. 52:2672-2681.
Ismail-Beigi, F., J.G. Reinhold, B. Faraji, and P. Abadi. 1977. Effects of cellulose added to diets of low and high fiber content upon the metabolism of calcium, magnesium, zinc and phosphorus by man. J. Nutr. 107:510-518.
Ivanovich, P., H. Fellows, and C. Rich. 1967. The absorption of calcium carbonate. Ann. Intern. Med. 66:917-923.
Izawa, Y., K. Sagara, T. Kadota, and T. Makita. 1985. Bone disorders in spontaneously hypertensive rat. Calcif. Tissue Int. 37:605-607.
Page 363
Jaffe, H.L., A. Bodansky, and J.P. Chandler. 1932. Ammonium chloride decalcification, as modified by calcium intake: relation between generalized osteoporosis and ostitis fibrosa. J. Exp. Med. 56:823-834.
Jain, M., G.M. Cook, F.G. Davis, M.G. Grace, G.R. Howe, and A.B. Miller. 1980. A case-control study of diet and colo-rectal cancer. Int. J. Cancer 26:757-768.
Jensen, O.M., R. MacLennan, and J. Wahrendorf on behalf of the IARC Large Bowel Cancer Group. 1982. Diet, bowel function, fecal characteristics, and large bowel cancer in Denmark and Finland. Nutr. Cancer 4:5-19.
Johansson, B., and A.P. Somlyo. 1980. Electrophysiology and excitation-contraction coupling. Pp. 301-323 in D.F. Bohr, A.P. Somlyo, and H.V. Sparks, Jr., eds. Handbook of Physiology, Section II: The Cardiovascular System, Vol. II—Vascular Smooth Muscle. American Physiological Society, Bethesda, Md.
Jowsey, J., and J. Gershon-Cohen. 1964. Effect of dietary calcium levels on production and reversal of experimental osteoporosis in cats. Proc. Soc. Exp. Biol. Med. 116:437-441.
Kageyama, Y., H. Suzuki, K. Hayashi, and T. Saruta. 1986. Effects of calcium loading on blood pressure in spontaneously hypertensive rats: attenuation of the vascular reactivity. Clin. Exp. Hypertens. 8:355-370.
Kanders, B., R. Lindsay, D. Dempster, L. Markhard, and G. Valiquette. 1984. Determinants of bone mass in young healthy women. Pp. 337-340 in C. Christiansen, C.D. Arnaud, B.E.C. Nordin, A.M. Parfitt, W.A. Peck, and B.L. Riggs, eds. Osteoporosis: Proceedings of the Copenhagen International Symposium on Osteoporosis. Department of Clinical Chemistry, Glostrup Hospital, Copenhagen.
Kaplan, N.M., and R.B. Meese. 1986. The calcium deficiency hypothesis of hypertension: a critique. Ann. Int. Med. 105: 947-955.
Kawashima, H. 1986. Altered vitamin D metabolism in the kidney of the spontaneously hypertensive rat. Biochem. J. 237:893-897.
Kelsey, J.L. 1984. Osteoporosis: Prevalence and Incidence. Pp. 25-28 in Osteoporosis, NIH Consensus Development Conference, April 2-4, 1984. National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases and the Office of Medical Applications of Research, National Institutes of Health, Bethesda, Md.
Kelsey, J.L. 1987. Epidemiology of osteoporosis and associated fractures. Pp. 409-444 in W.A. Peck, ed. Bone and Mineral Research, Annual 4: A Yearly Survey of Developments in the Field of Bone and Mineral Metabolism. Elsevier, New York.
Kesteloot, H. 1984a. Epidemiological studies on the relationship between sodium, potassium, calcium, and magnesium and arterial blood pressure. J. Clin. Cardiovasc. Pharmacol. 6:S192-S196.
Kesteloot, H. 1984b. Urinary cations and blood pressure—population studies. Ann. Clin. Res. 16 suppl. 43:72-80.
Knapp, EL. 1947. Factors influencing the urinary excretion of calcium. I. In normal persons. J. Clin. Invest. 26:182-202.
Kok, F.J., J.P. Vandenbroucke, C. Van der Heide-Wessel, and R.M. Van der Heide. 1986. Dietary sodium, calcium, and potassium, and blood pressure. Am. J. Epidemiol. 123: 1043-1048.
Krishnarao, G.V., and H.H. Draper. 1972. Influence of dietary phosphate on bone resorption in senescent mice. J. Nutr. 102:1143-1145.
Krolner, B., and S. Pors Nielsen. 1982. Bone mineral content of the lumbar spine in normal and osteoporotic women: cross-sectional and longitudinal studies. Clin. Sci. 62:329-336.
Krook, L. 1968. Dietary calcium-phosphorus and lameness in the horse. Cornell Vet. 58 suppl. 1:59-73.
Kune. S., G.A. Kune, and L.F. Watson. 1987. Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer study. Nutr. Cancer 9:21-42.
Kurtz, T.W., A.A. Portale. and R.C. Morris, Jr. 1986. Evidence for a difference in vitamin D metabolism between spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 8:1015-1020.
Lamke, B., H.E. Sjoberg, and M. Sylven. 1978. Bone mineral content in women with Colles' fracture: effect of calcium supplementation. Acta Orthop. Scand. 49:143-146.
Lau, K., and B. Eby. 1985. The role of calcium in genetic hypertension. Hypertension 7:657-667.
Lau, K., D. Zikos, J. Spirnak, and B. Eby. 1984a. Evidence for an intestinal mechanism in hypercaliuria of spontaneously hypertensive rats. Am. J. Physiol. 247:E625-E633.
Lau, K., S. Chen, and B. Eby. 1984b. Evidence for the role of PO4 deficiency in antihypertensive action of a high-Ca diet. Am. J. Physiol. 246:H324-H331.
Lau, K., C.B. Langman, U. Gafter, P.K. Dudeja, and T.A. Brasitus. 1986. Increased calcium absorption in prehypertensive spontaneously hypertensive rat. Role of serum 1,25-dihydroxyvitamin D3 levels and intestinal brush border membrane fluidity. J. Clin. Invest. 78:1083-1090.
Lavel-Jeanet, A.M., G. Paul, C. Bergot, J.L. Lamarque, and M.N. Ghiania. 1984. Correlation between vertebral bone density measurement and nutritional status. Pp. 305-309 in C. Christiansen, C.D. Arnaud, B.E.C. Nordin, A.M. Parfitt, W.A. Peck, and B.L. Riggs, eds. Osteoporosis: Proceedings of the Copenhagen International Symposium on Osteoporosis. Department of Clinical Chemistry, Glostrup Hospital, Copenhagen.
Leichsenring, J.M., LM. Norris, S.A. Lamison, E.D. Wilson, and M.B. Patton. 1951. The effect of level of intake on calcium and phosphorus metabolism in college women. J. Nutr. 45:407-418.
Lipkin, M., and H. Newmark. 1985. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colon cancer. N. Engl. J. Med. 313: 1381-1384.
Lucas, P.A., R.C. Brown, T. Drüeke, B. Lacour, J.A. Metz, and D.A. McCarron. 1986. Abnormal vitamin D metabolism, intestinal calcium transport, and bone calcium status in the spontaneously hypertensive rat compared with its genetic control. J. Clin. Invest. 78:221-227.
Lutwak, L. 1975. Metabolic and biochemical considerations of bone. Ann. Clin. Lab. Sci. 5:185-194.
Lutwak, L., and G.D. Whedon. 1963. Disease-a-Month: Osteoporosis. Year Books Medical Publ., Chicago. 39 pp.
Macquart-Moulin, G., E. Riboli, J. Cornee, B. Charnay, P. Berthezene, and N. Day. 1986. Case-control study on colorectal cancer and diet in Marseilles. Int. J. Cancer 38: 183-191.
Maierhofer, W.J., R.W. Gray, and J. Lemann, Jr. 1984. Phosphate deprivation increases serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 25:571-575.
Malm, O.J. 1953. On phosphates and phosphoric acid as dietary factors in the calcium balance of man. Scand. Clin. Lab. Invest. 5:75-84.
Page 364
Marcus, R. 1982. The relationship of dietary calcium to the maintenence of skeletal integrity in man—an interface of endocrinology and nutrition. Metabolism 31:93-102.
Marcus, R., P. Madvig, and G. Young. 1984. Age-related changes in parathyroid hormone and parathyroid hormone action in normal humans. J. Clin. Endocrinol. Metab. 58: 223-230.
Matkovic, V., K. Kostial, I. Simonovic, R. Buzina, A. Brodarec, and B.E. Nordin. 1979. Bone status and fracture rates in two regions of Yugoslavia. Am. J. Clin. Nutr. 32: 540-549.
Mazess, R.B. 1983. The noninvasive measurement of skeletal mass. Pp. 223-279 in W.A. Peck, ed. Bone and Mineral Research, Annual 1: A Yearly Survey of Developments in the Field of Bone and Mineral Metabolism. Excerpta Medica, Amsterdam.
McCance, R.A., and E.M. Widdowson. 1942. Mineral metabolism of healthy adults on white and brown bread dietaries. J. Physiol. 101:44-85.
McCarron, D.A., and C.D. Morris. 1985. Blood pressure response to oral calcium in persons with mild to moderate hypertension. A randomized, double-blind, placebo-controlled, crossover trial. Ann. Int. Med. 103:825-831.
McCarron, D.A., N.N. Yung, B.A. Ugoretz, and S. Krutzik. 1981. Disturbances of calcium metabolism in the spontaneously hypertensive rat. Hypertension 43:1162-1167.
McCarron, D.A., C.D. Morris, H.J. Henry, and J.L. Stanton. 1984. Blood pressure and nutrient intake in the United States. Science 224:1392-1398.
McCarron, D.A., P.A. Lucas, R.S. Schneidman, B. LaCour, and T. Drüeke. 1985. Blood pressure development of the spontaneously hypertensive rat after concurrent manipulations of dietary Ca2 and Na+: relation to Ca2+ intestinal fluxes. J. Clin. Invest. 76:1147-1154.
McCarron, D.A., P. Lucas, B. Lacour, and T. Drüeke. 1986. Ca2+ efflux rate constant (K°Ca) in isolated SHR enterocytes. Kidney Int. 29:252.
Merke, J., A. Slotkowski, H. Mann, P.H. Lucas, T. Drüeke, and E. Ritz. 1987. Abnormal 1,25(OH)2D3 receptor status in genetically hypertensive rats. Kidney Int. 31:303.
Miller, G. 1985. Magnesium deficiency syndrome. Compr. Ther. 11:58-64.
Miller, R.M. 1969. Nutritional secondary hyperparathyroidism. A review of etiology, symptomatology and treatment in companion animals. Vet. Med. Small Anim. Clin. 64:400-408.
Morris, C.D., H.J. Henry, and D.A. McCarron. 1984. Discordance of hypertensives' dietary Ca2+ intake and urinary Ca2+ excretion. Clin. Res. 32:57a.
NRC (National Research Council). 1980. Recommended Dietary Allowances, 9th ed. Report of the Committee on Dietary Allowances, Food and Nutrition Board, Division of Biological Sciences, Assembly of Life Sciences. National Academy Press, Washington, D.C. 185 pp.
Neri, L.C., and H.L. Johansen. 1978. Water hardness and cardiovascular mortality. Ann. N.Y. Acad. Sci. 304:203-221.
Nilas, L., C. Christiansen, and P. Rodbro. 1984. Calcium supplementation and postmenopausal bone loss. Br. Med. J. 289:1103-1106.
Nordin, B.E.C. 1960. Osteomalacia, osteoporosis and calcium deficiency. Clin. Orthop. 17:235-258.
Nordin, B.E.C. 1961. The pathogenesis of osteoporosis. Lancet 1:1011-1014.
Nordin, B.E.C. 1966. International patterns of osteoporosis. Clin. Orthop. 45:17-30.
Nordin, B.E.C., R. Wilkinson, D.H. Marshall, J.C. Gallagher, A. Williams, and M. Peacock. 1976. Calcium absorption in the elderly. Calcif. Tissue Res. 21:442-451.
Nordin, B.E.C., A. Horsman, D.H. Marshall, M. Simpson, and G.M. Waterhouse. 1979. Calcium requirement and calcium therapy. Clin. Orthop. 140:216-246.
Nottestad, S.Y., J.J. Baumel, D.B. Kimmel, R.R. Recker, and R.P. Heaney. 1987. The proportion of trabecular bone in human vertebrae. J. Bone Miner. Res. 2:221-229.
Ornoy, A., I. Wolinsky, and K. Guggenheim. 1974. Structure of long bones of rats and mice fed a low calcium diet. Calcif. Tissue Res. 15:71-76.
Orwoll, E.S., and D.E. Meier. 1986. Alterations in calcium, vitamin D, and parathyroid hormone physiology in normal men with aging: relationship to the development of senile osteopenia. J. Clin. Endocrinol. Metab. 63:1262-1269.
Pacifici, R., D. Droke, S. Smith, N. Susman, and L.V. Avioli. 1985. Quantitative computer tomographic (QCT) analysis of vertebral bone mass (VBM) in a female population. Clin. Res. 33:615A.
Parfitt, A.M. 1980. Morphologic basis of bone mineral measurements: transient and steady state effects of treatment in osteoporosis. Miner. Electrolyte Metab. 4:273-287.
Parfitt, A.M. 1983. Dietary risk factors for age-related bone loss and fractures. Lancet 2:1181-1185.
Pennington, J.A., B.E. Young, D.B. Wilson, R.D. Johnson, and J.E. Vanderveen. 1986. Mineral content of foods and total diets: the Selected Minerals in Foods Survey, 1982 to 1984. J. Am. Diet. Assoc. 86:876-891.
Petersen, B., M. Schroll, C. Christiansen, and I. Transbol. 1977. Serum and erythrocyte magnesium in normal elderly Danish people: relationship to blood pressure and serum lipids. Acta Med. Scand. 201:31-34.
Portale, A.A., B.E. Booth, B.P. Halloran, and R.C. Morris, Jr. 1984. Effect of dietary phosphorus on circulation concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderated renal insufficiency. J. Clin. Invest. 73:1580-1589.
Portale, A.A., B.P. Halloran, M.M. Murphy, and R.C. Morris, Jr. 1986. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J. Clin. Invest. 77:7-12.
Postnov, Y.V., and S.A. Orlov. 1985. Ion transport across plasma membrane in primary hypertension. Physiol. Rev. 65:904-945.
Rafter, J.J., V.W. Eng, R. Furrer, A. Medline, and W.R. Bruce. 1986. Effects of calcium and pH on the mucosal damage produced by deoxycholic acid in the rat colon. Gut 27:1320-1329.
Rasmussen, P. 1977. Calcium deficiency, pregnancy, and lactation in rats. Microscopic and microradiographic observations on bones. Calcif. Tissue Res. 23:95-102.
Recker, R.R., and R.P. Heaney. 1985. The effect of milk supplements on calcium metabolism, bone metabolism and calcium balance. Am. J. Clin. Nutr. 41:254-263.
Recker, R.R., P.D. Saville, and R.P. Heaney. 1977. Effect of estrogens and calcium carbonate on bone loss in postmenopausal women. Ann. Intern. Med. 87:649-655.
Reddy, B.S., A.R. Hedges, K. Laakso, and E.L. Wynder. 1978. Metabolic epidemiology of large bowel cancer: fecal bulk and constituents of high-risk North American and
Page 365
low-risk Finnish populations. Cancer 42:2832-2838.
Reed, D., D. McGee, K. Yano, and J. Hankin. 1985. Diet, blood pressure, and multicollinearity. Hypertension 7:405-410.
Resnick, L.M., R.K. Gupta, and J.H. Laragh. 1984a. Intracellular free magnesium in erythrocytes of essential hypertension: relation to blood pressure and serum divalent cations. Proc. Natl. Acad. Sci. U.S.A. 81:6511-6515.
Resnick, L.M., J.P. Nicholson, and J.H. Laragh. 1984b. Outpatient therapy of essential hypertension with dietary calcium supplementation. J. Am. Coll. Cardiol. 3:616.
Resnick, L.M., F.B. Muller, and J.H. Laragh. 1986. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann. Intern. Med. 105:649-654.
Riggs, B.L., and L.J. Melton III. 1986. Involutional osteoporosis. N. Engl. J. Med. 314:1676-1686.
Riggs, B.L., P.J. Kelley, W.R. Kinney, D.A. Scholz, and A.J. Bianco, Jr. 1967. Calcium deficiency and osteoporosis. Observations in one hundred and sixty-six patients and critical review of the literature. J. Bone Jt. Surg., Am. Vol. 49:915-924.
Riggs, B.L., J. Jowsey, P.J. Kelly, D.L. Hoffman, and C.D. Arnaud. 1976. Effects of oral therapy with calcium and vitamin D in primary osteoporosis. J. Clin. Endocrinol. Metab. 42:1139-1144.
Riggs, B.L., A. Hamstra, and H.F. DeLuca. 1981. Assessment of 25-hydroxyvitamin D I alpha-hydroxylase reserve in postmenopausal osteoporosis by administration of parathyroid extract. J. Clin. Endocrinol. Metab. 53:833-835.
Riggs, B.L, E. Seeman, S.F. Hodgson, D.R. Taves, and W.M. O'Fallon. 1982. Effect of the fluoride/calcium regimen on vertebral fracture occurrence in postmenopausal osteoporosis: comparison with conventional therapy. N. Engl. J. Med. 306:446-450.
Riggs, B.L., H.W. Wahner, L.J. Melton III, L.S. Richelson, H.L. Judd, and W.M. O'Fallon. 1987. Dietary calcium intake and rates of bone loss in women. J. Clin. Invest. 80: 979-982.
Riis, B., K. Thomsen, and C. Christiansen. 1987. Does calcium supplementation prevent postmenopausal bone loss? A double-blind, controlled clinical study. N. Engl. J. Med. 316:173-177.
Rosenthal, F.D., and S. Roy. 1972. Hypertension and hyperparathyroidism. Br. Med. J. 4:396-397.
Roullet, C., T. Drüeke, B. Lacour, and D. McCarron. 1986. Ca2+ influx of isolated enterocytes in adult SHRs and WKYs. Circulation 74:11-331.
Sakhaee, K., M.J. Nicar, K. Glass, J.E. Zerwekh, and C.Y. Pak. 1984. Reduction in intestinal calcium absorption by hydrochlorothiazide in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 59:1037-1043.
Sakhaee, K., M.J. Nicar, K. Glass, J.E. Zerwekh, and C.Y. Pak. 1985. Postmenopausal osteoporosis as a manifestation of renal hypercalciuria with secondary hyperparathyroidism. J. Clin. Endocrinol. Metab. 61:368-373.
Salomon, C.D. 1972. Osteoporosis following calcium deficiency in rats. Calcif. Tissue Res. 8:320-333.
Salomon, C.D., and G. Volpin. 1970. Fine structure of bone resorption in experimental osteoporosis caused by calcium deficient diet in rats. An electron microscopic study of compact bone. Calcif. Tissue Res. 4:80-82.
Sangal, A.K., and D.G. Beevers. 1982. Serum calcium and blood pressure. Lancet 2:493.
Saville, P.D., and L. Krook. 1969. Gravimetric and isotopic studies in nutritional hyperparathyroidism in beagles. Clin. Orthop. 62:15-24.
Schedl, H.P., D.L. Miller, J.M. Pape, R.L. Horst, and H.D. Wilson. 1984. Calcium and sodium transport and vitamin D metabolism in the spontaneously hypertensive rat. J. Clin. Invest. 73:980-986.
Schedl. H.P., D.L. Miller, R.L. Horst, H.D. Wilson, K. Natarajan, and T. Conway. 1986. Intestinal calcium transport in the spontaneously hypertensive rat: response to calcium depletion. Am. J. Physiol. 250:G412-G419.
Schroeder, H.A. 1960. Relation between mortality from cardiovascular disease and treated water supplies: variations in states and 163 largest municipalities of the United States. J. Am. Med. Assoc. 172:1902-1908.
Seelig, M.S. 1974. Magnesium interrelationships in ischemic heart disease: a review. Am. J. Clin. Nutr. 27:59-79.
Shah, B.G., G.V. Krishnarao, and H.H. Draper. 1967. The relationship of Ca and P nutrition during adult life and osteoporosis in aged mice. J. Nutr. 92:30-42.
Shils, M.E. 1988. Magnesium. Pp. 159-192 in M.E. Shils and V.R. Young, eds. Modem Nutrition in Health and Disease, 7th ed. Lea & Febiger, Philadelphia.
Singer, D.R.J., N.D. Markandu, F.P. Cappuccio, G.W. Beynon, A.C. Shore, S.J. Smith, and G.A. MacGregor. 1985. Does oral calcium lower blood pressure: a double-blind study. J. Hypertension 3:661.
Sissons, H.A., G.J. Kelman, and G. Marotti. 1985. Bone resorption in calcium-deficient rats. Bone 6:345-347.
Smith, E.L., Jr., W. Reddan, and P.E. Smith. 1981. Physical activity and calcium modalities for bone mineral increase in aged women. Med. Sci. Sports Exerc. 13:60-64.
Smith, R.W., Jr., and B. Frame. 1965. Concurrent axial and appendicular osteoporosis: its relation to calcium consumption. N. Engl. J. Med. 273:73-78.
Smith, R.W., Jr., and J. Rizek. 1966. Epidemiologic studies of osteoporosis in women of Puerto Rico and Southeastern Michigan with special reference to age, race, national origin and to other related or associated findings. Clin. Orthop. 45:31-48.
Spencer, H., and M. Lender. 1979. Adverse effects of aluminum-containing antacids on mineral metabolism. Gastroenterology 76:603-606.
Spencer, H., L. Kramer, D. Osis, and C. Norris. 1978. Effect of phosphorus on the absorption of calcium and on the balance in man. J. Nutr. 108:447-457.
Spencer, H., L. Kramer, C. Norris, and D. Osis. 1982. Effect of small doses of aluminum-containing antacids on calcium and phosphorus metabolism. Am. J. Clin. Nutr. 36:32-40.
Stern, N., D.B. Lee, V. Silis, F.W. Beck, L. Deftos, S.C. Manolagas, and J.R. Sowers. 1984. Effects of high calcium intake on blood pressure and calcium metabolism in young SHR. Hypertension 6:639-646.
Strazzullo, P., A. Siani, S. Guglielmi, A. Di Carlo, F. Galletti, M. Cirillo, and M. Mancini. 1986. Controlled trial of long-term oral calcium supplementation in essential hypertension. Hypertension 8:1084-1088.
Thulin, T., M. Abdulla, I. Dencker, M. Jagerstad, A. Melander, N. Schersten, and B. Akesson. 1980. Comparison of energy and nutrient intakes in women with high and low blood pressure levels. Acta Med. Scand. 208:367-373.
Toraason, M.A., and G.L. Wright. 1981. Transport of calcium by duodenum of spontaneously hypertensive rats. Am. J. Physiol. 241:G344-G347.
Page 366
Tsai, K.S., H. Heath III, R. Kumar, and B.L. Riggs. 1984. Impaired vitamin D metabolism with aging in women. Possible role in pathogenesis of senile osteoporosis. J. Clin. Invest. 73:1668-1672.
Tuyns, A.J., M. Haelterman, and R. Kaaks. 1987a. Colorectal cancer and the intake of nutrients: oligosaccharides are a risk factor, fats are not. A case-control study in Belgium. Nutr. Cancer 10:181-196.
Tuyns, A.J., E. Riboli, G. Doornbos, and G. Pequignot. 1987b. Diet and esophageal cancer in Calvados (France). Nutr. Cancer 10:81-92.
USDA (U.S. Department of Agriculture). 1984. Nationwide Food Consumption Survey. Nutrient Intakes: Individuals in 48 States, Year 1977-78. Report No. 1-2. Consumer Nutrition Division, Human Nutrition Information Service, Hyattsville, Md. 439 pp.
USDA (U.S. Department of Agriculture). 1986. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Men 19-50 Years, 1 Day, 1985. Report No. 85-3. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 94 pp.
USDA (U.S. Department of Agriculture). 1987. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Women 19-50 Years and Their Children 1-5 Years, 4 Days, 1985. Report No. 85-4. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 182 pp.
Vinther-Paulsen, N. 1953. Calcium and phosphorus intake in senile osteoporosis. Geriatrics 8:76-79.
Wallach, S., and R.L. Verch. 1986. Tissue magnesium in spontaneously hypertensive rats. Magnesium 5:33-38.
Wargovich, M.J., V.W. Eng, H.L. Newmark, and W.R. Bruce. 1983. Calcium ameliorates the toxic effect of deoxycholic acid on colonic epithelium. Carcinogenesis 4:1205-1207.
Weidmann, P., S.G. Massry, J.W. Coburn, M.H. Maxwell, J. Atleson, and C.R. Kleeman. 1972. Blood pressure effects of acute hypercalcemia. Studies in patients with chronic renal failure. Ann. Intern. Med. 76:741-745.
Wright, G.L., and G.O. Rankin. 1982. Concentrations of ionic and total calcium in plasma of four models of hypertension. Am. J. Physiol. 243:H365-H370.
Young, E.W., S.R. Patel, and C.H. Hsu. 1986. Plasma 1,25(OH)2D3 response to parathyroid hormone, cyclic adenosine monophosphate, and phosphorus depletion in the spontaneously hypertensive rat. J. Lab. Clin. Med. 6:562-566.
Young, E.W., R.D. Bukoski, and D.A. McCarron. 1988. Calcium metabolism in experimental hypertension. Proc. Soc. Exp. Biol. Med. 187:123-124.
Zemel, M.B., and H.M. Linkswiler. 1981. Calcium metabolism in the young adult male as affected by levels and form of phosphorus intake and level of calcium intake. J. Nutr. 111:315-324.




















