
20 Methanol Toxicity
|
Environmental ALERT …
|
This monograph is one in a series of self-instructional publications designed to increase the primary care provider’s knowledge of hazardous substances in the environment and to aid in the evaluation of potentially exposed patients. See page 21 for more information about continuing medical education credits and continuing education units.
|
Guest Contributor: |
Dennis Shusterman, MD, MPH |
|
Guest Editor: |
John D.Osterloh, MD |
|
Peer Reviewers: |
John Ambre, MD, PhD; Charles Becker, MD; Jonathan Borak, MD; Joseph Cannella, MD; Howard Kipen, MD, MPH; Richard J.Jackson, MD, MPH; Jonathan Rodnick, MD; Brian A.Wummer, MD |

U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES
Public Health Service
Agency for Toxic Substances and Disease Registry
Case Study
A 67-year-old man with headache, nausea, and visual disturbance
During an afternoon visit, you see a 67-year-old man for onset of headache, nausea, and visual disturbance. The friend who accompanies him explains that both of them frequent the same senior center and that they have been preparing for a fund-raising event during the past 2 days. During this time, the patient spent between 6 and 9 hours per day reproducing fliers using a “spirit duplicator” (mimeograph machine). This activity took place in a small, unventilated room with the patient working alone most of the time.
On questioning, the patient says that he had eye irritation and lightheadedness after the first few hours of activity but considered these symptoms to be a minor annoyance. He also had nausea by the end of the first day but noted that this cleared overnight. During the second day of activity, he was again troubled by eye irritation, this time accompanied by vertigo, tinnitus, visual blurring, and photophobia. He tried to ventilate the room by placing a small fan near the door but continued to feel poorly despite a prolonged break. Late in the afternoon his friend insisted that he seek medical attention.
The patient is a widower and retired insurance salesman with a smoking history of one pack per day from age 27 to 62 (none for the last 5 years). He typically consumes a six-pack of beer per day, but he has felt poorly and has been abstinent for the past 10 days. Medical history includes coronary artery bypass surgery at age 63 with subsequent medical management of stable angina and a transurethral prostatectomy at age 65 with no recurrence of obstructive symptoms. Current medications include nitroglycerine patches used before exercise (with no patches used in the previous 4 days) and sublingual nitroglycerine, which he takes rarely. The review of symptoms is negative for other cardiopulmonary complaints. There is no family history of glaucoma, myopia, or diabetes mellitus.
On examination, the patient is alert and oriented to time, space, and person, although he appears somewhat distracted. His breath has a faint solvent-like smell. Vital signs are within normal range with the exception of a respiratory rate of 30/minute. The cardiopulmonary examination is unremarkable, but abdominal examination reveals mild tenderness in the epigastrium without rebound or guarding. Muscle tone, strength, sensation (pinprick, light touch, position sense) and reflexes are symmetrically intact. His gait is unsteady with a wide-based stance, and he shows a positive Romberg sign, heel-to-shin, and rapid alternating movements (bilaterally).
Ophthalmologic examination reveals a visual acuity of 20/200 bilaterally despite newly prescribed corrective lenses. The conjunctivae appear somewhat injected, nystagmus is present on lateral gaze, and the pupils are large and poorly reactive to light. Examination also reveals hyperemia of the optic nerve head with no hemorrhages or exudates.
![]()
(a) What is the differential diagnosis for this patient?
_________________________________________________________________
(b) What additional information would you request regarding the patient’s activities in the last 2 days?
_________________________________________________________________
(c) What consultation(s) would you obtain to help you manage this case?
_________________________________________________________________
(d) What type of therapeutic intervention is indicated?
_________________________________________________________________
Answers can be found on page 17.
Exposure Pathways
❑ Methanol is used in a variety of commercial and consumer products.
❑ Increased use of methanol as a motor fuel may cause higher ambient air levels and a greater potential for ingestion from siphoning accidents.
Methanol (methyl alcohol) is a clear, colorless, flammable liquid with a faintly pleasant odor. Popularly known as wood alcohol, methanol has historically been referred to as wood spirit, wood naphtha, pyroligneous spirit, and carbinol. Despite these references to its derivation as a wood distillation product, methanol is currently produced almost exclusively by synthetic pathways. It ranks 22nd (by volume) among chemicals produced in the United States.
The largest quantities of methanol are used for the manufacture of other chemicals including methyl methacrylate, acetic acid, ethylene glycol, and methyl chloride. Methanol also is added to a variety of commercial and consumer products such as windshield washing and deicing solutions (35% to 95% concentration), duplicating fluids (95% concentration or greater), solid canned fuels (4% concentration), paint removers, model airplane fuels, and embalming fluids. Other methanol uses are as a denaturant for ethanol; as a solvent for shellacs, lacquers, adhesives, and inks; and, most recently, as an alternative motor fuel. Because methanol is a natural fermentation product, its concentration may be up to 300 milligrams per liter (mg/L) in wines and higher in brandies and other distilled fruit spirits. Although serious methanol toxicity has been most commonly associated with ingestions, exposures also occur via inhalation and skin absorption, which are a concern in both occupational and household settings.
Environmentally, methanol has been detected in concentrations ranging from less than 10 parts per billion (ppb) in rural air to nearly 30 ppb in urban air. If methanol-powered vehicles become more prevalent, ambient methanol levels could be thousands of times greater in residential and public parking garages. Currently, there is no enforceable atmospheric standard for methanol. Increased use of methanol as a motor fuel would probably result in the reduction of some air pollutants (e.g., particulates and ozone) but an increase in others (e.g., formaldehyde). Data regarding methanol levels in drinking water are lacking.
|
(1) In emergency situations, what reference sources could you use to aid in identifying the chemical constituents of consumer and commercial products such as the duplicating fluid used in the case study? _________________________________________________________________ _________________________________________________________________ |
Who’s at Risk
❑ Persons having prolonged skin contact with methanol are at risk of developing severe systemic effects.
❑ Persons ingesting adulterated alcoholic beverages are at great risk of methanol toxicity.
❑ Folate-deficient persons are potentially at increased risk for toxicity after methanol exposure.
According to estimates from the National Institute for Occupational Safety and Health (NIOSH), more than 2.5 million persons are regularly exposed to methanol on the job. Workers most likely to experience inhalation or skin exposures to methanol include bookbinders, bronzers, dyers, foundry workers, gilders, hatmakers, ink makers, laboratory technicians, painters, photoengravers, and chemical manufacturers. In addition, administrative aides or others using mimeograph machines may be exposed to methanol, as well as workers at refineries, fuel distribution centers, and service stations, if they handle methanol-containing fuels.
Householders, hobbyists, and motorists using methanol-containing products can be at risk for inhalation exposure; therefore, precautions must be taken to avoid using these products in poorly ventilated spaces. In addition, prolonged skin contact with methanol can produce systemic effects—a painter developed blindness after working in methanol-soaked clothes, and an 8-month-old child with a methanol-soaked pad placed on his chest developed signs of methanol toxicity.
Historically, the largest number of serious methanol exposures have occurred by ingestion. Methanol poisoning has been caused by materials such as shellac thinner, duplicator fluid, and denatured alcohol that have been drunk directly or have been used to adulterate beverages. In addition, about 35,000 gasoline ingestions are reported annually in the United States, most of which occur from fuel siphoning. Siphoning accidents could significantly increase the number of methanol ingestions if the use of methanol-containing automotive fuels becomes widespread.
One step in the metabolic detoxification of methanol is a folic acid-dependent process. Consequently, susceptibility to methanol toxicity may be higher among folate-deficient persons. Folate deficiency can occur not only in persons consuming inadequate diets, but also in those with intestinal malabsorption (e.g., inflammatory bowel disease) or hemolytic anemia, or in persons undergoing drug therapy (e.g., anticonvulsants, antibiotics). Because alcoholics have a greater likelihood of both methanol ingestion and folate deficiency, they may be at dual risk for methanol’s adverse effects. Up to 10% of the population may be folate-deficient.
|
Additional information for the case study: During the investigation, you request a listing of the contents on the label of the duplicating fluid used by the patient. You learn that it contains greater than 90% methanol. You also learn that the patient drank a small amount of the duplicating fluid (about 5 milliliters [mL]) on his second day of working at the senior center. (2) Discuss the factor(s) that may place this patient at increased risk of methanol toxicity. _________________________________________________________________ _________________________________________________________________ |
Biologic Fate
❑ Methanol is absorbed well by all exposure routes.
❑ Methanol is oxidized in the liver to formaldehyde, then formic acid, which contributes to the profound metabolic acidosis seen in acute methanol poisoning.
❑ Most methanol is eliminated via the lungs as carbon dioxide.
Gastrointestinal absorption of methanol is virtually complete, whereas lung retention averages 58%. Dermal absorption may occur if skin is abraded or methanol exposure is prolonged. There is evidence that methanol absorption through the skin is enhanced in gasoline-methanol mixtures. Once absorbed, methanol is distributed with total body water.
Metabolism of methanol is a three-step process taking place chiefly in the liver. The first metabolic step involves methanol’s oxidation to formaldehyde by alcohol dehydrogenase, which is a saturable, rate-limiting process (Figure 1, Step 1). In the next step (Figure 1, Step 2), formaldehyde is oxidized by aldehyde dehydrogenase to formic acid (or formate, depending upon pH). Since step 2 is rapid, little formaldehyde accumulates in the serum. Formic acid, a metabolite of formaldehyde, contributes to the development of metabolic acidosis both directly (i.e., via its acid load) and indirectly (i.e., through its inhibitory effects upon iron-containing cytochromes with subsequent accumulation of lactic acid [lactate]). In Step 3, formic acid is detoxified to carbon dioxide and water.
Some absorbed methanol is eliminated unchanged via the lungs (10% to 20%) and kidneys (about 3%). However, most absorbed methanol is oxidatively metabolized (75% to 85%). A small amount of the metabolic products is excreted in the urine as formate, but most is exhaled as carbon dioxide. Methanol elimination patterns are dose-dependent, with elimination half-lives ranging from 3 hours in volunteers who ingested small amounts of methanol to 30 hours in persons who overdosed.
Figure 1. Methanol metabolism to toxic intermediates—formaldehyde and formic acid (formate).
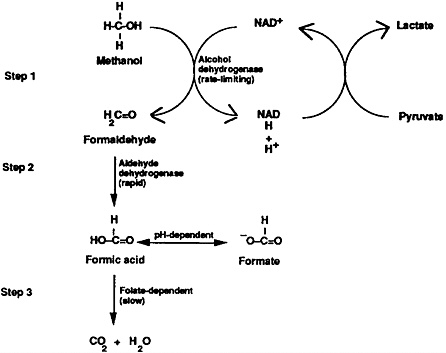
Discovery of methanol’s metabolic pathway has led to several practical treatments; among them are the therapeutic administration of ethanol and folic acid. Alcohol dehydrogenase, the enzyme responsible for the first step of methanol metabolism, has an approximately ninefold greater affinity for ethanol than for methanol. Administration of ethanol blocks the oxidation of methanol, preventing the lethal synthesis of formaldehyde and formic acid and increasing the amount of methanol that is eliminated unchanged (now approximately equal amounts in urine and exhaled breath). Administration of folic acid and its analogues, which affect Step 3, enhances the conversion of toxic formic acid to carbon dioxide and water (Figure 1).
Physiologic Effects
Acute Exposure
❑ The acute effects of inhaling methanol vapor, which are similar to those caused by many other organic solvents, include upper respiratory tract irritation and inebriation.
Methanol shares with many other hydrocarbon solvents the ability to produce reversible sensory irritation, headache, nausea, and narcosis at airborne levels below those producing specific organ system pathology. Headaches were a frequent complaint in one study of office workers in the vicinity of duplicating machines where airborne methanol levels were in the range of 200 to 375 parts per million (ppm). In another study, exposed administrative aides were
❑ The metabolic products of methanol can produce a syndrome of delayed-onset acidosis, obtundation, visual disturbance, and death.
❑ Partial or total blindness, dementia, or a Parkinson-like syndrome are potential sequelae in survivors of acute methanol intoxication.
significantly more likely to report blurred vision, headache, dizziness, and nausea than were controls. Workers reported that the symptoms improved when they were away from the workplace.
Most methanol-related metabolic and ophthalmologic alterations have been associated with exposure through ingestion. Although the most frequently cited dosage for a lethal methanol ingestion is 1 milliliter per kilogram (mL/kg) of body weight, permanent blindness and deaths have been reported with ingestions as low as 0.1 mL/kg (6 to 10 mL in adults).
Metabolic Effects
After a characteristic latent period of 6 to 30 hours, severe metabolic acidosis may occur in victims of methanol poisoning. The acidosis is due to formic acid, and less often, lactic acid. Formic acid is metabolically produced from methanol, while lactic acid results from hypotension and from formate’s interference with cellular respiration.
Ophthalmologic and Neurologic Effects
Experimental evidence suggests that formate is responsible for optic nerve damage in methanol overexposure. In fatal cases, the optic nerve shows central necrosis in the distal (orbital) portion with the central optic tracts intact. In nonfatal cases, visual function can normalize completely after treatment, although central and peripheral scotomata or complete blindness may persist, depending on several variables. Occasional neurologic sequelae of methanol poisoning can include polyneuropathy, a Parkinson-like extrapyramidal syndrome, and mild dementia. Hemorrhages in the putamen have been documented on computerized tomography (CT) scanning and on pathologic examination.
Chronic Exposure
Respiratory and Ophthalmologic Effects
❑ Chronic exposures to methanol have not been thoroughly studied, although anecdotal reports of chronic visual effects have been published in the medical literature.
Despite methanol’s widespread use, there are few rigorous studies of workers chronically exposed to methanol. Some reports can be found of permanent visual effects due to chronic inhalation or dermal exposure, but many of these reports date to the early part of the century and lack exposure data. Inhalation studies in experimental animals do not demonstrate significant pathology with chronic exposures at levels up to 50 times the current occupational Permissible Exposure Limit (PEL) of 200 ppm. The only consistent effects in rats and monkeys exposed for 4 weeks to levels up to 5000 ppm methanol vapor were mucoid nasal discharge and upper respiratory tract irritation; no ophthalmologic alterations were found. Because formic acid is rapidly metabolized and does not accumulate in
experimental animals, they may not be good models for the ophthalmologic effects of methanol.
Other Effects
❑ Data regarding the potential developmental effects of methanol exposure are inconclusive.
❑ Data regarding the carcinogenic potential of methanol in humans are lacking.
Published data on animal and human developmental effects of methanol are limited and inconclusive. One case-control study of pregnant women in the workplace shows a possible association of fetal central nervous system defects with exposures to a mixed solvent that included methanol. The general medical literature contains no references to methanol’s carcinogenic potential in humans.
|
(3) Do the symptoms and appearance of the patient in the case study suggest acute methanol intoxication? Explain. _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ |
Clinical Evaluation
History and Physical Examination
❑ In cases of suspected methanol intoxication, the goal is to determine exposure route and neurologic and ocular status.
History-taking in methanol intoxications should focus on exposure route. In suspected ingestions, the clinician should ask about the consumption of illicit alcoholic beverages (or beverages that may have been adulterated) and about other potential accidental or intentional ingestion scenarios. In suspected inhalational and dermal exposures, emphasis should be placed on identifying specific methanol-containing products (e.g., canned fuel, windshield washer solution, duplicator fluid, shellac thinner, alternative fuels) and on documenting unusual conditions of prolonged and extensive skin contact or inhalation. The symptom history should emphasize disturbances in visual, neurologic, and gastrointestinal function. The physical examination should focus particularly on neurologic status and ocular findings.
Signs and Symptoms
Acute Exposure
❑ Timely evaluation of a patient who may be over-exposed to methanol is essential to prevent severe and permanent sequelae.
Persons acutely exposed to high levels of methanol via ingestion, inhalation, or extensive skin contact may develop severe metabolic, ocular, and neurologic toxicity. The initial intoxicating effects of methanol are similar to those of ethanol in producing cognitive slowing and cloudy sensorium, which extends to impaired brain stem function at very high doses. After a latent period of 12 to 24 hours, methanol toxicity may result in progressive visual disturbance and impairment of consciousness due to the gradual build-up of toxic metabolites. Unusual ocular symptoms, such as a sensation of “being in a snowstorm,” may be reported. Ophthalmologic examination may reveal central or peripheral visual field defects and dilated pupils that react poorly to light but accommodate normally. Erythema of the optic nerve may occur, with peripapillar edema early in the course. Rarely, flame-shaped hemorrhages may be seen. Necrosis of the distal portion of the optic nerve leads to atrophy, which may be evidenced by optic nerve pallor days or weeks after exposure.
Chronic Exposure
❑ Symptoms of chronic, low-level methanol exposure are generally reversible.
Persons intermittently or chronically exposed to airborne methanol at levels insufficient to cause systemic acidosis may complain of eye irritation and visual blurring, upper respiratory irritation, headache, nausea, and lightheadedness—all of which are reversible under these conditions. Chronic short-term cutaneous exposures may result in skin irritation and defatting. Chronic ingestion of methanol at levels documented in commercially distilled beverages or in drinking water have not been linked with specific symptoms or pathology.
Laboratory Tests
In light of methanol’s profound metabolic effects, numerous standard laboratory tests are useful in documenting acute toxicity. These include the following:
Blood methanol and blood ethanol
Arterial blood gases
Serum electrolytes (Na+, K+, Cl−, HCO3−) and calculation of anion gap
BUN and serum creatinine
Serum glucose
Serum ketones
Serum osmolarity and calculation of osmolar gap
Urinalysis
CBC
Direct Biologic Indicators
❑ Immediately after an acute exposure, a blood methanol level serves as the best predictor of the severity of the clinical course.
❑ Chronic methanol exposure can be documented by measuring urinary methanol.
Although methanol can be detected in both urine and exhaled breath, blood methanol levels are more widely available and serve as the best predictor of toxicity immediately after acute exposure. The normal blood concentration of methanol from endogenous sources is less than 0.05 milligrams per deciliter (mg/dL). Generally, central nervous system effects appear above blood methanol levels of 20 mg/dL; ocular symptoms appear above 100 mg/dL; and fatalities in untreated patients have occurred in the range of 150 to 200 mg/dL.
After the latency period, blood methanol level alone is not a reliable prognostic indicator because toxicity results from the metabolites. A methanol level below 20 mg/dL in a symptomatic patient, for example, does not rule out serious intoxication since the methanol may already have been completely metabolized to formate. When considerable time has elapsed after ingestion, mortality correlates best with severity of acidosis rather than with blood methanol levels.
In the workplace, where intermittent or chronic exposures are likely to occur, the American Conference of Governmental Industrial Hygienists (ACGIH) recommends a urinary methanol level of less than 15 mg/L at the end of an 8-hour workshift.
Indirect Biologic Indicators
❑ Formate levels are useful as indicators of methanol exposure, although they are not widely available.
❑ Both the anion and osmolar gaps are increased in methanol poisoning.
Of methanol’s metabolites, only formate is present in biologic fluids at concentrations useful for monitoring exposures. When serum formate levels exceed 20 mg/dL, ocular injury and metabolic acidosis are likely. In acute intoxications, elevated serum formate concentrations can confirm the diagnosis and aid in clinical decisionmaking regarding the institution of hemodialysis. However, laboratory tests for serum formate levels are not widely available.
The ACGIH considers a urinary formic acid level of less than 80 milligrams per gram (mg/g) creatinine, obtained preshift at the end of a workweek, as indicative of exposures below the 8-hour time-weighted average (TWA) of 200 ppm.
The anion gap and osmolar gap aid in the diagnosis of acute methanol poisoning. The serum anion gap (AG) may be defined by the formula
AG=(Na++K+)−(Cl−+HCO3−)
with all ions measured in milliequivalents per liter (mEq/L). The normal anion gap is 12 to 16.
An approximation of the serum osmolar gap (OG) is most commonly defined as
OG=Osmolarity (measured)−(2 Na++[BUN+2.8]+[Glucose+18])
with measured osmolarity expressed in milliosmoles per liter (mOsm/L), Na+ in mEq/L, and BUN and glucose in mg/dL. The normal osmolar gap is 0 to 10.
The conditions that can produce an elevated anion-gap acidosis are summarized by the mnemonic MUDPILES:
|
M |
Methanol intoxication |
|
U |
Uremia |
|
D |
Diabetic ketoacidosis |
|
P |
Propylene glycol poisoning |
|
I |
Iron and isoniazid overdoses; inhalants (carbon monoxide, cyanide, hydrogen sulfide) |
|
L |
Lactic acidosis |
|
E |
Ethanol (alcoholic) ketoacidosis, ethylene glycol poisoning |
|
S |
Salicylate overdose |
Of the various pathophysiologic states and toxic agents listed above, only diabetic ketoacidosis, ethanol ketoacidosis, and methanol and ethylene glycol poisoning produce elevations of both the anion and osmolar gaps. Identification of diabetic ketoacidosis is based on the findings of elevated serum glucose and ketones, particularly in a person with pre-existing diabetes mellitus. Ethanol ketoacidosis is characterized by a history of chronic, excessive ethanol intake with anorexia and vomiting and acidosis out of proportion to the apparent degree of ketonemia.
Differentiation of methanol and ethylene glycol poisoning is based on the exposure history and on specific toxicologic testing. In ethylene glycol poisoning, there is an absence of eye complaints; oxalate crystals are found in the urine; and hypocalcemia may be present.
Findings that may accompany secondary complications of methanol poisoning include myoglobinuric renal failure (with elevations in serum creatinine and CPK, a positive test for occult blood in the urine, and rare or absent red blood cells in the urine sediment), pancreatic or salivary gland pathology (with hyperamylasemia), and central nervous system pathology (as evidenced by diffuse cerebral edema or hemorrhages of the putamen on CT scanning). Mean corpuscular volume (MCV) is elevated in severe methanol poisoning, probably resulting from a primary increase in red blood cell size from poisoning rather than megaloblastic anemia.
|
(4) The patient in the case study has a serum sodium of 140, potassium 4.0, chloride 102, and bicarbonate 10 (all measured in mEq/L). The glucose level is 90 mg/dL, BUN 14 mg/dL, and measured osmolarity 320 mOsm/L. What is the calculated anion gap? Osmolar gap? Are these gaps consistent with methanol poisoning? _________________________________________________________________ _________________________________________________________________ (5) What is the differential diagnosis for a wide anion-gap acidosis? _________________________________________________________________ (6) What conditions can produce an elevated serum osmolar gap? _________________________________________________________________ (7) What neuro-ophthalmologic findings might be anticipated in the patient? _________________________________________________________________ |
Treatment and Management
Acute Exposure
❑ With methanol poisoning, substantial treatment delays may occur because the clinician is falsely reassured by the initial lack of severe symptoms.
❑ Intravenous sodium bicarbonate therapy should be considered if the blood pH is below 7.2.
❑ Symptoms and history determine whether intravenous ethanol therapy and hemodialysis should be instituted.
Acute methanol Intoxication constitutes a medical emergency. Effective therapy requires attention to both clinical and laboratory data, as well as anticipation of events that may be latent at the time of initial examination. Methanol intoxication, like that of ethylene glycol, acetaminophen, and lithium, may deceive the clinician by the initial lack of severe toxic manifestations.
For recent, suspected methanol ingestions, gut decontamination should be carried out even in the absence of clinical or laboratory abnormalities. Emesis should be induced if the patient is conscious and if a substantial ingestion has occurred within 30 to 45 minutes of first medical care; alternatively, gastric lavage may be performed, particularly if the patient is obtunded. There is no evidence that activated charcoal or cathartics significantly reduce methanol absorption.
Formate’s diffusion across cell membranes, particularly in the optic nerve, is facilitated by a low systemic pH; hence, therapy should include partial correction of acidosis via direct alkalinization. Intravenous sodium bicarbonate therapy, which is aimed at reversing acidosis (by titrating the blood pH) to avert circulatory collapse and
impede the intracellular penetration of formic acid, should be considered if the pH is below 7.2. A reduction of blood pH of 0.15 corresponds to a base deficit of 10 mEq/L bicarbonate. The target should be a pH in the range of 7.36 to 7.40. Sodium bicarbonate solution should be administered slowly to allow the resulting carbon dioxide to dissipate via hyperventilation. Sodium overload is a constant hazard of sodium bicarbonate therapy, and electrolytes must be monitored frequently.
In cases of suspected methanol exposure, the following are indications for starting an intravenous ethanol infusion: a blood methanol level of greater than 20 mg/dL; a history of ingesting more methanol than 0.4 mL/kg body weight; any ingestion history, with delayed access to toxicologic testing; or metabolic acidosis with otherwise unexplained elevated anion and osmolar gaps, especially if eye symptoms are present. Ethanol, usually as a 10% solution (10 mL of 100% ethanol in 90 mL of 5% aqueous dextrose), is first administered intravenously in a loading dose of approximately 7.5 to 10 mL/kg over 20 to 60 minutes. If the patient is conscious, oral loading doses can be given since intravenous doses may be painful.
The subsequent ethanol infusion rate varies with the patient’s ethanol metabolism and should be adjusted to keep the blood ethanol level between 100 and 150 mg/dL. Typically, rates between 0.8 to 1.4 mL/kg/hr suffice. In chronic alcoholics and during hemodialysis (see paragraph below), higher rates may be required. Infusions are continued until the methanol level drops below 20 mg/dL.
Criteria for combined ethanol infusion and hemodialysis include visual disturbance, or a methanol level exceeding 50 mg/dL, or a severe acidosis unresponsive to intravenous bicarbonate. Peritoneal dialysis is less effective than hemodialysis in clearing methanol from the blood. During hemodialysis, ethanol infusions should not only continue, but also should be increased slightly to compensate for the increased ethanol clearance. Even during dialysis, the target blood ethanol concentration should remain at 100 to 150 mg/dL.
An adjunctive treatment for methanol poisoning is the administration of folate (in the form of folic acid or folinic acid) to increase the conversion of formate to carbon dioxide and water. Folate administration is considered safe and may be efficacious if the patient is folate-deficient. Suggested dosage regimens include folic acid, 50 to 70 mg intravenously every 4 hours for the first 24 hours of treatment, or folinic acid (also known as leucovorin, Citrovorum factor, or 5-formyl-5,6,7,8-tetrahydrofolate), 1 to 2 mg/kg intravenously every 4 to 6 hours. Any attempt to replenish folic acid stores by administering multiple vitamins is likely to be frustrated by their low folate content (typically 1 mg per tablet).
An experimental drug, 4-methylpyrazole, is being investigated in animals and humans. This orally administered drug, which combines with the enzyme alcohol dehydrogenase, may replace ethanol as a
safe means to block methanol metabolism while patients are prepared for hemodialysis. Administration of 4-methylpyrazole does not appear to add to the patient’s CNS depression as does ethanol. In addition, the metabolism of 4-methylpyrazole is more predictable and prolonged than is that of ethanol, making administration less difficult technically. Investigation of 4-methylpyrazole is currently in Phase I in the United States.
Chronic Exposure
❑ Patients chronically exposed to methanol should be treated symptomatically.
Because a clearly defined clinical syndrome does not exist for chronic methanol exposure, treatment should be symptomatic. Patient management should include removal from exposure, supportive counseling, and a consideration of alternative diagnoses.
|
Additional information for the case study: Consistent with acute methanol intoxication, the patient’s arterial blood gases indicate a pH of 7.25; bicarbonate is 10 mEq/L, pCO223 mm Hg, and pO292 mm Hg. The blood methanol level is 83 mg/dL. (8) What type of therapeutic intervention is indicated? _________________________________________________________________ (9) You are a rural practitioner, and the nearest hospital is 90 minutes away by ambulance. Although you stock standard intravenous rehydration solutions in your clinic pharmacy and have a “crash cart” with standard resuscitative drugs, there is no intravenous ethanol or parenteral folate available. There is, however, a liquor store nearby. If vodka is used as a substitute for ethanol, explain how you would prepare the ethanol dosing solution. _________________________________________________________________ _________________________________________________________________ |
Standards and Regulations
Workplace
Air
❑ Currently, EPA does not regulate the amount of methanol in public drinking water supplies.
❑ EPA has not promulgated an air emission standard for methanol.
❑ OSHA regulations for worker exposures to methanol include a requirement that skin contact be minimized.
Methanol is volatile at room temperature and has an odor threshold at approximately 100–250 ppm concentration in air. The Occupational Safety and Health Administration (OSHA) maintains a workplace limit for airborne exposures to methanol of 200 ppm (as an 8-hour TWA) and 250 ppm for short-term (15-minute) excursions not to exceed four such excursions in an 8-hour day (Table 1). Identical standards are recommended by ACGIH and NIOSH. The odor of methanol may not be perceived by some persons until levels exceed acceptable workplace limits. Concentrations exceeding 25,000 ppm are considered “immediately dangerous to life or health” (i.e., they may result in irreversible health effects or impair the ability of an individual to escape from the exposure environment). In general, airborne exposures can be controlled through engineering measures or by appropriate personal protective equipment or both.
Significant dermal absorption of methanol can occur. Workers using methanol should be protected against dermal exposures by engineering controls (e.g., by isolating the work process) and by using personal protective equipment (impervious gloves, aprons, boots, and other appropriate equipment).
Environment
Air
EPA does not have an emission standard for methanol. However, under EPA’s generic standards for the synthetic organic chemical manufacturing industry, all volatile organic chemical (VOC) emissions, including methanol releases, are to be kept to a technologically feasible minimum.
Drinking Water
Neither EPA nor the states maintain standards for methanol in drinking water.
Table 1. Standards and regulations for methanol
|
Agency* |
Focus |
Level |
Comments |
|
OSHA |
Air-workplace |
200 ppm |
|
|
|
250 ppm |
Regulation: PEL (STEL¶) |
|
|
NIOSH |
Air-workplace |
200 ppm |
Advisory: REL** (8-hr TWA) |
|
|
250 ppm |
Advisory: REL (STEL) |
|
|
ACGIH |
Air-workplace |
200 ppm |
Advisory: TLV†† (8-hr TWA) |
|
|
250 ppm |
Advisory: TLV (STEL) |
|
|
EPA |
Air-environment |
N/A |
Covered under the “best available technology” clause for VOC emissions from new or modified facilities. |
|
|
Water-drinking |
N/A |
|
|
FDA |
Food |
N/A |
Approved only as an “indirect food additive” (i.e., in food packaging adhesives). |
|
*ACGIH=American Conference of Governmental Industrial Hygienists; EPA=Environmental Protection Agency; FDA=Food and Drug Administration; NIOSH=National Institute for Occupational Safety and Health; OSHA=Occupational Safety and Health Administration †PEL (Permissible Exposure Limit)=highest level of methanol in air to which a worker may be exposed during a normal workshift. §TWA (Time-Weighted Average)=time-weighted average concentration for a normal 8-hour workday and 40-hour workweek to which nearly all workers may be repeatedly exposed. ¶STEL (Short-Term Exposure Limit)=usually a 15-minute sampling period. In the case of methanol, not to exceed four 5-minute excursions in an 8-hour workday, with at least 1 hour between excursions. **REL (Recommended Exposure Limit)=highest recommended level of methanol in air to which a worker may be exposed during a normal workshift. ††TLV (Threshold Limit Value)=exposure guideline recommended by ACGIH. |
|||
Suggested Reading List
Environmental Sources and Routes of Exposure
Bindler F, Voges E, Laugel P. The problem of methanol concentration admissible in distilled fruit spirits. Food Addit Contam 1988;5:343–51.
Frederick LJ, Schulte PA, Apol A. Investigation and control of occupational hazards associated with the use of spirit duplicators. Am Ind Hyg Assoc J 1984;45:51–5.
Kahn A, Blum D. Methyl alcohol poisoning in an 8-month-old boy: an unusual route of intoxication. Pediatrics 1979;94:841–3.
Diagnosis and Treatment
Becker CE. Methanol poisoning. J Emerg Med 1983;1:51–8.
Ekins BR, Rollins DE, Duffy DP, Gregory MC. Standardized treatment of severe methanol poisoning with ethanol and hemodialysis. West J Med 1985;142:337–40.
Enger E. Acidosis, gaps and poisonings [Editorial]. Acta Med Scand 1982;212:1–3.
Gonda A, Gault H, Churchill D, Hollomby D. Hemodialysis for methanol intoxication. Am J Med 1978;64:749–58.
Jacobsen D, Bredesen JE, Eide I, Ostborg J. Anion and osmolal gaps in the diagnosis of methanol and ethylene glycol poisoning. Acta Med Scand 1982;212:17–20.
Jacobsen D, McMartin KE. Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol 1986;1:309–34.
Osterloh JD, Pond SM, Grady S, Becker CE. Serum formate concentrations in methanol intoxication as a criterion for hemodialysis. Ann Intern Med 1986;104:200–3.
Suit PF, Estes ML. Methanol intoxication: clinical features and differential diagnosis. Cleve Clinic J Med 1990;57:464–71.
Complications of Intoxication
Aquilonius SM, Askmark H, Enoksson P, Lundberg PO, Mostrom U. Computerised tomography in severe methanol intoxication. Br Med J 1978;2:929–30.
Eckfeldt JH, Kershaw MJ. Hyperamylasemia following methyl alcohol intoxication. Source and significance. Arch Intern Med 1986;146:193–4.
Grufferman S, Morris D, Alvarez J. Methanol poisoning complicated by myoglobinuric renal failure. Am J Emerg Med 1985;3:24–6.
McLean DR, Jacobs H, Mielke BW. Methanol poisoning: a clinical and pathological study. Ann Neurol 1980;8:161–7.
Ley CO, Gali FG. Parkinsonian syndrome after methanol intoxication. Eur Neurol 1983;22:405–9.
Phang PT, Passerini L, Mielke BH, Berendt R, King EG. Brain hemorrhage associated with methanol poisoning. Crit Care Med 1988;16:137–40.
Rastogi SP, Gold RM, Arruda JA. Fanconi’s syndrome associated with carburetor fluid intoxication. Am J Clin Pathol 1984;82:124–5.
Related Government Document
National Institute for Occupational Safety and Health. Criteria for a recommended standard: occupational exposure to methyl alcohol. Washington, DC: US Department of Health, Education and Welfare, 1976; Publication no. (NIOSH) 76–148. NTIS no. P8–273 806/0.
Sources of Information
More information on the adverse effects of methanol and treating and managing cases of exposure to methanol can be obtained from ATSDR, your state and local health departments, and university medical centers. Case Studies in Environmental Medicine: Methanol Toxicity is one of a series. For other publications in this series, please use the order form on the back cover. For clinical inquiries, contact ATSDR, Division of Health Education, Office of the Director, at (404) 639–6204.
Answers to Pretest Questions and Challenge Questions
Pretest questions begin on page 1. Challenge questions begin on page 2.
Answers to Pretest
-
Acute visual loss in this age group can occur with central retinal artery or vein occlusion, internal carotid emboli, vitreous hemorrhage, retinal/macular hemorrhage, retinal detachment, temporal arteritis, cerebrovascular accidents of the posterior circulation, acute angle-closure glaucoma, idiopathic optic neuritis, head trauma, and carbon monoxide and methanol poisoning. Of these conditions, only cerebrovascular events, head trauma, temporal arteritis, and carbon monoxide and methanol poisoning commonly affect vision bilaterally. Other symptoms such as hyperpnea, and later, Kussmaul breathing, are indicative of acidosis (see page 10 for a differential diagnosis of acidosis). The patient also manifests signs of inebriation. Methanol poisoning could account for all of these effects.
-
The following information should be sought in any occupational or avocational history: (1) a full description of the activity in question, with identification of all chemical products used (including ethanol) either by chemical or trade name; (2) documentation of potential routes of exposure including inhalation, skin contact, and ingestion; and (3) type of ventilation employed and use of personal protective equipment.
-
Confirmation of suspected methanol poisoning should take place in an emergency department or inpatient setting with rapid laboratory tests and the opportunity for prompt therapeutic intervention. Physicians with special expertise who might be consulted in this case include clinical toxicologists, nephrologists, and ophthalmologists. In geographic areas with restricted access to specialists or with exposures of questionable toxicologic significance, informational assistance can be obtained from the nearest regional poison control center.
-
The clinical findings and the acknowledged methanol exposure (see Challenge question 2, page 4) are indicative of methanol intoxication of potentially life-threatening severity. Appropriate therapeutic interventions for methanol intoxication include intravenous ethanol infusion, sodium bicarbonate and folate administration, and hemodialysis.
In addition, the patient should receive psychosocial care for his substance abuse tendencies. Referral to a substance abuse program is appropriate.
Answers to Challenge Questions
-
In emergency situations, the most complete and up-to-date information is usually available through a regional poison control center. Common reference books that contain information on the chemical composition of consumer and commercial products include Gosselin’s Clinical Toxicology of Commercial Products and Sax’s Dangerous Properties of Industrial Materials, among others. Many local hospital emergency departments have access to toxicologic reference information either on microfiche or CD-ROM. Some hospital libraries also have access to online clinical toxicology databases.
-
The patient used a methanol-containing product for an extended period in a small, unventilated room. Thus, the inhaled dose alone is significant. The patient also admits to ingesting a small amount of the product. Furthermore, as an alcohol abuser, the patient may be folate-deficient, thus increasing his risk of methanol toxicity.
-
Yes, the symptoms and appearance of the patient in the case study do suggest acute methanol intoxication. The patient manifests symptoms of inebriation and complains of visual disturbance several hours after the start of a significant methanol exposure. He also is showing signs of acidosis. These factors constitute a classic presentation for acute methanol intoxication.
-
The calculated anion gap is 32 (normal: 12 to 16) and the calculated osmolar gap is 30 (normal: less than 10). These values are consistent with acute methanol intoxication. For calculations, see below.
Anion Gap=(Na++K+)−(Cl−+HCO3−)
=(140+4)−(102+10)
=144−112
=32
Osmolar Gap=Osmolarity (measured)−(2Na++[BUN÷2.8]+[Glucose÷18])
=320−(2×140+[14÷2.8]+[90÷18])
=320−(280+5+5)
=320−290
=30
-
The conditions that can produce an elevated anion-gap acidosis are summarized by the mnemonic MUDPILES:
|
M |
Methanol intoxication |
|
U |
Uremia |
|
D |
Diabetic ketoacidosis |
|
P |
Propylene glycol poisoning |
|
I |
Iron and isoniazid overdoses; inhalants (carbon monoxide, cyanide, hydrogen sulfide) |
|
L |
Lactic acidosis |
|
E |
Ethanol (alcoholic) ketoacidosis, ethylene glycol poisoning |
|
S |
Salicylate overdose |
-
Intoxication by the following agents (or accumulation, in the case of acetone in diabetic or alcoholic ketoacidosis) produces an elevated osmolar gap: methanol, ethanol, ethylene glycol, acetone, and isopropanol.
Although several drugs can potentially contribute to the osmolar gap (e.g., salicylates, paraldehyde, and chloral hydrate), they are rarely present at concentrations sufficient to raise osmolarity.
-
A neuro-ophthalmologic examination of the patient might reveal several findings. Results of examination of visual fields, as determined by perimetry, typically indicate central scotomata early in the course of methanol poisoning (soon after onset of acidosis), with peripheral constriction of visual fields a late finding. Dilated, unreactive pupils and dim vision are characteristic. The result can be bilateral blindness, which is usually permanent.
-
See the answer to Pretest question (d) above.
-
One-hundred proof vodka is actually 50% ethanol by volume. A loading dose equivalent to the required 7.5 mL/kg of 10% ethanol can be achieved with vodka as follows: In a 70 kg person, a total of 525 mL of 10% ethanol would be needed (7.5 mL/kg×70 kg=525 mL).
525 mL×10%=X mL×50%
52.5 mL=0.5X mL
X=105 mL of the 50% ethanol
In summary, 105 mL of 50% ethanol with 5% dextrose in water added to total 525 mL will produce a 10% ethanol solution. (See Treatment and Management, page 11.) This quantity of vodka or an equivalent amount of ethanol from another distilled spirit can be initially administered orally or by gavage.




















