7
Neuroethology of Primate Social Behavior
STEVE W. C. CHANG,*† LAUREN J. N. BRENT,*† GEOFFREY K. ADAMS,*† JEFFREY T. KLEIN,‡ JOHN M. PEARSON,*†§ KARLI K. WATSON,*† AND MICHAEL L. PLATT*†∥#**
A neuroethological approach to human and nonhuman primate behavior and cognition predicts biological specializations for social life. Evidence reviewed here indicates that ancestral mechanisms are often duplicated, repurposed, and differentially regulated to support social behavior. Focusing on recent research from nonhuman primates, we describe how the primate brain might implement social functions by co-opting and extending preexisting mechanisms that previously supported nonsocial functions. This approach reveals that highly specialized mechanisms have evolved to decipher the immediate social context, and parallel circuits have evolved to translate social perceptual signals and nonsocial perceptual signals into partially integrated social and nonsocial motivational signals, which together inform general-purpose mechanisms that command behavior. Differences in social behavior between species, as well as between individuals within a species, result in part from neuromodulatory regulation of these neural circuits, which itself appears to be under partial genetic control. Ultimately, intraspecific variation in social behavior has differential fitness consequences, providing fundamental building blocks of natural selection. Our review suggests that the neu-
_____________
Departments of *Neurobiology and §Neurosurgery, Duke University School of Medicine, Durham, NC 27710; †Duke Institute for Brain Sciences, Center for Cognitive Neuroscience and Departments of ∥ Psychology and Neurosciences and #Evolutionary Anthropology, Duke University, Durham, NC 27708; and ‡Bowles Center for Alcohol Studies, University of North Carolina, Chapel Hill, NC 27599. **To whom correspondence should be addressed. E-mail: platt@neuro.duke.edu.
roethological approach to primate behavior may provide unique insights into human psychopathology.
Sensitivity and responsiveness to information about others is critical for human health (Berkman, 2000; Cohen, 2004), survival (Barefoot et al., 2005), and even financial success (Baron and Markman, 2003). To navigate our social worlds, we track the behavior of others and form models of their intentions and emotional states, we actively seek out and exchange information about others, and we flexibly alter our behavior in response to what we know about others. These faculties are so important to human behavior that their disruption constitutes psychopathology (Adolphs, 2003; Meyer-Lindenberg et al., 2011). These specializations for social behavior reflect a rich evolutionary heritage of adaptation to group life (Byrne and Whiten, 1989; Dunbar, 1998; Allman, 1999). Like humans, many nonhuman primates also live in large groups characterized by patterns of social behaviors like grooming, imitative and cooperative foraging, differentiated affiliative relationships, ritualized courtship and mating behavior, and competitive interactions structured by social dominance (Wilson, 1975; Smuts et al., 1987). Not surprisingly, the ability to deftly navigate the social environment has observable consequences for reproductive success in some nonhuman primates (Silk et al., 2003).
EVOLUTIONARY PERSPECTIVE ON SOCIAL BEHAVIOR
Social behavior places strong and unique demands on the nervous system. Across primate species, group size (a potential proxy of social complexity) is correlated with forebrain volume, after correcting for body size (Dunbar, 1998). Additional brain tissue beyond that required to maintain a body of a particular size is costly, in both developmental complexity and metabolic demands (Aiello and Wheeler, 1995; Allman, 1999; Lennie, 2003; Leonard et al., 2003). Indeed, social complexity and the elaboration of neural mechanisms to support it are associated with diets high in dependable calorie-rich foods (Harvey et al., 1980; Harding, 1981; Milton, 1981). Major expansion of the hominine brain during human evolution appears to have coincided with the development of new behaviors that added more calories to the diet, such as eating meat (Homo habilis, ~2.3 Mya) (Leakey et al., 1964) and cooking (Homo erectus, ~1.5 Mya) (Wrangham, 2009).
Social behavior seems likely to depend on homologous neural mechanisms in humans and nonhuman primates (Rushworth et al., 2013). Novel behaviors can evolve by connecting, repurposing (i.e., shifted to serve a new function), or elaborating upon ancestral mechanisms that originally served a different function (Katz and Harris-Warrick, 1999), and the evo-
lution of social behaviors seems likely to follow this pattern. A striking example of such elaboration and repurposing is the electrocommunication system of mormyrid fish. These fish have electrosensory receptors that are part of their lateral line system, which originally evolved to aid orienting and the detection of motion (Montgomery, 1991; Katz, 2006). In mormyrids, the cerebellum, where sensations from the lateral line system are processed, is greatly enlarged and serves an important role in electrocommunication, a social function absent in the ancestral state (Montgomery, 1991; Katz, 2006). The evolution of the neuropeptide oxytocin (OT) is another excellent example of repurposing for social functions. The ancestral anxiolytic (Neumann et al., 2000; Yoshida et al., 2009), approach- and tolerance-enhancing (Young, 2002; Averbeck, 2010; Kemp and Guastella, 2010) roles of OT in early vertebrates may have been co-opted to support parental behavior and social bonding in mammals.
In this review, we discuss recent evidence supporting the idea that social behavior can be constructed from the basic building blocks of nonsocial behaviors. In some cases, sociality is supported by general-purpose mechanisms whereas others may require special-purpose mechanisms. By “general purpose,” we mean that a given mechanism is used generally across both social and nonsocial domains, whereas, by “special purpose,” we mean that a given mechanism has a privileged role in the social domain. Specialized mechanisms, such as the electrosensory receptor organ of mormyrid fish tuned for species communication and face identification cells in the temporal lobes of primates (Perrett et al., 1982; Desimone et al., 1984; Desimone, 1991; Tsao et al., 2003) and ungulates (Kendrick, 1994), are more frequently found near the input stages of social processing (i.e., receiving social information) whereas generalized mechanisms are more common near the output stages of effector control (Klein et al., 2008). By contrast, a mixture of specialized and generalized mechanisms appear to characterize intermediate computational stages of processing that translate socially specific inputs into motivational signals that guide learning and decision making, ultimately resulting in motor commands that generate behavior (Watson and Platt, 2012; Chang et al., 2013; Klein and Platt, 2013). Our review focuses on recent behavioral, neurobiological, and genetic findings supporting these general principles. Selected examples used in this review to support our claim are summarized in Table 7.1.
PARALLELS BETWEEN SOCIAL AND NONSOCIAL BEHAVIORS
Many of our behaviors are driven by reinforcement, and we and other animals seek a variety of rewards by foraging. Foraging is one of the most primitive and basic behavioral states, being a feature of essentially all motile, heterotrophic life. It is therefore unsurprising that foraging strate-
TABLE 7.1 Summary List of Selected Examples from the Current Paper on How Nonsocial Functions Are Repurposed to Serve Social Functions Throughout Evolution
| Biological Units | Type/ Region | Nonsocial Functions | Social Functions | ||||||
| Behaviors | Foraging | Reward seeking, information seeking (Charnov, 1976; Stephens and Krebs, 1986; Miller and Remington, 2004; Pirolli, 2007; Lawrance et al., 2013) | Social information seeking (Keating and Keating, 1982; Johnson et al., 1991; Emery, 2000; Deaner et al., 2005; Hayden et al., 2007; Adams et al., 2012) | ||||||
| Imminent threat response | Reflexive, escape behavior (Fanselow and Lester, 1988) | Gaze aversion (Deaner et al., 2005; Watson and Platt, 2012) | |||||||
| Distant threat response | Cautious exploratory behavior (Dielenberg et al., 2001) | Social exploration (Deaner et al., 2005; Watson and Platt, 2012) | |||||||
| Neural circuits | Posterior superior sulcus (pSTS) | Multisensory integration, perceiving intention from animacy (Bruce et al., 1981; Gao et al., 2012) | Gaze perception, gaze following (Hoffman and Haxby, 2000; Roy et al., 2014) | ||||||
| Lateral intraparietal area (LIP) | Spatial orienting, motor planning (Snyder et al., 2000; Bisley and Goldberg, 2010) | Gaze direction, social value associated with space (Klein et al., 2008; Roy and Platt, 2009; Shepherd et al., 2009) | |||||||
| Striatum (medial) | Reward and learning (Moll et al., 2006; Izuma et al., 2008) | Social image category, reward donation (Izuma et al., 2008; Klein and Platt, 2013) | |||||||
| Orbitofrontal cortex (OFC) | Social image category, received reward during social interactions, social network size (Dunbar, 1995; Lewis et al., 2011; Watson and Platt, 2012; Chang et al., 2013) | ||||||||
| Biological Units | Type/ Region | Nonsocial Functions | Social Functions | ||||||
| Anterior cingulate sulcus (ACCs) | Foraging decisions, performance monitoring (Hayden et al., 2011) | Foregone reward during social interactions (Chang et al., 2013) | |||||||
| Anterior cingulate gyrus (ACCg) | Reward and learning (A m e m o r i a n d G r a y b i e l, 2 0 1 2 ) | Shared and donated reward during social interactions, social evaluation, other-regard, mentalizing about others’ states of mind (Singer et al., 2004; Rudebeck et al., 2006; Saxe, 2006; Mobbs et al., 2009; Waytz et al., 2012; Chang et al., 2013) | |||||||
| Neuromodulators | Oxytocin/ vasopressin | Water regulation, reproduction, anxiolysis (Belin and Moos, 1986; Neumann et al., 2000; Donaldson and Young, 2008; Yoshida et al., 2009) | Pair-bonding, parental care, selective aggression, social salience, generosity, trust (Pedersen et al., 1982; Winslow et al., 1993; Cho et al., 1999; Young, 2002; Kosfeld et al., 2005; Zak et al., 2007; Heinrichs et al., 2009; Averbeck, 2010; Kemp and Guastella, 2010; Bartz et al., 2011; Chang et al., 2012; Crockford et al., 2013) | ||||||
| HPA axis | Physical stress | Psychosocial stress (social status) (Abbott et al., 2003; Blumstein et al., 2006; Tung et al., 2012) | |||||||
| HPG axis | Reproduction | Social regulation/ control, social opportunity (social status) (Wingfield et al., 1990; Burmeister et al., 2005; Hirschenhauser and Oliveira, 2006; Fernald, 2012; Higham et al., 2013) | |||||||
| Biological Units | Type/ Region | Nonsocial Functions | Social Functions | ||||||
| Serotonin | Cardiac and gastrointestinal functions, mood, memory, reward and learning (Sirviö et al., 1994; Hayes and Greenshaw, 2011) | Social network integration, social structure, social information processing (Wendland et al., 2006; Watson et al., 2009; Brent et al., 2013) | |||||||
gies are under strong selective pressure for maximizing returns on investment. Animals often forage for foods sparsely distributed in locally dense patches (Charnov, 1976). As an animal forages in a patch, resources are depleted and the rate of energy intake slows. However, traveling to a new patch may be costly and accompanied by uncertain outcomes, leading to a decision to abandon a patch to maximize its overall rate of consumption. The same principle applies to many everyday decisions made by people. Because resources are often patchily distributed, this model has broad applicability. The optimal solution, known as Charnov’s Marginal Value Theorem, is that a patch should be abandoned when the current rate of consumption falls to the average for the overall environment (Charnov, 1976). This model has been remarkably successful at describing the foraging behavior of a wide variety of organisms (Stephens and Krebs, 1986) and recently has been applied to understand neural correlates of foraging decisions (Hayden et al., 2011; Kolling et al., 2012). In fact, foraging theory has also been applied to problems far afield from its original purpose, including the efficient design of websites (Miller and Remington, 2004) and a description of how computer programmers search for errors in code (Lawrance et al., 2013).
Organisms searching for information can be said to be “information foraging” (Pirolli, 2007). Like foraging for primary rewards, information foraging presents opportunities as well as costs. Costs come in the form of missed opportunities to eat, drink, or sleep because information-seeking behaviors often demand certain postures or behavioral states incompatible with attentive orienting, as well as social costs, such as aggression from conspecifics and missed opportunities to interact with partners. Because social information has reinforcement value (either positive or negative), the basic problems studied by foraging theory may apply to the acquisition of social information. A wealth of behavioral data indicates that both humans and nonhuman primates actively seek social information. Humans and nonhuman primates find social stimuli to be intrinsically rewarding, and certain types of social stimuli are more interesting and
reinforcing than others (Emery, 2000; Deaner et al., 2005; Hayden et al., 2007). For instance, even shortly after birth, human infants look longer at faces than at similar nonface stimuli (Johnson et al., 1991). Likewise, nonhuman primates spend more time looking at pictures of faces directed toward them compared with pictures of faces with averted gaze (Keating and Keating, 1982), and direct their gaze more often toward higher-ranking than lower-ranking animals (McNelis and Boatright-Horowitz, 1998). Furthermore, active social interactions such as cooperative transactions (Rilling et al., 2002; Rand et al., 2012) or the opportunity to punish a traitor (de Quervain et al., 2004), which can be understood using a game theoretic framework (Lee, 2008), can be as motivating as primary rewards in humans. These observations support the hypothesis that the brains of many animals, especially those of primates, have evolved mechanisms that find social information rewarding and worth foraging.
We propose that, because a major function of the brain is to seek resources, it is likely that mechanisms that evolved to support foraging are readily repurposed to solve other, formally similar computational problems. With respect to social behavior, if information about others is a valuable resource, then the biological mechanisms underlying foraging decisions will be used to support social information seeking (Adams et al., 2012). For example, opportunities and costs associated with social information foraging are likely to engage fundamental biological mechanisms for computing opportunities and costs. Foraging mechanisms seem likely to have become further specialized to cope with the unique demands of interindividual dynamics that arise as a consequence of group living.
Another potential example of similarities between social and nonsocial behaviors arises from the comparison of behavioral responses to predators and social threats. In both cases, an imminent threat evokes fast, reflexive behaviors, such as freezing, defensive aggression, or escape behavior (Fanselow and Lester, 1988). A distant threat, however, elicits cautious exploratory behavior of the threatening object (Dielenberg et al., 2001). Rhesus macaques, when given the opportunity, will opt to view pictures of dominant monkeys, a potentially threatening social stimulus, over pictures of subordinates (Deaner et al., 2005; Watson and Platt, 2012). Despite this interest, low-status monkeys typically avert their gaze from high-status monkey faces when confronted (Deaner et al., 2005) and look quickly away from dominant male pictures after choosing to see them (Deaner et al., 2005). This behavior is reminiscent of the exploratory behavior of rodents confronted with cat odor (Dielenberg et al., 2001) and the avoidance behavior in the presence of an actual predator. Indeed, many fundamental behavioral strategies designed for nonsocial settings seem to resonate across behavioral strategies used in social settings.
NEURAL CIRCUITS GUIDING SOCIAL DECISIONS
The neural mechanisms supporting social behaviors are broadly distributed throughout the primate forebrain, overlapping with areas involved in more general-purpose functions (Fig. 7.1A). Current evidence suggests that most neural circuits involved in social behavior are not dedicated exclusively to “social” functions. Rather, such circuitry is also typically engaged in related nonsocial behaviors, regardless of whether social information is processed in a privileged manner (i.e., special purpose) or not (i.e., general purpose). This evidence supports the hypothesis that the evolution of novel social behaviors has occurred by co-opting existing neural hardware for the purpose of interacting with others.
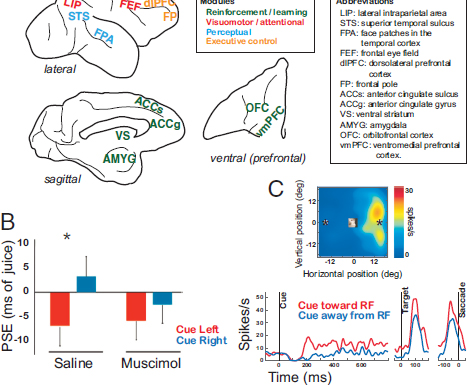
FIGURE 7.1 Example neural circuits co-opted to serve social functions. (A) Representative brain regions in rhesus macaques whose preexisting functions encompass reward, attention, perception, and executive control. (B) Point of subjective equality (PSE), bias for socially cued target in terms of foregone juice, after saline or muscimol injections in pSTS. Reproduced from Roy et al. (2014) with permission from Oxford University Press. (C) LIP neuron showing firing rate enhancement by observed gaze directed toward the receptive field (RF). (Upper) RF map. (Lower) Neuronal activity as a function of time. Reproduced with permission from Shepherd et al. (2009). [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
Broadly speaking, these circuits can be thought of as organized into input, integrative, and output stages of social processing. The input stage of social processing comprises specialized sensory channels that transduce socially important information, including face-selective (Tsao et al., 2006) and identity-specific cells (Quiroga et al., 2005) in primates, pheromone-sensing systems like the vomeronasal organ in rodents (Keverne, 1999), and specialized regions for species-specific vocalizations in birds (Doupe and Konishi, 1991) and mammals (Ghazanfar and Hauser, 2001; Eliades and Wang, 2008), and language in humans (Damasio and Geschwind, 1984). The output stage of social processing comprises socially specific motor patterns, including highly stereotyped behaviors like allogrooming (Schino et al., 1988), ritualized play (Grant and Mackintosh, 1963), and threat and submission gestures (Deag, 1977). In the integrative stages of social information processing, studies in humans have shown that phenomena such as opprobrium and moral disgust rely in large part on circuits involved in nociception and interoception, particularly those linking the amygdala, periaqueductal gray, insular cortex, and anterior cingulate cortex (ACC) (Moll et al., 2005). Experiments in both humans and other animals have shown that information about socially relevant stimuli such as attractive faces, bodies, and rewards delivered to others activate regions likewise implicated in nonsocial reward (Moll et al., 2006; Izuma et al., 2008; Klein et al., 2008; Mobbs et al., 2009; Smith, 2010; Azzi et al., 2012; Watson and Platt, 2012; Chang et al., 2013). These results are consistent with the idea that social processing is largely built upon and extended from other nonsocial computations by these neural circuits.
The demands of dynamic social interactions are likely to have further shaped the functions of neural circuits involved in social behavior (i.e., selection on a mechanism for a specific function). Humans and other primates clearly elaborate upon the aforementioned basic, relatively stereotyped patterns of social behavior. For example, both human and nonhuman primates can covertly attend to a specific location in space without looking at it directly (Eriksen and Yeh, 1985; Herrington and Assad, 2010), a behavior that seems likely to have evolved to support monitoring of others in social groups (Moore et al., 2003; Hunnius, 2007). Watching another individual shift gaze to an object or location in space typically evokes a gaze shift, as well as a shift in covert attention, in the same direction, in humans and other nonhuman primates (Shepherd, 2010). This gaze-following response depends upon neural circuits involved in decoding where another individual is looking, and circuits that orient attention and plan gaze shifts. Neurons in the primate superior temporal sulcus (STS) are involved in the integration of converging inputs from multiple sensory modalities (Bruce et al., 1981). A posterior portion of STS (pSTS) seems to have evolved the specialized function of perceiving the
gaze of other individuals (Hoffman and Haxby, 2000) as well as intention implied from animacy (Gao et al., 2012). Consistent with its role in gaze perception, inactivating pSTS with muscimol abolishes gaze following in rhesus macaques (Roy et al., 2014) (Fig. 7.1B). Neurons in the primate lateral intraparietal area (LIP), an area important for spatial attention and the oculomotor planning (Snyder et al., 2000; Bisley and Goldberg, 2010), are activated by the mere observation of a monkey looking toward the region of space covered by the neurons’ receptive fields (Shepherd et al., 2009) (Fig. 7.1C). Unlike pSTS, however, inactivating LIP has no specific impact on gaze following (Roy and Platt, 2009), consistent with a more generalized role in visuomotor behavior.
As mentioned previously, both human and nonhuman primates are highly motivated by social information. Social information activates key reward areas in humans and nonhuman primates, including the ACC, orbitofrontal cortex (OFC), nucleus accumbens, and caudate nucleus (Moll et al., 2006; Izuma et al., 2008; Mobbs et al., 2009; Smith, 2010; Azzi et al., 2012; Watson and Platt, 2012; Chang et al., 2013; Klein and Platt, 2013). These observations suggest the possibility that social information and information about primary motivators like food are translated into a common framework or currency that drives both learning and decision making (Levy and Glimcher, 2012). When monkeys choose between fluid rewards and information about others (Deaner et al., 2005; Watson and Platt, 2012), neurons in area LIP simultaneously encode the social value and fluid value associated with a target in space, consistent with a common currency of target/action value (Klein et al., 2008). By contrast, neurons in the primate striatum, particularly the medial aspect, appear to be more specialized for signaling social information (Klein and Platt, 2013). In monkeys choosing between fluid rewards and information about others, similar proportions of neurons (~30–35 percent) carried information about fluid outcomes and social image outcomes, but these populations were largely nonoverlapping. Thus, multiple, unique, small ensembles of striatal neurons appear to convey idiosyncratic yet highly specific information about motor responses, contexts, cues, outcomes, or combinations thereof, and this organization extends to social behavior.
The OFC also encodes the value of rewards like food and money (Padoa-Schioppa and Assad, 2006). Like the striatum, OFC also contains neurons specialized for social interaction. We found that even when monkeys’ choices were dominated by the value of fluid rewards, the responses of ~50 percent of neurons encoded social information, but only ~20 percent encoded information about fluid rewards (Fig. 7.2A) (Watson and Platt, 2012). As in striatum, these populations of neurons were largely distinct, but, unlike striatum, they were anatomically intermingled. Notably, individual OFC neurons also signaled categorical information with respect
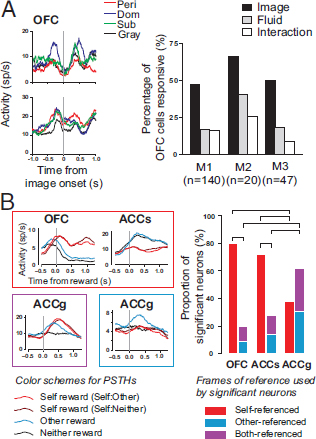
FIGURE 7.2 Reward circuits co-opted to serve social functions. (A, Left) Firing rates aligned to social image onset for OFC neurons in a social choice task. (Right) Percentage of OFC neurons with activity significantly modulated by social image category (black bar), fluid amount (gray bar), or their interaction (white bar) for three monkeys (M1–M3). Reproduced from Watson and Platt (2012) with permission from Elsevier. (B, Left) Firing rates of example neurons from each area, aligned to reward delivery. Box color signifies the category to which these neurons belong in the bar graphs. (Right) Proportion of significant neurons from OFC, ACCs, and ACCg using self, other, and shared frames of reference to encode reward outcomes during a reward-allocation task. Horizontal lines indicate significant differences (P < 0.05, χ2 test). Reproduced from Chang et al. (2013) with permission. [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
to images of other monkeys (Fig. 7.2A). On the basis of its connections to gustatory, olfactory, interoceptive, and limbic systems, OFC has been proposed to function as a feeding circuit (Carmichael and Price, 1995a,b, 1996). Thus, the observation that more neurons responded to social information than to fluid reward supports the idea that ancestral neural adaptations are repurposed to serve social functions. These findings, along with the observed relationship of OFC size to social network size in humans (Lewis et al., 2011) and group size across primates (Dunbar, 1995), suggest
that OFC is part of a specialized neural circuit that evolved concomitantly with increasing sophistication of social behavior.
Highly specialized neural mechanisms may be required to support complex social interactions that depend on the behavior and intentions of other individuals. This process may require the brain to encode sensory, motor (Rizzolatti and Fabbri-Destro, 2008), and even reward information in multiple frames of reference (Chang et al., 2011). We recently investigated how neurons in three frontal cortical areas—anterior cingulate gyrus (ACCg), anterior cingulate sulcus (ACCs), and OFC—encoded reward information while monkeys decided to deliver juice to themselves, to a recipient monkey, or to no one (Chang et al., 2013). In this social reward-allocation task, monkeys tend to prefer to reward someone over no one, and this prosocial preference is magnified by familiarity and dominance status (Chang et al., 2011) and significantly modulated by neuropeptide OT (Chang et al., 2012). We found remarkable specializations in the way neurons in these three areas encoded reward information in this social task. OFC neurons predominantly signaled rewards directly received by the donor monkey, revealing its egocentric encoding scheme; ACCs neurons predominantly signaled rewards foregone by the donor monkey, a process critical for monitoring outcomes and learning; and ACCg neurons signaled rewards delivered to the recipient or mirrored rewards delivered to either the donor or the recipient, indicating specialized functions for other-regarding social behaviors (Chang et al., 2013) (Fig. 7.2B). These findings resonate with previous work showing that lesions in ACCg, but not ACCs or OFC, lead to deficits in understanding the meaning of social cues in monkeys (Rudebeck et al., 2006) and the activation of medial prefrontal and gyral portions of ACC in humans by observing events occurring to others or thinking about others’ states of mind (Singer et al., 2004; Saxe, 2006; Mobbs et al., 2009; Waytz et al., 2012). Together, these observations suggest that ACCg is a key structure supporting shared experience and social reward and may be specialized in human and nonhuman primates to support complex social interactions.
NEUROMODULATORY INFLUENCES ON SOCIAL BEHAVIOR
Differences between species or between individuals within a species may reflect neuromodulatory influences on the development and function of neural circuits mediating social and nonsocial behaviors. Hormones strongly influence brain development (Keverne, 2004; McEwen, 2007) and shape the expression of fundamental behaviors like feeding, fleeing, fighting, and mating (Adkins-Regan, 2005). Neuropeptides (peptides used by neurons to communicate with one another) set the tone for state-specific neuronal signaling by altering chemical transmission within individual
neurons as well as across networks of neurons (Adkins-Regan, 2005). For example, OT cells in the paraventricular and supraoptic nuclei synchronize their activity to achieve coordinated neurosecretory bursts required for milk ejection during lactation (Belin and Moos, 1986).
Neuropeptides involved in these primary functions are often recruited to mediate social behavior. The nonapeptides OT and arginine vasopressin (AVP) nicely illustrate this principle. Both OT and AVP are involved in basic reproductive functions in mammals, including parturition and lactation in females and erection and ejaculation in males (Donaldson and Young, 2008). Building on pioneering work demonstrating a role for OT in maternal behavior in rats (Pedersen et al., 1982), a series of elegant studies in voles has revealed that OT and AVP also regulate social behaviors like pair bonding (Cho et al., 1999) and selective aggression (Winslow et al., 1993). More recently, it has been shown that exogenous application of OT can promote emotions like trust (Kosfeld et al., 2005) and encourage generosity (Zak et al., 2007), in a context-dependent and sometimes idiosyncratic fashion (Bartz et al., 2011).
Recently, we demonstrated that OT inhaled via a nebulizer effectively penetrates the central nervous system in rhesus macaques (Chang et al., 2012) (Fig. 7.3A), endorsing the potential promise of OT inhalation therapy in individuals with neuropsychiatric disorders marked by social deficits (Meyer-Lindenberg et al., 2011). Increasing OT levels in the brain via inhalation also promotes prosocial decisions in monkeys as well as their attention to a social partner (Fig. 7.3B and C). Surprisingly, OT also promotes selfish decisions in the same task when there is a perceived cost (Chang et al., 2012) (Fig. 7.3B). Furthermore, a recent study in chimpanzees has shown that a rise in OT levels following grooming depends on whether the two animals have strong bonds (Crockford et al., 2013), suggesting that the OT system has been further specialized to process partner-specific affiliative interactions. Together, these observations endorse the idea that neuropeptides like OT, which serves basic sexual and parenting functions, can be co-opted to regulate more complex social behaviors in species that live in large, complex groups, like humans and rhesus macaques.
Ultimately, neuropeptides like OT may impact even complex social behavior via a basic set of mechanisms. The anxiolytic effects (Neumann et al., 2000; Yoshida et al., 2009) of OT may have served as a preadaptation for the prolonged interaction necessary for high-intensity parental care in mammals by promoting approach behavior and enhancing tolerance (Young, 2002; Averbeck, 2010; Kemp and Guastella, 2010). These basal functions could then serve as building blocks for more complex social behaviors. Suppressing vigilance and increasing tolerance to non-offspring may permit extended interactions with others. Ultimately, complex emotions like trust may arise via reduced social apprehension and
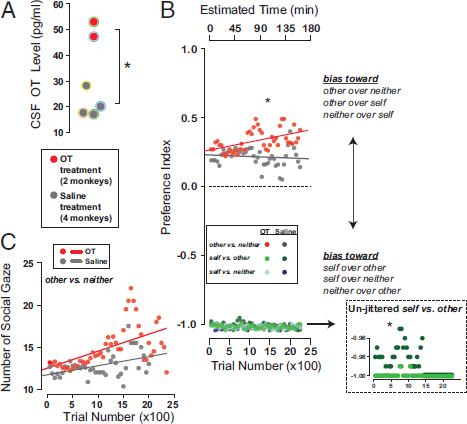
FIGURE 7.3 Social functions of neuropeptide OT. (A) OT concentration in cerebrospinal fluid (CSF) after inhaling OT (red) or saline (dark gray; *P < 0.05, Welch two-sample t test). Colored outlines on data points indicate animal IDs. (B) Choice preference index for OT (red) and saline (gray) for rewards delivered to: other (recipient) vs. neither, self (actor) vs. other, and self vs. neither in the social reward-allocation task. Data points from self vs. other and self vs. neither are jittered for visibility. Inset shows unjittered data from self vs. other trials. (C) Number of gaze shifts to recipient after reward delivery over the course of each session for other vs. neither choice trials. Reproduced from Chang et al. (2012) with permission. [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
enhanced tolerance, under the regulatory influence of neuropeptides like OT (Heinrichs et al., 2009).
Other neuromodulatory systems also contribute to variation in social behavior. For example, the hypothalamic–pituitary–adrenal (HPA) axis has long been associated with social status in primates (Abbott et al., 2003; Tung et al., 2012) and may play a critical role in the production of behavior. Yellow-bellied marmots were shown to be more likely to emit alarm calls during periods in which their HPA axis activity (measured by fecal cortisol concentrations) was high compared with periods during which it was low (Blumstein et al., 2006). The hypothalamic–pituitary–gonadal
(HPG) axis also shapes social behavior in vertebrates. According to the “challenge hypothesis,” males’ androgen levels are modulated according to context-dependent requirements for aggressive behavior (Wingfield et al., 1990), and this prediction has been substantiated broadly among vertebrates (Hirschenhauser and Oliveira, 2006). In rhesus macaques, modulations of testosterone levels in response to social challenge are also dependent on social rank (Higham et al., 2013). Male social status in African cichlid fish is regulated by gonadotropin-releasing hormone 1, a hormone critical for reproduction, at various levels of neuronal processing (Burmeister et al., 2005; Fernald, 2012). These findings resonate with the idea that preexisting signaling pathways, in this case pathways that regulate stress and mating behaviors, are repurposed to shape the development and function of neural circuits mediating social behavior. Through duplication, repurposing, and dynamic regulation of elements, a relatively limited toolkit of basic hormonal mechanisms can be used to generate a wide array of social behavior.
GENETIC REGULATION OF SOCIAL BEHAVIOR
The influence of genes on social behavior is undeniable because genes shape the neural circuits that produce behavior (Plomin, 2001). The adoption of preexisting biological mechanisms for social purposes, and indeed the evolution of social behavior in general, must, therefore, have roots in genetic change, or, in more Darwinian terms, must be based on modification through descent of inherited material. One hint that social behavior influences change in gene pools over time is a handful of studies linking sociality with fitness. In species such as baboons and rhesus macaques, engaging in social interactions is correlated with reproductive output; the offspring of individuals that spend a greater amount of time grooming and associating with others are more likely to survive to 1 year of age (Silk et al., 2003; Brent et al., 2013). This correlation, in female baboons at least, seems to be driven by the quality of social relationships as individuals with the strongest, most enduring social bonds have higher offspring survival (Silk et al., 2009) and greater longevity (Silk et al., 2010) than others. These findings suggest that there are adaptive benefits to interacting with others and that social behavior is shaped by natural selection.
However, such findings beg confirmation that social tendencies actually have a genetic basis and ask that we explore the roles of environment and experience in shaping the impact of genes on behavior. Quantitative genetic analysis is a tool that allows researchers to determine the amount of variance in a trait that can be attributed to genes, otherwise known as the amount of additive genetic variance or heritability. Using this technique, dimensions of human personality, including sociability, have been
shown to be heritable (Johnson et al., 2008). Similar findings show that the behavioral tendencies of a number of vertebrate species, including some nonhuman primates (Weiss et al., 2000; Williamson et al., 2003; Fairbanks et al., 2004), are heritable, thus pointing to a (partly) genetic basis for primate social behavior. Not only are social components of personality heritable, but so too is the extent to which individuals are integrated into their social networks in both humans (Fowler et al., 2009) and rhesus macaques (Brent et al., 2013). This integration includes social network connections mediated by multiagent relationships, such as friend-of-a-friend relationships. Such indirect social connections might be emergent properties of a social network or reflect meaningful aspects of the way individuals navigate large groups. Nevertheless, humans exploit these connections, and our actions (consciously or not) are influenced by them via reputation, one of the primary mechanisms believed to underlie the evolution of cooperation in humans (Nowak, 2006b).
Genetic information also shapes the specific proximate mechanisms that underlie the processing of social information and expression of social behavior. An excellent example is the serotonin pathway. Serotonin is involved in a host of peripheral functions, including cardiac and gastrointestinal functions (Hayes and Greenshaw, 2011). Centrally, serotonin regulates mood, memory, and reward (Sirviö et al., 1994; Hayes and Greenshaw, 2011). The serotonin pathway is also involved in the expression of social behavior. Genetic studies have tied this neuromodulatory pathway to social behavior in humans and other primates, with variants of two serotonergic genes having been examined in particular depth: a variable insertion in the gene encoding tryptophan hydroxylase (TPH2), the rate-limiting enzyme in serotonin synthesis, and the 5HTTLPR (serotonin-transporter-linked polymorphic region) polymorphism within the promoter region of the serotonin transporter gene (SLC6A4, solute carrier family 6 member 4). Both variants have orthologs in humans and rhesus macaques, have been associated with altered development of several brain regions (Hariri and Holmes, 2006; Jedema et al., 2010), and may influence the intensity and duration of signaling at serotonergic synapses (Hariri and Holmes, 2006; Chen et al., 2006).
Both TPH2 and SLC6A4 have been associated with social behavior phenotypes and endophenotypes, many of which are likely to have strong ties to serotonin’s central functions, such as the regulation of reward. For example, both genes have been implicated in neuropsychiatric diseases, such as autism and depression (Caspi et al., 2003; Coon et al., 2005), which are partly characterized by disruptions in social attention and interaction, and are accompanied by differences in brain response to social stimuli (Canli et al., 2008). The 5-HTTLPR polymorphism predicts social avoidance in rhesus macaques in response to familiar dominant face images
across many contexts. Specifically, rhesus macaques with a copy of the “short” allele spend less time looking at the eyes of other monkeys, show greater pupil dilation—a peripheral index of arousal—when viewing dominant faces, shy away from risk after being primed with dominant faces, and typically avoid dominant faces during a reward-guided decision-making task (Watson et al., 2009) (Fig. 7.4A).
Across the genus Macaca, the 5-HTTLPR polymorphism has been related to social structure, with more despotic species, such as the rhesus macaque, possessing both long and short numbers of repeats, whereas purportedly less despotic macaque species are monomorphic for the long allele (Wendland et al., 2006). This finding may suggest that this polymor-
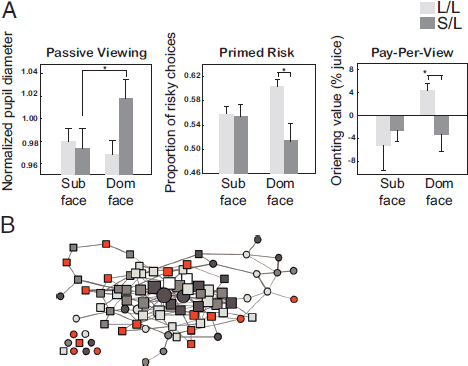
FIGURE 7.4 Genetic variations in the serotonergic system predict social behavior. (A) Monkeys with a “short” copy of the 5-HTTLPR polymorphism (S/L) show increased pupil dilation to a dominant face (Left), suppressed risk following a dominant face flash (Center), and do not forego juice to view a dominant face (Right). (B) Serotonergic gene profiles predict social network position in free-ranging rhesus macaques. Squares, females; circles, males; lines, presence of a grooming interaction between monkeys. Increasing line thickness indicates frequency of interaction. Node size and position reflect social centrality; largest nodes are the most socially central. Monkeys most central in the network were less likely to carry the minor allele for both the 5-HTTLPR or TPH2-length polymorphisms (gray nodes). A was reproduced from Watson et al. (2009), and B was reproduced from Brent et al. (2013) with permission. [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
phism confers resilience to psychosocio challenges (Suomi, 2006) but may also point to the interplay between serotonin, social behavior, reward, and risk aversion. Heightened social vigilance may confer particular advantages in the competitive situations that occur more frequently in despotic societies (Homberg and Lesch, 2011). The 5-HTTLPR polymorphism is associated with differential activation of a number of brain regions associated with affiliative behavior (e.g., ACC, insular cortex) (Canli and Lesch, 2007), leading to speculation that serotonergic gene profiles play a role not only in competition, but also in positive social interactions (Canli and Lesch, 2007).
We recently found preliminary evidence supporting this hypothesis in a study of rhesus macaques living in a free-ranging colony on Cayo Santiago Island, Puerto Rico. An individual monkey’s position in the social (grooming) network was predicted by the interaction between the 5-HTTLPR and TPH2-length polymorphisms. Either mutation alone had no effect on network position, but monkeys with the rare allele of both genes were less well integrated socially (Brent et al., 2013) (Fig. 7.4B). Overall, these results suggest that genetic factors that influence the development and functioning of the serotonin system shape primate social behavior. Serotonin-related genes therefore may be viewed as a valuable example of “candidate genes” that provide tractability to empirical questions about the interaction of genes, neural circuits, and social behavior. These tantalizing findings require further study to understand the specific genetic contributions of this system and other neuromodulatory systems to various aspects of social behavior and cognition.
It is fitting to end a survey of the neuroethology of social behavior on a genetic note, as in doing so we return to the very roots of evolutionary change. Genetic information not only represents a powerful tool to investigate the proximate bases of social behavior, but also allows us to establish direct links between sociality and evolutionary fitness, the ultimate driving force behind natural selection. Genetic information exposes the dynamic contingencies upon which sociality is based, where the interactions between genes that lay the foundations of neural architecture and the social, physical, and biochemical environments in which those genes exist are brought to light, and wherein lie some of the greatest challenges facing future researchers hoping to understand this complex and enigmatic trait.
CONCLUDING REMARKS
Social information is clearly valuable—it is worth foraging, often receives privileged attention over other types of information, and is inherently rewarding. The social environment is rife with information and tinged with uncertainty, and as a result much of our mental machinery is
applied to reducing the cognitive load of social interaction. Social behaviors impact evolutionary fitness (Silk et al., 2003; Brent et al., 2013), suggesting they are critical for survival and reproduction. Biological mechanisms that primarily functioned to mediate nonsocial behaviors in the ancestral state have been repurposed in some species, like humans and rhesus macaques, to mediate social behavior. Biological mechanisms are rededicated and further modified for social functions at multiple levels of organization, from neurons and circuits, to hormones and genes. It is important to note, however, that social behavior also feeds back upon these mechanisms to shape their structure and function. Manipulations of social network size in rhesus macaques alter cortical thickness and functional coupling across brain areas that support social functions (Sallet et al., 2011). Epigenetics and gene regulation are also essential to guiding changes in neural development and social behavior (Curley et al., 2011; Fernald, 2012; Tung et al., 2012). Epigenetic changes that are related to reinforcement and learning might be particularly powerful and are important directions for future research.
A neuroethological approach to the study of human and nonhuman primate social behavior is powerful in the extent to which it is encompassing and holistic. By presenting the evolution of social behavior through a lens of nonsocial functions, we have provided evolutionarily parsimonious lines of reasoning and evidence, along with tractable avenues for future research. For many human psychopathologies, the interactions between social and nonsocial deficits are poorly understood. Greater comprehension of the general-purpose mechanisms that generate social action and translate social signals may therefore improve disease diagnosis and treatment.
ACKNOWLEDGMENTS
We thank Monica L. Carlson for general technical assistance. This work was collectively supported by National Institute of Mental Health Grants K99-MH099093 (to S.W.C.C.), R01-MH095894 (to M.L.P. and S.W.C.C.), R01-MH086712 (to M.L.P., K.K.W., and G.K.A.), R01-MH096875 and R01-MH089484 (to M.L.P. and L.J.N.B.), and F31-MH081443 (to J.T.K.); Department of Defense CDMRP Grant W81XWH-11-1-0584 (to M.L.P. and S.W.C.C.); National Eye Institute Grant R01-EY019303 (to J.M.P.); Cure Autism Now (K.K.W.); The Davis Foundation (K.K.W.); a Duke Institute for Brain Sciences Incubator Award (to M.L.P.); and a Duke Center for Interdisciplinary Decision Sciences Fellowship (to L.J.N.B.).




















