The Interaction of Biology and Environment
Among the most compelling stories emerging in the early 21st century from the science of child development have been the extraordinary developmental processes by which a microscopic assembly of embryonic neural cells gives rise to the human brain—the most complex physical object in the known universe (Fox et al., 2010; IOM and NRC, 2009; National Scientific Council on the Developing Child, 2004). These remarkable developmental events contribute to the entire, elaborate array of individual life attributes and trajectories, from personality, intelligence, and individual achievement to lifelong risks for disease, disorder, and criminality. The course of brain development also shapes a child’s growing capacities (or incapacities) for learning; complex thought; and supportive, empathic involvement with others—capacities that powerfully influence life chances for success, productivity, and satisfaction. The profusion of possible futures and life paths grounded in the character, course, and timing of early brain development is guided and sustained by continuous, bidirectional interactions between human biology and social and educational environments. Such interactions, strongly influenced by the quality of the care and teaching and the learning environments that families and societies provide, co-determine over time the developmental, educational, biological, and health outcomes that progressively characterize individual lives. Although much complexity—at the behavioral, neurobiological, cellular, and molecular levels—awaits further elucidation, much has already been learned about the nature, timing, and consequences of neurodevelopmental events and how they interact with the environments in which children develop, learn, and engage with adults and peers. New knowledge of these
developmental processes has yielded a rich and useful harvest of insights for those who care for, teach, protect, and support young children.
THE DEVELOPING BRAIN, THE DEVELOPING SCIENCE
The publication of From Neurons to Neighborhoods: The Science of Early Childhood Development (NRC and IOM, 2000) proved a pivotal moment in the integration and dissemination of the insights gained from new knowledge of developmental processes. That report assembles and compellingly presents the evidence that early child development is critically dependent upon relationships with caring and teaching adults, that individual biology and social experiences are equally influential in determining developmental outcomes, and that infants are born able and ready to learn. The report also made clear that growing children’s social experiences of adversity and stress—experiences disproportionately prevalent in impoverished communities of low socioeconomic status—have direct effects on the structure and function of the developing brain. Emotion and the social experiences of early life are deeply and enduringly represented within behavioral development and are “biologically embedded” in the anatomic structure and function of the growing brain (Hertzman, 2012).
In the decade and a half since From Neurons to Neighborhoods was published, enormous strides have been taken toward a neurobiological accounting of how young brains develop and how both perturbations of experience and support can fundamentally alter the trajectories of normative and maladaptive development. Four broad categories of insight have emerged in developmental neuroscience with specific implications for learning, care, and behavior in early childhood.
First, the past decade of research has converged on an understanding that in many or perhaps even most instances, causality with respect to disease, disorders, and maladaptive development—as well as the preservation of health and maintenance of normative, adaptive development—is best viewed as an interplay between genome-based biology and environmental exposures. This understanding represents a clear departure from the historical views that human morbidities are attributable to either pathogenic environments or faulty genes. Thus whereas it was once viewed as sufficient to ascertain, through genetically informed (e.g., twin) studies, the proportion of variation in an individual’s observable characteristics, or “phenotype,” attributable to genes and to environments, it is now generally accepted that the key to a deeper, richer understanding of pathogenesis and adaptive development is elucidation of how genes and environments work together.
Second, the role of developmental time in the dramatic unfolding of brain structure and function and the acquisition of concomitant human capacities has become increasingly important in explaining early development.
Critical and sensitive periods—time windows in which experience-related developmental transitions must or can most readily occur—create a temporal mapping of anticipated early childhood exposures that guide the timing and sequencing of developmental change. As the molecular substrates for such critical periods and events have become known and tractable, altering their timing and manipulating the opening and closing of specific developmental windows have become increasingly plausible (Greenough and Black, 1992; Greenough et al., 1987; NRC and IOM, 2000).
Third, there is now strong evidence that early psychosocial adversities—beginning even during fetal development—can have important short- and long-term effects on the brain’s development, the regulation of stress-responsive hormone systems, and the calibration of stress reactivity at a variety of levels from behavioral to gene expression responses. Stress triggers activation of the hypothalamic–pituitary–adrenocortical (HPA) axis, which results in cortisol secretion from the adrenal gland, as well as activation of the autonomic nervous system (ANS), which ignites the so-called fight or flight sequence of physiologic changes, including increases in blood pressure and heart rate, sweating, and pupil dilation. Together, these two systems exert important effects on the cardiovascular and immune systems that anticipate and prepare the individual for stress or challenge, including affecting the regulation of glucose levels and altering the activation levels for a variety of genes.
Fourth, inquiry into the sources of special vulnerability and resilience with respect to early adversity has led to the discovery of substantial individual differences in children’s susceptibilities to both negative and positive environmental exposures. This discovery has reinforced the unique character of each child’s responses to the physical and social worlds, has offered perspectives on why some children thrive within environments of great adversity, and has illuminated seemingly contradictory findings about how social conditions affect health and development. It also has informed a better understanding of children’s differential responsiveness to interventions (Belsky and van Ijzendoorn, 2015). These individual differences in context sensitivity, like health and developmental outcomes, are likely due to the joint, interactive effects of nature and nurture.
Taken together, these four broad categories of insights have reshaped understanding of the formative experiences of early life—in families, communities, health care settings, childcare centers, and schools—and are changing societal approaches to crafting, managing, and monitoring those experiences. Following a synopsis of the emerging neuroscience of childhood learning and development, each of these new groups of insights is considered in turn, along with its implications for those who raise, teach, and care for young children. Given the foundational and rapid processes of brain development during foundational periods of early development, this
is a window of both great risk of vulnerability to developmental disruption and great potential for receptivity to positive developmental influences and interventions.
THE NEUROSCIENCE OF LEARNING AND DEVELOPMENT
The fundamental insights derived from developmental and educational psychology about child development from birth through age 8 are enhanced by an increasingly elegant neuroscience defining the cerebral, neural circuit, cellular, and molecular processes that attend early learning, cognition, and socioemotional development. Even greater insight into these processes will inevitably result from innovative approaches to imaging the growing brain and from federal investments in collaborative scientific projects such as the National Institutes of Health’s BRAIN Initiative.1 This section summarizes some of the major recent advances in brain science of relevance to this report. For a more comprehensive review, the interested reader is referred to previous National Research Council reports (see IOM and NRC, 2009; NRC and IOM, 2000), as well as overviews appearing in the developmental neuroscience literature (e.g., Bloom et al., 2001; Boyce and Kobor, 2015; Fox et al., 2010).
Early Brain Development
The early central nervous system appears in embryologic development at 2 to 3 weeks postconception. Over the remaining weeks of gestation, primitive cells differentiate into specialized cells and brain regions with distinctive forms and functions. Precursor neural cells differentiate into neurons and glia cells; the former appear at 5 to 25 weeks of gestation and play key roles in the execution of brain functions, while the latter appear later in prenatal development and have key structural and functional supportive functions in the brain and nervous system.
New neurons must migrate to new locations within the developing brain to serve specific roles within particular functional regions, such as the motor cortex, which coordinates bodily movement, or the auditory cortex, which serves hearing. In moving from their site of origin to their precise correct position in the brain, neurons are guided along structural “maps” created by molecular signals from neighboring cells. Failures of neuronal migration have now been implicated in the genesis of neurological and psychiatric disorders, such as some seizure disorders and intellectual deficits.
As neurons move toward their final brain positions, they grow long, tubular extensions called axons along which an electrical signal can be
_____________
1 See http://www.nih.gov/science/brain/index.htm (accessed March 24, 2015).
propagated to another neuron. They also develop branched projections from the neuronal cell body called dendrites, which are capable of receiving such signals from other neurons (see Figure 3-1). The point of physical communication between neurons is the synapse, a microscopic cleft across which a chemical signal—a neurotransmitter—is released, resulting in the activation of the downstream neuron. Many of the psychotropic medications currently used for disorders such as depression and anxiety act upon the molecular mechanisms involved in synaptic communication.
The rate of formation of both new neurons and new synapses during prenatal brain development is staggering. As shown in Figure 3-2, during a period of neuronal proliferation between 5 and 25 weeks of gestation, new neurons are generated from neural stem cells at a rate as high as 250,000 per minute. In a slightly later but overlapping period, synapses are produced at a rate of 40,000 per second. Both periods are followed by a systematic pruning of both neurons and synapses, the former through a phase of programmed cell death called apoptosis, and the latter through attrition of the least utilized synaptic connections. Both the striking overproduction of neurons and synapses and the subsequent, rapid elimination of those that are underutilized must occur in sequence and to the proper
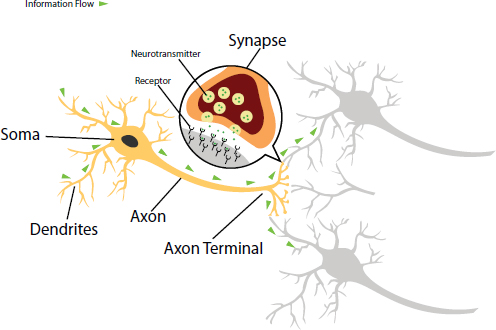
FIGURE 3-1 The structure of neurons and neuronal connections.
SOURCE: Kellett, 2015.
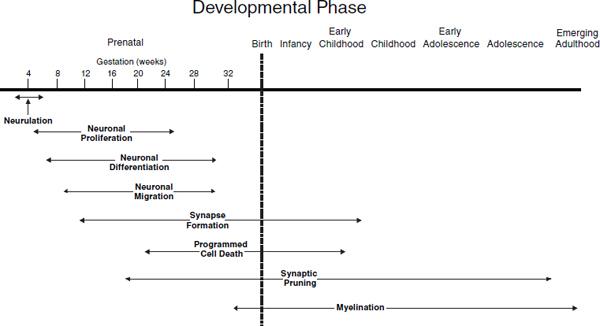
FIGURE 3-2 Developmental phases of neural development.
SOURCE: IOM and NRC, 2009, p. 122.
degree for normal intellectual and socioemotional development to occur. Schizophrenia has been linked, for example, to abnormal synaptic pruning during the adolescent years of development.
Myelination—the progressive “insulation” of the neuronal axons with a myelin sheath produced by specialized neural cells—increases the speed and efficiency of neuronal activation. Myelination occurs at different developmental rates in different areas of the brain; the prefrontal cortex, responsible for the slowly acquired “executive” functions of reasoning, decision making, and attentional skills, becomes fully myelinated as late as early adulthood. It is the white myelin sheath, with its cholesterol and lipoprotein components, that is responsible for the increasing, maturational presence of “white matter” in the developing brain.
The early development of the brain also progresses at the level of cortical and subcortical organization and signaling circuits that are integrated into networks with similar functions. The cortical structures and signaling circuitry of the brain underlie neural systems for complex cognitive and socioemotional functions such as learning and memory, self-regulatory control, and social relatedness (IOM and NRC, 2009). During this development, specialization occurs with different anatomical regions of the brain involved in different functions, including both those that are explicit and conscious, which have been the focus of much developmental science research to understand cognitive development, as well as those that are implicit and automatic or unconscious, which are increasingly being studied for their foundational importance for socioemotional development (Schore, 2010).
A related feature of brain development is lateralization, in which specialized functions are predominant in one hemisphere, or side, of the brain. For example, growing evidence points to the specialized dominance of the right side of the brain in processing social and emotional information, including nonverbal information, which are the foundation of important functions such as interpreting social stimuli, understanding the emotions and intentions of others, and engaging in social interactions, including the important development of attachment in very young children (De Pisapia et al., 2014; Decety and Lamm, 2007; Hecht, 2014; Schore, 2014; Semrud-Clikeman et al., 2011).
Although structural specialization does develop in the brain, it is also becoming increasingly well understood that brain functioning is more complex than discretely assigned anatomical areas. Language, for example, has been tied to the left hemisphere, in what are known as Broca’s and Wernicke’s areas. However, there is emerging recognition that aspects of communicating through language, which requires nonverbal information and interpretation of meaning and inference, are also linked to right hemisphere functions (Ross and Monnot, 2008). Similarly, cortical functions
are also interconnected with subcortical systems that underlie arousal systems and autonomic function. Developmental neuroscience has also been increasingly focused on the importance of the maturation of these brain systems prenatally and early in life, which like the cortical regions, undergo a rapid growth in the first year of life (Knickmeyer et al., 2008).
The brain has capacity for change in anatomy and function as a result of experience and stimulation, a function known as neural plasticity. Such plasticity takes place at multiple levels of organization and scale, ranging from synaptic changes in neurotransmitter production and release to regional increases in the size of a specific cortical region following the acquisition of new skills. For example, the cortical area controlling the fingers of the left hand expands in students of the violin at a level commensurate with their years of study and increasing virtuosity. Learning and mastery thus are physically represented, at both the micro and macro levels, in the changes in brain structure and function resulting from neural plasticity.
As a consequence of the exquisite precision of the timing, spatial resolution, and sequencing of brain development, enriching experiences in the early years will support healthy brain development, while conversely, a variety of disturbances or deficiencies prenatally or in early childhood can interrupt or perturb the growing brain, resulting in functional changes that range from subtle incapacities to generalized developmental disabilities. Prenatally, such disturbances can include, for example, deficiencies in folate in the maternal diet, which can result in severely disordered formation of the brain and spine, and infection with such organisms as toxoplasmosis or cytomegalovirus, which can produce severe forms of psychopathology such as schizophrenia or autism. In early childhood, one perturbation that occurs with great prevalence in human populations is the developing brain’s exposure, directly or indirectly through the parents’ experiences, to substantial psychosocial adversity and stress, such as abuse or neglect, the death of a parent, or exposure to violence in the home or neighborhood. Because of its early sensitivity to such adversity, the developing brain can sustain profound effects on structure; function; and capacities for learning, cognition, and adaptive behavior. Although children across populations and socioeconomic levels can experience these kinds of stressors, exposure to many of them is unevenly distributed within populations, which can result in disproportionate risk for the marginalized and the poor.
GENE–ENVIRONMENT INTERPLAY AND DEVELOPMENT
As noted earlier, poor health and maladaptive development have historically been attributed to either experiential or heritable causes, depending on the prevailing scientific and cultural view. Proponents of environmental determinism, the predominant view in the 1960s and 1970s, claimed that,
with few heritable exceptions, aspects of context and environmental exposure were the principal forces shaping developmental outcomes. In other periods, such as that following the Human Genome Project in the 1980s and 1990s, proponents of genetic determinism alleged that all the major determinants of disease and developmental disorder were single-gene or polygenic variations. Based on more recent research, however, it is now understood that the interaction of genes and experiences guides development and that the key to a richer understanding of pathogenesis is an elucidation of how genes and environments work together to produce—or to protect from—illness and disorder, i.e., gene–environment interplay (Boyce et al., 2012; Rutter, 2010).
The Interplay of Genetic and Environmental Variation
Gene–environment interplay is a category of interactive processes comprising gene–environment correlation (rGE), gene–environment interaction (GxE), and epigenetic modification of the DNA packaging that regulates gene expression. The first of these, rGE, denotes the influence of genetic variation on environmental exposures, referring to how individuals may select, alter, and generate experiences that are in keeping with their own genetic proclivities. For example, a child who has a more inhibited temperament will be inclined toward less intensive social environments. The second, GxE, describes genetic or environmental effects that are conditional upon each other—for example, the effects of genetic variation that become apparent only in the presence of specific environmental conditions, or the effects of social contexts that are more or less potent depending on the underlying genotype of the individual who experiences them. Third, epigenetic processes that stem from environmental exposures modify chromatin—the structural packaging of the genome—through the chemical “tagging” of DNA or the histone proteins around which it is wound. These chromatin modifications or “marks” alter gene activity, control the production of the protein for which the gene codes, and thereby modify the observable, phenotypic characteristics of the child—all without affecting the DNA sequence itself (Boyce and Kobor, 2015).
The exploration of these domains of gene–environment interplay has become one of the most prolific, engaging, and controversial areas of biomedical and social science research. On the one hand, such research holds promise for illuminating how differences in individual susceptibility and environmental conditions operate together to initiate disorders of development, behavior, and health or to sustain health, resilience, and adaptive well-being. On the other hand, this arena of biomedical research also is marked by ongoing, sometimes divisive, controversies over methods and the interpretation of findings.
Initial reports of GxE interaction in developmental psychopathology (Caspi et al., 2002, 2003), now a decade past, revealed for the first time the potential and long-theorized capacity for DNA sequence variants to amplify or constrain the health and developmental risks of disadvantaged or abusive early environments. In the decade that followed, a large number of scientific papers reported GxE interaction in which DNA sequence variations (called single nucleotide polymorphisms, or SNPs) statistically moderated the influence of risky social contexts on the incidence of disordered development and psychopathology. Studies of both human children (see, e.g., Dunn et al., 2011; Molenaar et al., 2013) and animal species (see, e.g., Barr et al., 2004; Burns et al., 2012) continue to identify statistical interactions between variation in genotype and aspects of the rearing environment, although ongoing, legitimate concerns remain about the reliability of findings on GxE interaction (Manuck and McCaffery, 2014).
How GxE interaction exerts effects on developmental and behavioral outcomes has been explained in part by studies showing how variations in DNA sequence are linked to connectivity in specific brain regions (Thompson et al., 2010). Recent functional magnetic resonance imaging studies, for example, have demonstrated the heritability of task-related brain region activation and shown how a functional mutation in an important gene is associated with differences in the function of the prefrontal cortex, where executive skills reside (Egan et al., 2001). Such studies of GxE interaction have employed “candidate” SNPs in genes relevant to brain development and psychopathology or in genes sharing candidate biological pathways (e.g., pathways involved in inflammation). Other researchers have developed experimental animal models for testing the effects of GxE interaction on cognitive and behavioral outcomes (Koch and Britton, 2008; Turner and Burne, 2013) or focused on families of environment-responsive intracellular molecules, called “transcription factors,” that control the activation or expression of multiple genes (Slavich and Cole, 2013).
Epigenetics
Among the most compelling, emergent stories in developmental biology is the discovery of the molecular, epigenetic processes by which environmental conditions can regulate the activation or deactivation of genes. It is increasingly understood that development is driven not only by the joint, additive, or interactive effects of genetic and contextual variation but also by the direct regulation of gene expression by environmental events and experiences (see, e.g., Lam et al., 2012; Pezawas et al., 2005; Rutter, 2012). Research in epigenetics has shown that experiences can alter gene expression through their effects on molecular regulators that interact with the DNA molecule.
As described earlier, an epigenetic mark is a chemical change in DNA packaging, or chromatin, that affects gene transcription (i.e., the decoding of the gene) without changing the DNA sequence itself. Chromatin modifications that occur as a result of experiences and exposures in an individual’s social and physical environments constitute a molecular pathway by which context can influence gene expression and phenotype. This physical conformation of chromatin, which resembles “beads on a string” (see Figure 3-3), allows or disallows access to gene coding regions by RNA polymerase, the enzyme that decodes DNA sequences. Which chromatin conformation exists at a given time depends on epigenetic processes of chemical modification or “marking” that modifies either the DNA itself or the histone proteins around which the DNA is wrapped.
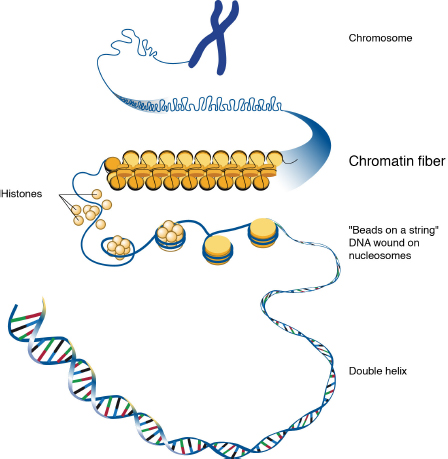
FIGURE 3-3 Chromatin structure.
SOURCE: Leja and NHGRI, 2010.
Early in development, the maturation of the embryo itself depends upon epigenetic programming that shapes cell differentiation and development (Strachan and Read, 2011). The early embryonic genome undergoes several phases of genome-wide epigenetic change that establish and maintain the distinctive, somatic cell lines that make up specific tissues. These early modifications create a kind of genetic tabula rasa for the epigenetic reprogramming of cellular diversity (Boyce and Kobor, 2015). Because the body’s approximately 200 different cell types contain the same genomic DNA sequence, epigenetic processes must control the tracking of primitive, undifferentiated cells into distinctive cell types through differential expression of each cell’s approximately 20,000 genes. Only by such divergent activation of genes could so many tissue types emerge from a single, common genome and ensure the stability of each cell type over generations of cell division. Differential gene expression also guides the differentiation of cellular functions, for example, the development of neurons into unique subsets, the guidance of axon growth, and the spatial organization of brain development (Fox et al., 2010).
At the same time, epigenetic processes also are called upon for adaptive, dynamic responses later in development, such as those a child makes to changing environmental conditions like exposures to severe adversity and stress. In research entailing the calibration of rat pups’ stress reactivity, for example, high levels of maternal care resulted in increased production of the glucocorticoid receptor in pups’ brains through an epigenetic change in chromatin structure (Weaver et al., 2004). This epigenetic modification of a regulatory region in the glucocorticoid receptor gene increases its expression, thereby blunting cortisol reactivity. Thus, a single set of molecular processes serve both stability and change—an “epigenetic paradox” of the same molecular mechanisms providing for contrasting cellular needs.
Paradoxical though they may be, the uses and functions of epigenetic processes play critically important roles in the successful emergence of social, educational, and biological capacities. A new body of research addressing the genomic and neurobiological bases for complex social cognitions, for example, has shown how inferences about others’ thoughts and emotions, the processing of facial information, and control of socially evoked emotion all require functional connectivity between a variety of brain structures, including the amygdala, hippocampus, and prefrontal cortex (Adolphs, 2009; Blakemore, 2010, 2012; Lesch, 2007; Norman et al., 2012; Robinson et al., 2005, 2008). There is evidence that perturbations in such brain circuits are related to genetic and epigenetic processes (Lesch, 2007; Norman et al., 2012). Environmental conditions produce patterns of cellular signals in the brain, and these neural signals remodel epigenetic marks, which modify the expression of genes controlling brain development. Because some of these epigenetic marks are chemically stable,
environmental influences during childhood can become “biologically embedded” within the genome of the growing child (Hertzman, 2012).
Further, the processes that influence postnatal development, learning, and health also can be mediated by epigenetic events controlling neuroregulatory genes. Social dominance and rearing conditions in nonhuman primates, for example, are associated with epigenetic variation in the immune system (Cole et al., 2012; Provencal et al., 2012; Tung et al., 2012). In human research, epigenetic changes in the glucocorticoid receptor gene in brain cells have been identified in suicide victims with a history of child abuse (McGowan et al., 2009; Sasaki et al., 2013), and longitudinal associations have been found between socioeconomic disadvantage and stress in early life and both genome-wide and gene-specific epigenetic changes in later life (Borghol et al., 2012; Essex et al., 2013; Lam et al., 2012).
Most recently, new research has revealed that epigenetic, molecular processes may sometimes underlie GxE interactions (Klengel et al., 2013; Mehta et al., 2013). Specifically, an epidemiologically observed interaction between childhood trauma and an SNP in a cortisol response-regulating gene predicts symptoms of posttraumatic stress disorder in adulthood. Laboratory investigation of this GxE interaction revealed that the effect is mediated through epigenetic changes in a cortisol response element in the gene. This observation shows how chromatin modification and epigenetic marks may be a molecular mechanism for GxE interactions.
Interplay of Genes and Environment: Implications for Adults
For adults who work with children, it is important to recognize that “nature” and “nurture” are not parallel tracks. Instead, the tracks are woven together and influence each other’s pathways in ways that may vary greatly depending on the individual child. The adaptations that occur as a result of these mutual interactions mean that the early experiences and early learning environments that adults provide can affect all domains of human development.
In sum, a new and promising body of research is producing evidence, in both animal and human studies, that many variations in human developmental and educational trajectories have early origins in early childhood (Shonkoff and Garner, 2012); are the products of gene–environment interplay (Rutter, 2006); and influence developing neural circuits and processes that are directly linked to long-term trajectories of health, disease, and life achievement (Fox et al., 2010). This research may signal a period of remarkable progress in understanding the extensive interplay among social
environments, genes, and epigenetic processes and how genetic and environmental variations converge in typical and atypical development (Boyce and Kobor, 2015).
The central role of time is a recurrent theme in developmental science. The effects of experience change dynamically across the life span, as critical and sensitive periods open and close, especially in the early years. During critical periods of development, important experiences or exposures result in irreversible changes in brain circuitry. During sensitive periods, the brain is especially responsive to such experiences (Fox et al., 2010). These are defined windows of early life when there is plasticity highly dependent on experience (Takesian and Hensch, 2013). In a classic example of this, when children lack patterned visual stimulation because of cataracts, strabismus, or other occlusions of vision during the early development of the brain’s visual circuitry (i.e., birth to 7-8 years of age), the result is deprivation amblyopia (dimness of sight). The developing brain is also especially vulnerable to the effects of physical and social environmental exposures during early developmental periods. For example, during critical periods of neurodevelopment children are more prone than adults to toxic chemical injury (Nelson et al., 2014; Zeanah et al., 2011). In a random-assignment trial of children in orphanages, neurobiological and developmental outcomes were dramatically improved for children whose foster care placements occurred prior to 2 years of age (Nelson et al., 2014; Zeanah et al., 2011).
The molecular mechanisms for such critical and sensitive periods are being studied in animal models involving experimental manipulations at the neuronal and molecular levels. Recent research has shown how plasticity in the brain over time is initiated and constrained by molecular “triggers” and “brakes” (Takesian and Hensch, 2013). Such findings have led to a fundamental shift from assuming that brain plasticity arises during sharply defined critical periods to a new understanding that the brain is instead intrinsically plastic and normal development requires a timed, molecular suppression of that plasticity. The onset and offset of critical periods are due to epigenetic molecular mechanisms (Fagiolini et al., 2009). These discoveries together reveal a complex time sensitivity within development that is initiated, guided, and curtailed by epigenetic, molecular events affecting the neuroregulatory genes that govern brain development (Boyce and Kobor, 2015).
BIOLOGICAL CONSEQUENCES OF PSYCHOSOCIAL ADVERSITIES IN EARLY LIFE
As discussed earlier, there is now strong evidence that psychosocial and other stressors in early life—beginning even in the prenatal period—can have important effects on development. This section focuses in depth on the biological consequences of these stressors. Chapter 4 places those biological consequences in the context of broader considerations and consequences having to do with chronic stress and adversity, focusing in particular on the stressors associated with economic adversity; social buffering of stress; and the relationships among stress, learning, and mental health. Importantly, children experience stress—and the biological dysregulation that can occur—not only as a result of the active stressors of chronic threat or danger but also because of the unavailability of nurturing, supportive care on which children rely, especially early in life. Both conditions appear to constitute significant stressors for young children.
Multiple biological systems are affected by chronic adversity because these systems are activated by stressful events and their persistence. As noted earlier, adverse early experiences can have significant consequences for a child’s brain development through the “biological embedding” of such experiences in the stress response systems of the child’s brain (Hertzman, 1999). In studies of children who have a depressed parent, live in poverty, witness persistent conflict, are abused or neglected, are in foster care, or experience other kinds of significant chronic stress, developmental researchers have documented important consequences for neurocognitive development (for reviews, see Blair and Raver, 2012; Hertzman and Boyce, 2010; Lupien et al., 2009; Thompson, 2014). The biological effects of chronic stress, especially when it occurs early in life, influence not only brain development but also immunologic functioning; autonomic reactivity; the development of stress reactivity and coping; and memory, learning, and thinking (McEwen, 2012; Ulrich-Lai and Herman, 2009).
The specific effects of early stressors depend critically upon the timing, intensity, and duration of the exposure. Chronic stressors are characterized by prolonged activation of the physiologic stress response systems and are particularly harmful when experienced in the absence of the protection afforded by stable, responsive relationships (Garner and Shonkoff, 2012). Evidence that early adverse experiences can have lasting effects on multiple biological systems helps explain the well-documented association between early adversity and later problems in physical and mental health in adulthood (Boyce et al., 2012; Danese and McEwen, 2012; Edwards et al., 2005). Chronic adversity has these effects because of the cumulative biological “wear and tear” that results from the prolonged activation and
overburdening of biological systems that are designed primarily for short-term activation (Geronimus et al., 2006; McEwen, 2012).
Effects on the Neuroendocrine Stress Response System
The HPA axis both contributes to coping with stress and is affected by chronic stress. The HPA axis is activated when the brain detects threatening events, leading to the production of cortisol, which mobilizes energy, enhances cardiovascular tone, alters immune functioning, and orients an individual to danger attentionally and cognitively. These biological responses have important psychological consequences that together provide immediate resources for coping with adversity, including heightened motivation for self-defense, threat vigilance, and motivational and emotional arousal. Over time, however, chronic stress and repeated exposures to adversity can alter the brain centers and neuroendocrine circuitry that underlie the regulation of stress responses and change the functioning of the HPA axis (Ulrich-Lai and Herman, 2009).
Considerable variability in stress reactivity is observed in children facing adversity (see, e.g., Essex et al., 2011). The HPA system is altered in two ways through experiences of adversity (Bruce et al., 2013; Hertzman and Boyce, 2010), and both reflect poor regulation of HPA responses as the result. One is when the HPA axis becomes hyperresponsive to perceived threats, so that cortisol levels rise quickly and are slow to decline, as the result of repeated shocks to the stress system. Children who are hyperresponsive may show heightened vigilance to threat, greater reactivity and poorer self-regulation when challenges ensue, and difficulties maintaining cognitive and attentional focus (Blair and Raver, 2012; Evans and Kim, 2013). This heightened reactivity has been observed in children who have been maltreated (Cicchetti and Rogosch, 2001), in infants and toddlers growing up in poverty (Blair et al., 2008, 2011), and in the young children of chronically depressed mothers (Essex et al., 2002).
Another way in which the HPA system can be dysregulated is when stress reactivity becomes blunted or underresponsive. In this case, cortisol levels are low, and the typical daily rhythm of cortisol secretion that regulates physiologic functioning is diminished or absent, as if the system is beginning to shut down. This pattern has been observed in young children living within deprived, institutional care (Carlson and Earls, 1997); neglected children placed in foster care (Dozier et al., 2006); and young children living in homes characterized by domestic violence and maternal emotional unavailability (Sturge-Apple et al., 2012). Thus, the effects of chronic, severe deprivation produce a dampening of the HPA axis, possibly due to changes in the hormonal feedback system by which cortisol production is controlled (Bruce et al., 2013; Nelson et al., 2014).
Chronic dysregulation of the HPA axis also alters the immune system, increasing vulnerability to infections, boosting levels of the cytokines by which immune cells communicate with each other, and embedding a biological “bias” toward inflammatory responses (Miller et al., 2011). The effects of HPA dysregulation contribute to the well-known association between stressors and both acute and chronic illness.
Chronic cortisol output also alters the functioning of other brain systems that help regulate HPA activity, including the prefrontal cortex (the seat of executive functions such as planning and emotion regulation), hippocampus (memory and learning), amygdala (emotion activation and regulation), and hypothalamus (multiple neuroendocrine functions) (Lupien et al., 2009; Ulrich-Lai and Herman, 2009). These linkages of stress exposure with brain areas that influence self-regulation, memory, emotion, and behavioral motivation help explain the associations between chronic stress and impairments in focused attention, learning, memory, and self-regulation in children and adults.
Stress also is associated with acute increases in ANS reactivity. The stress effects of ANS activation can result in elevated blood pressure (El-Sheikh and Erath, 2011); poor control of blood sugar levels; and immune system and inflammation dysregulation, through the effects of ANS molecular signals on white blood cell functions.
Prenatal Stressors
Although this report focuses on children beginning at birth rather than on the prenatal period, it is important to note in some depth that child development and early learning also are affected by prenatal exposures. There is growing evidence that the biological embedding of chronic stress begins prenatally because fetal development is affected by the hormonal, autonomic, and other physiologic correlates of maternal stress. Prenatal exposure to cortisol, for example, can have profound influences on the developing brain, as some portion of maternally secreted cortisol moves through the placenta and affects the fetus’s neurodevelopment. In animal models, treating the pregnant mother with corticosterone (the rodent equivalent of cortisol) delays the maturation of neurons, myelination, glia cell formation, and blood supply to brain structures (Lupien et al., 2009). In humans, observed effects of prenatal stressors, including maternal depression and anxiety, include smaller birth weights, perturbations in postnatal development and behavior, and increased reactivity of the HPA axis. Heightened prenatal exposure to stress is associated with greater stress reactivity in infancy, as well as longer-term difficulties in emotional and cognitive functioning (Oberlander et al., 2008; Sandman et al., 2012).
The biological embedding of maternal stress in fetal development is
consistent with a variety of other biological influences on prenatal growth arising from the mother’s diet and nutrition, exposure to environmental pollutants, use of controlled substances, and other aspects of maternal care (Almond and Currie, 2011). The importance of prenatal experience to long-term development is sometimes described as “fetal programming” because prenatal conditions appear to calibrate or program a variety of fetal brain systems involved in responses to stress and adversity.
Socioeconomic Status and Early Brain Development
For children, poverty often entails the confluence of multiple sources of chronic stress. For this reason, considerable research on the effects of chronic stress on children’s development has focused on children in families living in poverty or with low income. Studies of children in these conditions indicate that the stressors associated with poverty can contribute to problems with coping, self-regulation, health, emotional well-being, and early learning (Blair and Raver, 2012; Evans and Kim, 2013).
Neuroimaging studies show that socioeconomic status is especially associated with brain functioning in areas related to language and self-regulation (Hackman and Farah, 2009; Kishiyama et al., 2009). Luby and colleagues (2013) found that early childhood poverty was associated with smaller volume in a brain structure involved in the formation of new memories from current experience (the hippocampus) and that this association derived from the impact of stressful childhood events and hostile parenting. Hanson and colleagues (2013) found that preschool children growing up in poverty had lower volumes of gray matter—tissue that is important to information processing, especially in areas of the brain relevant to self-regulation and higher-order thinking.
Children growing up in conditions of economic adversity often sustain stress-related perturbations in the development of brain areas associated with important cognitive and self-regulatory functions. Further, these changes may contribute to academic and social-behavioral problems associated with neurocognitive functions, and may also affect the acquisition of learning skills associated with self-regulation and persistence. In other words, in addition to other disadvantages they experience, one reason children in stressful circumstances fall behind academically is that the biological effects of stress impair their capacities for concentrated attention, memory, cognitive self-regulation, language, and focused thinking. One of the reasons these children experience social difficulties, such as peer conflict or poor compliance with teachers, is that the biological effects of stress enhance their emotional reactivity, heighten their threat vigilance, and undermine their emotion regulation and impulse control.
Interaction Between Exposure to Stress and Gene Expression
There is increasing evidence that chronic stress has the biological effects described above because of its consequences for gene expression, and studies of the developmental biology of social adversity contribute to understanding the mechanisms of the combined, interactive influences of genes and experiences (Gilbert, 2002; Gottlieb, 1991; Karmiloff-Smith, 2007; Meaney, 2010; Waddington, 1959, 2012). Stress constitutes one of the most powerful experiential catalysts of epigenetic influences on gene expression in studies of animals and humans, and epigenetic modifications may be the basis for some of the biological and behavioral effects of stress described here. For example, Oberlander and colleagues (2008) described an association between maternal depression during pregnancy and heightened cortisol reactivity when infants were 3 months old. They also found that heightened cortisol was associated with decreases in the expression of the glucocorticoid receptor gene in the infants. Changes in gene expression in the child helped account, in other words, for the enduring influence of prenatal maternal stress.
INDIVIDUAL DIFFERENCES IN SUSCEPTIBILITY TO ENVIRONMENTAL FACTORS
Variability in the effects of context can be seen at the levels of both behavior and biology, and there is an emerging understanding that this is due to differences among individuals in their susceptibility to environmental influence, in which a subset of individuals appears to be more sensitive to the influences of both negative and positive environmental factors. The most intensive study in this area has focused on the sometimes dramatic differences in individual variation in the consequences of exposure to early adversity and stress. Among children who face these challenges, many children show immediate and long-term negative effects on health and development, while others thrive and survive with little detrimental effect. Understanding such differences is important as a means to explain stress-related disorders, account for uneven distributions of disorders within populations, shed light on the sources of individual resilience and vulnerability, and provide insights to lead to effective intervention strategies (Boyce and Kobor, 2015).
Early perspectives on such differences in stress response concluded that individuals experienced variable effects of adversity because of either heritable or acquired vulnerabilities to stress and challenge, referred to as the “stress diathesis” model. More recently, a now substantial body of literature suggests that it is not just that some children are more vulnerable to the effects of adversity, but rather that some children are more susceptible, or responsive, to the social environment; these children show either more
maladaptive or more positive outcomes, depending on the exposure (e.g., Belsky, 2005; Boyce and Ellis, 2005; Ellis et al., 2011a). Studies have demonstrated this greater susceptibility of neurobiologically responsive children to both positive and negative aspects of their environments in the context of a range of stressors and adversities, including overall family distress (Obradovic et al., 2010), marital conflict (El-Sheikh, 2005; El-Sheikh et al., 2007), paternal depression (Cummings et al., 2007), and parental psychopathology (Shannon et al., 2007). They also have done so in the context of a wide variety of positive environmental features, including parental warmth (Ellis et al., 1999), beneficial experiences and exposures (Pluess and Belsky, 2011), and supportive interventions (Bakermans-Kranenburg et al., 2008a). These studies have examined this variable susceptibility in light of a range of defining biological parameters, including genetic variations (Bakermans-Kranenburg et al., 2008b; Knafo et al., 2011; Manuck et al., 2011), differences in brain circuitry (Whittle et al., 2011), and physiological reactivity (e.g., Alkon et al., 2006; Boyce et al., 1995).
One of the most important findings has been that outcomes for highly susceptible children are affected in both directions in low- and high-stress settings—not just an attenuation of negative effects in low-stress circumstances. Examples of such bidirectional effects have included differential rates of violent injuries among high- and low-reactivity rhesus macaques before and during a prolonged period of confinement stress (Boyce et al., 1998); children’s sensitivity to a socioemotional intervention (Bakermans-Kranenburg et al., 2008a); adolescents’ susceptibility to parenting influence (Belsky and Beaver, 2011); and trajectories of pubertal development among girls with high- versus low-quality parent relationships (Ellis et al., 2011b). In each case, the “risky phenotype” showed high levels of maladaptive outcomes under stressful conditions but also lower levels of such outcomes than their low-risk counterparts in positive, low-stress conditions.
Together, these findings indicate that while all children exhibit responsiveness to environmental influences, a subset of children show an exaggerated susceptibility to the character of their social environments—heightened risk for morbidity and developmental deviation when reared in harsh, unsupportive conditions but higher levels of health and positive development if reared in environments characterized by nurturance and support. Such children almost certainly contribute substantially to the uneven distribution of ill health, learning difficulties, and troubled development found within childhood populations. However, they may also benefit disproportionately from positive early interventions (Belsky and van Ijzendoorn, 2015).
The mechanisms, consequences, and intervention opportunities related to these kinds of individual differences, including the interplay among environmental exposures and biological and genetic factors, emerge as essential for fully understanding the biology of social adversity.
Conclusion About the Interaction of Biology and the Environment
The capacity for learning is grounded in the development of the brain and brain circuitry. Rather than a structure built from a static “blueprint,” the brain architecture that underlies learning is developed through a continuous, dynamic, adaptive interaction between biology and environment that begins at conception and continues throughout life. This accounts for how early experiences (including supports and stressors) affect gene expression and how the brain develops, and it also accounts for how the effects of environmental factors on a child’s development may vary depending on underlying individual genetic characteristics. The adaptations that occur as a result of the mutual interactions between “nature” and “nurture” mean that early experiences and early learning environments affect all domains of human development.
Adolphs, R. 2009. The social brain: Neural basis of social knowledge. Annual Review of Psychology 60:693-716.
Alkon, A., S. Lippert, N. Vujan, M. E. Rodriquez, W. T. Boyce, and B. Eskenazi. 2006. The ontogeny of autonomic measures in 6- and 12-month-old infants. Developmental Psychobiology 48(3):197-208.
Almond, D., and J. Currie. 2011. Killing me softly: The fetal origins hypothesis. The Journal of Economic Perspectives 25(3):153-172.
Bakermans-Kranenburg, M. J., M. H. Van Ijzendoorn, J. Mesman, L. R. Alink, and F. Juffer. 2008a. Effects of an attachment-based intervention on daily cortisol moderated by dopamine receptor D4: A randomized control trial on 1- to 3-year-olds screened for externalizing behavior. Development and Psychopathology 20(3):805-820.
Bakermans-Kranenburg, M. J., I. M. H. Van, F. T. Pijlman, J. Mesman, and F. Juffer. 2008b. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology 44(1):293-300.
Barr, C. S., T. K. Newman, S. Lindell, C. Shannon, M. Champoux, K. P. Lesch, S. J. Suomi, D. Goldman, and J. D. Higley. 2004. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Archives of General Psychiatry 61(11):1146-1152.
Belsky, J. 2005. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In Origins of the social mind: Evolutionary psychology and child development, edited by B. J. Ellis and D. F. Bjorklund. New York: Guilford Press. Pp. 139-163.
Belsky, J., and K. M. Beaver. 2011. Cumulative-genetic plasticity, parenting and adolescent self-regulation. Journal of Child Psychology and Psychiatry and Allied Disciplines 52(5):619-626.
Belsky, J., and M. H. van Ijzendoorn. 2015. What works for whom? Genetic moderation of intervention efficacy. Development and Psychopathology 27(Special Issue 01):1-6.
Blair, C., and C. C. Raver. 2012. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist 67(4):309-318.
Blair, C., D. A. Granger, K. T. Kivlighan, R. Mills-Koonce, M. Willoughby, M. T. Greenberg, L. C. Hibel, and C. K. Fortunato. 2008. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology 44(4):1095-1109.
Blair, C., C. C. Raver, D. Granger, R. Mills-Koonce, and L. Hibel. 2011. Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology 23(3):845-857.
Blakemore, S. J. 2010. The developing social brain: Implications for education. Neuron 65(6):744-747.
———. 2012. Development of the social brain in adolescence. Journal of the Royal Society of Medicine 105(3):111-116.
Bloom, F. E., C. A. Nelson, and A. Lazerson. 2001. Brain, mind, and behavior. 3rd ed. New York: Worth Publishers.
Borghol, N., M. Suderman, W. McArdle, A. Racine, M. Hallett, M. Pembrey, C. Hertzman, C. Power, and M. Szyf. 2012. Associations with early-life socio-economic position in adult DNA methylation. International Journal of Epidemiology 41(1):62-74.
Boyce, W. T., and B. J. Ellis. 2005. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology 17(2):271-301.
Boyce, W. T., and M. S. Kobor. 2015. Development and the epigenome: The “synapse” of gene-environment interplay. Developmental Science 18(1):1-23.
Boyce, W. T., M. Chesney, A. Alkon, J. M. Tschann, S. Adams, B. Chesterman, F. Cohen, P. Kaiser, S. Folkman, and D. Wara. 1995. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine 57(5):411-422.
Boyce, W. T., P. O’Neill-Wagner, C. S. Price, M. Haines, and S. J. Suomi. 1998. Crowding stress and violent injuries among behaviorally inhibited rhesus macaques. Health Psychology 17(3):285-289.
Boyce, W. T., M. B. Sokolowski, and G. E. Robinson. 2012. Toward a new biology of social adversity. Proceedings of the National Academy of Sciences of the United States of America 109(Suppl. 2):17143-17148.
Bruce, J., M. R. Gunnar, K. C. Pears, and P. A. Fisher. 2013. Early adverse care, stress neurobiology, and prevention science: Lessons learned. Prevention Science 14(3):247-256.
Burns, J. G., N. Svetec, L. Rowe, F. Mery, M. J. Dolan, W. T. Boyce, and M. B. Sokolowski. 2012. Gene-environment interplay in drosophila melanogaster: Chronic food deprivation in early life affects adult exploratory and fitness traits. Proceedings of the National Academy of Sciences of the United States of America 109(Suppl. 2):17239-17244.
Carlson, M., and F. Earls. 1997. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences 807:419-428.
Caspi, A., J. McClay, T. E. Moffitt, J. Mill, J. Martin, I. W. Craig, A. Taylor, and R. Poulton. 2002. Role of genotype in the cycle of violence in maltreated children. Science 297(5582):851-854.
Caspi, A., K. Sugden, T. E. Moffitt, A. Taylor, I. W. Craig, H. Harrington, J. McClay, J. Mill, J. Martin, A. Braithwaite, and R. Poulton. 2003. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 301(5631):386-389.
Cicchetti, D., and F. A. Rogosch. 2001. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology 13(3):677-693.
Cole, S. W., G. Conti, J. M. Arevalo, A. M. Ruggiero, J. J. Heckman, and S. J. Suomi. 2012. Transcriptional modulation of the developing immune system by early life social adversity. Proceedings of the National Academy of Sciences of the United States of America 109(50):20578-20583.
Cummings, E. M., M. El-Sheikh, C. D. Kouros, and P. S. Keller. 2007. Children’s skin conductance reactivity as a mechanism of risk in the context of parental depressive symptoms. Journal of Child Psychology and Psychiatry and Allied Disciplines 48(5):436-445.
Danese, A., and B. S. McEwen. 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior 106(1):29-39.
De Pisapia, N., M. Serra, P. Rigo, J. Jager, N. Papinutto, G. Esposito, P. Venuti, and M. H. Bornstein. 2014. Interpersonal competence in young adulthood and right laterality in white matter. Journal of Cognitive Neuroscience 26(6):1257-1265.
Decety, J., and C. Lamm. 2007. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist 13(6):580-593.
Dozier, M., M. Manni, M. K. Gordon, E. Peloso, M. R. Gunnar, K. C. Stovall-McClough, D. Eldreth, and S. Levine. 2006. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment 11(2):189-197.
Dunn, E. C., M. Uddin, S. V. Subramanian, J. W. Smoller, S. Galea, and K. C. Koenen. 2011. Research review: Gene-environment interaction research in youth depression—a systematic review with recommendations for future research. Journal of Child Psychology and Psychiatry and Allied Disciplines 52(12):1223-1238.
Edwards, V., R. Anda, S. Dube, M. Dong, D. F. Chapman, and I. V. Felitt. 2005. The wide-ranging health consequences of adverse childhood experiences. In Victimization of children and youth: Patterns of abuse, response strategies, edited by K. Kendall-Tackett and S. Giacomoni. Kingston, NJ: Civic Research Institute.
Egan, M. F., T. E. Goldberg, B. S. Kolachana, J. H. Callicott, C. M. Mazzanti, R. E. Straub, D. Goldman, and D. R. Weinberger. 2001. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 98(12):6917-6922.
El-Sheikh, M. 2005. The role of emotional responses and physiological reactivity in the marital conflict-child functioning link. Journal of Child Psychology and Psychiatry and Allied Disciplines 46(11):1191-1199.
El-Sheikh, M., and S. A. Erath. 2011. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology 23(2):703-721.
El-Sheikh, M., P. S. Keller, and S. A. Erath. 2007. Marital conflict and risk for child maladjustment over time: Skin conductance level reactivity as a vulnerability factor. Journal of Abnormal Child Psychology 35(5):715-727.
Ellis, B. J., S. McFadyen-Ketchum, K. A. Dodge, G. S. Pettit, and J. E. Bates. 1999. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: A longitudinal test of an evolutionary model. Journal of Personality and Social Psychology 77(2):387-401.
Ellis, B. J., W. T. Boyce, J. Belsky, M. J. Bakermans-Kranenburg, and M. H. van Ijzendoorn. 2011a. Differential susceptibility to the environment: An evolutionary—neurodevelopmental theory. Development and Psychopathology 23(1):7-28.
Ellis, B. J., E. A. Shirtcliff, W. T. Boyce, J. Deardorff, and M. J. Essex. 2011b. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology 23(1):85-99.
Essex, M. J., M. H. Klein, E. Cho, and N. H. Kalin. 2002. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry 52(8):776-784.
Essex, M. J., E. A. Shirtcliff, L. R. Burk, P. L. Ruttle, M. H. Klein, M. J. Slattery, N. H. Kalin, and J. M. Armstrong. 2011. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology 23(4):1039-1058.
Essex, M. J., W. T. Boyce, C. Hertzman, L. L. Lam, J. M. Armstrong, S. M. Neumann, and M. S. Kobor. 2013. Epigenetic vestiges of early developmental adversity: Childhood stress exposure and DNA methylation in adolescence. Child Development 84(1):58-75.
Evans, G. W., and P. Kim. 2013. Childhood poverty, chronic stress, self-regulation, and coping. Child Development Perspectives 7(1):43-48.
Fagiolini, M., C. L. Jensen, and F. A. Champagne. 2009. Epigenetic influences on brain development and plasticity. Current Opinion in Neurobiology 19(2):207-212.
Fox, S. E., P. Levitt, and C. A. Nelson, 3rd. 2010. How the timing and quality of early experiences influence the development of brain architecture. Child Development 81(1):28-40.
Garner, A. S., and J. P. Shonkoff. 2012. Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into lifelong health. Pediatrics 129(1):e224-e231.
Geronimus, A. T., M. Hicken, D. Keene, and J. Bound. 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health 96(5):826-833.
Gilbert, S. F. 2002. The genome in its ecological context: Philosophical perspectives on inter-species epigenesis. Annals of the New York Academy of Sciences 981:202-218.
Gottlieb, G. 1991. Experiential canalization of behavioral development: Theory. Developmental Psychology 27(1):4-13.
Greenough, W. T., and J. E. Black. 1992. Induction of brain structure by experience: Substrates for cognitive development. In Developmental behavioral neuroscience, Minnesota Symposium on Child Psychology, edited by M. R. Gunnar and C. A. Nelson. Hillsdale, NJ: Lawrence Erlbaum Associates.
Greenough, W. T., J. E. Black, and C. S. Wallace. 1987. Experience and brain development. Child Development 58(3):539-559.
Hackman, D. A., and M. J. Farah. 2009. Socioeconomic status and the developing brain. Trends in Cognitive Sciences 13(2):65-73.
Hanson, J. L., N. Hair, D. G. Shen, F. Shi, J. H. Gilmore, B. L. Wolfe, and S. D. Pollak. 2013. Family poverty affects the rate of human infant brain growth. PLoS ONE 8(12):e80954.
Hecht, D. 2014. Cerebral lateralization of pro- and anti-social tendencies. Experimental Neurobiology 23(1):1-27.
Hertzman, C. 1999. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences 896:85-95.
———. 2012. Putting the concept of biological embedding in historical perspective. Proceedings of the National Academy of Sciences of the United States of America 109(Suppl. 2):17160-17167.
Hertzman, C., and W. T. Boyce. 2010. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health 31:329-347.
IOM (Institute of Medicine) and NRC (National Research Council). 2009. Preventing mental, emotional, and behavioral disorders among young people: Progress and possibilities. Washington, DC: The National Academies Press.
Karmiloff-Smith, A. 2007. Atypical epigenesis. Developmental Science 10(1):84-88.
Kellett, C. 2015. Neurons, neural networks and neural pathways. http://www.achoice2live.com/know-your-addiction/neurons-neural-networks-and-neural-pathways (accessed March 23, 2015).
Kishiyama, M. M., W. T. Boyce, A. M. Jimenez, L. M. Perry, and R. T. Knight. 2009. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience 21(6):1106-1115.
Klengel, T., D. Mehta, C. Anacker, M. Rex-Haffner, J. C. Pruessner, C. M. Pariante, T. W. Pace, K. B. Mercer, H. S. Mayberg, B. Bradley, C. B. Nemeroff, F. Holsboer, C. M. Heim, K. J. Ressler, T. Rein, and E. B. Binder. 2013. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience 16(1):33-41.
Knafo, A., S. Israel, and R. P. Ebstein. 2011. Heritability of children’s prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 gene. Development and Psychopathology 23(1):53-67.
Knickmeyer, R. C., S. Gouttard, C. Kang, D. Evans, K. Wilber, J. K. Smith, R. M. Hamer, W. Lin, G. Gerig, and J. H. Gilmore. 2008. A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience 28(47):12176-12182.
Koch, L. G., and S. L. Britton. 2008. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity (Silver Spring) 16(Suppl. 3):S28-S32.
Lam, L. L., E. Emberly, H. B. Fraser, S. M. Neumann, E. Chen, G. E. Miller, and M. S. Kobor. 2012. Factors underlying variable DNA methylation in a human community cohort. Proceedings of the National Academy of Sciences of the United States of America 109(Suppl. 2):17253-17260.
Leja, D., and NHGRI (National Human Genome Research Institute). 2010. Chromatin. https://www.genome.gov/dmd/img.cfm?node=Photos/Graphics&id=85280 (accessed March 25, 2015).
Lesch, K. P. 2007. Linking emotion to the social brain. The role of the serotonin transporter in human social behaviour. EMBO Reports 8 Spec No:S24-S29.
Luby, J., A. Belden, K. Botteron, N. Marrus, M. P. Harms, C. Babb, T. Nishino, and D. Barch. 2013. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics 167(12):1135-1142.
Lupien, S. J., B. S. McEwen, M. R. Gunnar, and C. Heim. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience 10(6):434-445.
Manuck, S. B., and J. M. McCaffery. 2014. Gene-environment interaction. Annual Review of Psychology 65:41-70.
Manuck, S. B., A. E. Craig, J. D. Flory, I. Halder, and R. E. Ferrell. 2011. Reported early family environment covaries with menarcheal age as a function of polymorphic variation in estrogen receptor-alpha. Development and Psychopathology 23(1):69-83.
McEwen, B. S. 2012. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America 109(Suppl. 2):17180-17185.
McGowan, P. O., A. Sasaki, A. C. D’Alessio, S. Dymov, B. Labonte, M. Szyf, G. Turecki, and M. J. Meaney. 2009. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience 12(3):342-348.
Meaney, M. J. 2010. Epigenetics and the biological definition of gene x environment interactions. Child Development 81(1):41-79.
Mehta, D., T. Klengel, K. N. Conneely, A. K. Smith, A. Altmann, T. W. Pace, M. Rex-Haffner, A Loeschner, M. Gonik, K. B. Mercer, B. Bradley, B. Muller-Myhsok, K. J. Ressler, and E. B. Binder. 2013. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America 110(20):8302-8307.
Miller, G. E., E. Chen, and K. J. Parker. 2011. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin 137(6):959-997.
Molenaar, D., S. van der Sluis, D. I. Boomsma, C. M. Haworth, J. K. Hewitt, N. G. Martin, R. Plomin, M. J. Wright, and C. V. Dolan. 2013. Genotype by environment interactions in cognitive ability: A survey of 14 studies from four countries covering four age groups. Behavior Genetics 43(3):208-219.
National Scientific Council on the Developing Child. 2004. Children’s emotional development is built into the architecture of their brains: Working paper #2. http://www.developingchild.net (accessed April 22, 2014).
Nelson, C. A., N. A. Fox, and C. H. Zeanah. 2014. Romania’s abandoned children: Deprivation, brain development, and the struggle for recovery. Cambridge, MA: Harvard University Press.
Norman, G. J., L. C. Hawkley, S. W. Cole, G. G. Berntson, and J. T. Cacioppo. 2012. Social neuroscience: The social brain, oxytocin, and health. Social Neuroscience 7(1):18-29.
NRC (National Research Council) and IOM (Institute of Medicine). 2000. From neurons to neighborhoods: The science of early childhood development, edited by J. P. Shonkoff and D. A. Phillips. Washington, DC: National Academy Press.
Oberlander, T. F., J. Weinberg, M. Papsdorf, R. Grunau, S. Misri, and A. M. Devlin. 2008. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3(2):97-106.
Obradovic, J., N. R. Bush, J. Stamperdahl, N. E. Adler, and W. T. Boyce. 2010. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development 81(1):270-289.
Pezawas, L., A. Meyer-Lindenberg, E. M. Drabant, B. A. Verchinski, K. E. Munoz, B. S. Kolachana, M. F. Egan, V. S. Mattay, A. R. Hariri, and D. R. Weinberger. 2005. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience 8(6):828-834.
Pluess, M., and J. Belsky. 2011. Prenatal programming of postnatal plasticity? Development and Psychopathology 23(1):29-38.
Provencal, N., M. J. Suderman, C. Guillemin, R. Massart, A. Ruggiero, D. Wang, A. J. Bennett, P. J. Pierre, D. P. Friedman, S. M. Cote, M. Hallett, R. E. Tremblay, S. J. Suomi, and M. Szyf. 2012. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. Journal of Neuroscience 32(44):15626-15642.
Robinson, G. E., C. M. Grozinger, and C. W. Whitfield. 2005. Sociogenomics: Social life in molecular terms. Nature Reviews: Genetics 6(4):257-270.
Robinson, G. E., R. D. Fernald, and D. F. Clayton. 2008. Genes and social behavior. Science 322(5903):896-900.
Ross, E. D., and M. Monnot. 2008. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain and Language 104(1):51-74.
Rutter, M. 2006. Genes and behaviour: Nature-nurture interplay explained. Oxford: Blackwell. ———. 2010. Gene-environment interplay. Depression and Anxiety 27(1):1-4.
———. 2012. Achievements and challenges in the biology of environmental effects. Proceedings of the National Academy of Sciences of the United States of America 109(Suppl. 2):17149-17153.
Sandman, C. A., E. P. Davis, C. Buss, and L. M. Glynn. 2012. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology 95(1):7-21.
Sasaki, A., W. C. de Vega, and P. O. McGowan. 2013. Biological embedding in mental health: An epigenomic perspective. Biochemistry and Cell Biology 91(1):14-21.
Schore, A. N. 2010. Synopsis, the impact of childhood trauma: Psychobiological sequelae in adults. In The impact of early life trauma on health and disease: The hidden epidemic, edited by R. A. Lanius, E. Vermetten, and C. Pain. Cambridge, UK: Cambridge University Press. Pp. 142-148.
———. 2014. The right brain is dominant in psychotherapy. Psychotherapy (Chicago, Illinois) 51(3):388-397.
Semrud-Clikeman, M., J. Goldenring Fine, and D. C. Zhu. 2011. The role of the right hemisphere for processing of social interactions in normal adults using functional magnetic resonance imaging. Neuropsychobiology 64(1):47-51.
Shannon, K. E., T. P. Beauchaine, S. L. Brenner, E. Neuhaus, and L. Gatzke-Kopp. 2007. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology 19(3):701-727.
Shonkoff, J. P., and A. S. Garner. 2012. The lifelong effects of early childhood adversity and toxic stress. Official Journal of the American Academy of Pediatrics 129(1):e232-e246.
Slavich, G. M., and S. W. Cole. 2013. The emerging field of human social genomics. Clinical Psychological Science 1(3):331-348.
Strachan, T., and A. P. Read. 2011. Human molecular genetics. New York: Garland Science.
Sturge-Apple, M. L., P. T. Davies, D. Cicchetti, and L. G. Manning. 2012. Interparental violence, maternal emotional unavailability and children’s cortisol functioning in family contexts. Developmental Psychology 48(1):237-249.
Takesian, A. E., and T. K. Hensch. 2013. Balancing plasticity/stability across brain development. Progress in Brain Research 207:3-34.
Thompson, P. M., N. G. Martin, and M. J. Wright. 2010. Imaging genomics. Current Opinion in Neurology 23(4):368-373.
Thompson, R. A. 2014. Stress and child development. The Future of Children 24(1):41-59.
Tung, J., L. B. Barreiro, Z. P. Johnson, K. D. Hansen, V. Michopoulos, D. Toufexis, K. Michelini, M. E. Wilson, and Y. Gilad. 2012. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proceedings of the National Academy of Sciences of the United States of America 109(17):6490-6495.
Turner, K. M., and T. H. Burne. 2013. Interaction of genotype and environment: Effect of strain and housing conditions on cognitive behavior in rodent models of schizophrenia. Frontiers in Behavioral Neuroscience 7:97.
Ulrich-Lai, Y. M., and J. P. Herman. 2009. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience 10(6):397-409.
Waddington, C. H. 1959. Canalization of development and genetic assimilation of acquired characters. Nature 183(4676):1654-1655.
———. 2012. The epigenotype. 1942. International Journal of Epidemiology 41(1):10-13.
Weaver, I. C., J. Diorio, J. R. Seckl, M. Szyf, and M. J. Meaney. 2004. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Annals of the New York Academy of Sciences 1024:182-212.
Whittle, S., M. B. Yap, L. Sheeber, P. Dudgeon, M. Yucel, C. Pantelis, J. G. Simmons, and N. B. Allen. 2011. Hippocampal volume and sensitivity to maternal aggressive behavior: A prospective study of adolescent depressive symptoms. Development and Psychopathology 23(1):115-129.
Zeanah, C. H., M. R. Gunnar, R. B. McCall, J. M. Kreppner, and N. A. Fox. 2011. VI. Sensitive periods. Monographs of the Society for Research in Child Development 76(4):147-162.
This page intentionally left blank.




























