4
Coral Population and Community Interventions
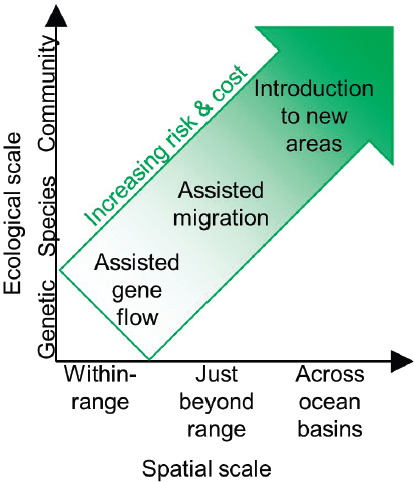
In contrast to most genetic and physiological interventions, which target individual corals with the ultimate purpose of changing entire coral populations, this chapter discusses interventions that seek to directly alter entire populations or communities of coral. This chapter groups together three interventions: assisted gene flow, assisted migration, and introduction to new areas. In practice, these three interventions can be seen as tiered scales of managed relocation in support of varying goals. Managed relocation is the introduction of a particular genotype or an entire species to areas outside of the historical bounds for a given genotype or species. Managed relocation is a component of the broader concept of translocation—the movement of individuals in space—which also includes reintroduction following habitat restoration in historically occupied locations (Armstrong and Seddon, 2007; Griffith et al., 1989). Assisted gene flow supports the expansion of resilient genotypes within a coral’s current range. Assisted migration supports movement of corals to areas just outside their range, which they may be better suited to as climate change causes preferred habitat to shift to higher latitudes. Introduction to new areas involves the introduction of non-native coral that may be more tolerant to stressed conditions, in order to maintain the presence of the coral reef.
Large-scale relocation of coral has not been trialed, but lessons can be drawn from other taxa or from corals that have been moved to or outplanted from coral nurseries, reciprocal transplant experiments, reef restoration efforts, accidental introductions, and natural range expansions.
These lessons provide a significant knowledge base for evaluating the approach in detail, including potential benefits, feasibility, risks, and limitations.
MANAGING CORAL PREDATORS, COMPETITORS, AND FACILITATORS
Corals are not the only potential targets when managing a coral reef community, and the health and diversity of other members of the community have a direct influence on the health and resilience of the coral species. The diversity of reef communities derives from the hundreds of species of fish, invertebrates, algae, protists, and microbes that typically live among corals. In almost all cases, these species also play a huge role in the value of reefs to humans—through recreation, fishing, algal farming, and other services. As a result, managing coral communities is more than just managing corals: Though corals play a fundamental role, management of other species is also important.
Maintaining ecological processes and community dynamics has been identified as an important factor in facilitating coral restoration success (Ladd et al., 2018; Shaver et al., 2018). This includes managing herbivory by fish and urchins to minimize algal competition, coral predation by fish and invertebrates, and nutrient cycling by fish. Noncoral individuals and species may be targets of managed relocation in combination with coral. While the focus of this chapter is on the coral-specific goals, the committee does discuss some considerations of moving reef-associated exosymbionts and herbivores in order to increase the likelihood of coral relocation success. Additionally, some reef-associated species are targets of existing management practices described in Chapter 1, such as management of overfishing and invasive species.
MANAGED RELOCATION
What It Is
Managed relocation is the movement of species, populations, genotypes, or phenotypes from a source area to locations outside of their historical distribution, sometimes with different environmental parameters (Richardson et al., 2009; Schwartz et al., 2012). Managed relocation typically focuses on moving individuals to promote adaptive response to climate change (Schwartz et al., 2012), by moving populations or species to locations with future climatic conditions analogous to what they historically experienced.
Managed relocation to promote adaptive responses to climate change has already occurred in a variety of taxa, and more are being planned.
Examples of species where relocations have occurred include an endangered conifer (Torreya taxifolia) in the southwestern United States (Barlow, 2010) and two butterfly species in the United Kingdom (Willis et al., 2009), and discussions are under way for relocations in the forestry industry (McKenney et al., 2009) and in commercial fisheries (e.g., lobsters; Green et al., 2010). There are no examples of large-scale managed relocation of corals, but assessments of its potential may be gleaned from corals that have been moved to or outplanted from coral nurseries, reciprocal transplant experiments, reef restoration efforts, accidental introductions, and natural range expansions. With concerns about coral reef persistence under future climate change and multiple anthropogenic stressors, managed relocation of corals has been considered albeit with caution (Hoegh-Guldberg et al., 2008).
Managed relocation is categorized into three types (depicted in Figure 4.1):
Assisted gene flow is the movement of genotypes within a population’s range (Aitken and Whitlock, 2003). Assisted gene flow typically focuses on the relocation of individuals with genotypes that confer higher stress tolerance, which requires that populations exhibit genetically-based variation in stress tolerance across locations with different historical levels of stress exposure. For example, some Pacific populations of corals live in highly variable or warm water microhabitats (Oliver and Palumbi,
2011). These corals can provide nursery stocks with high heat tolerance (Morikawa and Palumbi, in press).
Assisted migration (also called assisted colonization) is the movement of individuals beyond a species’ range boundaries (Schwartz et al., 2012). Climate change can cause locations just outside of a species’ range to have environmental conditions analogous to historic conditions within a species’ range, such that range shifts to these locations can contribute to species’ persistence under climate change (Davis and Shaw, 2001). Corals at some locations, including corals in Japan, Florida, and Australia (Baird et al., 2012; Greenstein and Pandolfi, 2008; Precht and Aronson, 2004; Yamano et al., 2011), already exhibit natural and detectable poleward range shifts in response to climate change (Parmesan and Yohe, 2003). Therefore, the typical focus is movement just beyond a species’ range in the poleward, or otherwise cooler or lower-stress, direction.
Introduction to new areas is the movement of highly stress-tolerant individuals between regions, such as, in the extreme, between ocean basins (Coles and Riegl, 2013; Sheppard, 2003). For example, corals in the Red Sea can maintain photosynthetic performance up to 32°C, nearly 6°C above average summer maximum (26.1°C) but within natural fluctuations for shallow regions (Fine et al., 2013). Corals from the Gulf of Oman can exist over a wide range of temperatures from 11.4°C to 36°C (Coles, 1997).
Because of the overlap in goals, methodologies for How to Do It, limitations, and risks among these three types of relocations are discussed together in this chapter, with any differences highlighted.
Benefit and Goals
Managed relocation often focuses on one or both of two distinct ultimate goals. First, the most commonly invoked goal of managed relocation across taxa is to reduce the likelihood of extinction for a species, population, or genotype vulnerable to climate change (Hewitt et al., 2011; Schwartz et al., 2012). This could involve moving corals that are vulnerable to extinction to locations where future predicted oceanographic conditions are expected to be more suitable. Second, managed relocation might promote the maintenance of the ecosystem state in a particular location, such as coral-dominated reefs and the associated species assemblage and ecosystem services (Hewitt et al., 2011; Schwartz et al., 2012). This is particularly relevant to foundational taxa such as corals, which provide the structure of reef communities (Wild et al., 2011). This could involve importing individuals from populations with temperature
tolerance (Bay and Palumbi, 2014; Loya et al., 2001), disease resistance (Vollmer and Kline, 2008), and tolerance to sedimentation or acidification (Fabricius, 2005). The overarching goal of promoting the maintenance of a particular ecosystem state might take on many forms, from having corals as the primary benthic taxa regardless of species composition, to maintaining a complex topography that supports diverse fish and invertebrate communities (Graham et al., 2006; Gratwicke and Speight, 2005a; Wilson et al., 2006) and associated ecosystem functions such as reducing wave stress (Storlazzi et al., 2017), maintaining tourism, and providing sand production. Therefore, managed relocation to promote an ecosystem state might involve the relocation of multiple species in a coral community assemblage.
Management actions may include some combination of these goals, focused on both preserving specific species and maintaining place-based reefs, but their clear definition at the outset will clarify potential costs, benefits, and risks. In addition to the two overarching goals that can apply to all relocations, proximate goals vary with relocation type.
For assisted gene flow, a key proximate goal is to enhance the spread of stress-tolerant alleles and their transmission into the next generation. An additional potential goal is to enhance genetic diversity within target populations to overcome low fertilization success at low colony densities (Allee effects) and at low intraspecific genetic diversity due to obligate outcrossing in some species (including the major reef-building corals of the Caribbean) (Miller et al., 2018), thereby enhancing reproductive success during spawning events (Baums, 2008; Miller et al., 2018). These goals align when the addition of new, stress-tolerant, genotypes to an area also increases genetic variation generally and when sexual reproduction further increases genetic variation in stress tolerance. There is overlap in goals of assisted gene flow and managed breeding (described in Chapter 2), where assisted gene flow is specifically focused on the transport between locations as an approach to achieve these goals.
Natural levels of gene flow vary widely among coral species (Ayre and Hughes, 2000) but can, in some cases, be low over scales of hundreds to thousands of kilometers (Sheets et al., 2018; Torda et al., 2013; Wood et al., 2014). Assisted gene flow can promote genetic connectivity between reefs along gradients of thermal stress (Baskett et al., 2010; Bay et al., 2017; Matz et al., 2018). A genomic model by Bay et al. (2017) showed that incorporation of 10 adult breeding colonies of heat-tolerant coral from Samoa could enhance evolution of heat tolerance in nominally cool-adapted populations in the Cook Islands, but that natural dispersal between these localities, about 1,000 kilometers apart, was far smaller than that. Therefore, assisted gene flow can be relevant if frequencies of known adaptive alleles are low and if diversity or abundance of native colonies is low.
For assisted migration, a key proximate goal is to promote range shifts along latitudinal or analogous gradients to track changes in climate. Poleward relocations are frequently proposed in the literature (Baird and Thomson, 2018; Hoegh-Guldberg et al., 2008; Riegl, 2003; Riegl and Piller, 2003; Tuckett et al., 2017). Surveys of genetic differentiation in corals suggest that natural migration more than 500-1,000 kilometers is rare (usually less than one successful migrant per generation). As an example, only four species of the most speciose genus of corals, Acropora, is established in Hawaii (Grigg et al., 1981; Walsh et al., 2014). Increased clonality of edge populations (Baums et al., 2014; Foster et al., 2013; Miller et al., 2018) might further limit natural range expansions for obligate outcrossers.
Migration along north-south coastlines might occur more rapidly by a stepping-stone mechanism such as along the west coast of Australia or along the Ryukyu Islands toward the main islands of Japan. Range shifts are typically more rapid in the oceans than on land despite slower overall climate velocity (the movement of zones of suitable climate) in the ocean, which might be due to lower heterogeneity in climate velocity among locations (Burrows et al., 2011) as well as greater dispersal potential. While geological evidence suggests that subtropical reefs have acted as important refugia in the past for tropical corals (Greenstein and Pandolfi, 2008; Halfar et al., 2005; Kiessling, 2009), some have questioned the likelihood that high-latitude ecosystems would actually be able to support viable tropical populations (Beger et al., 2014), in part because they are (at present) marginal environments for corals, for reasons such as reduced aragonite saturation (Guinotte et al., 2003), higher temperature variability, and limited winter light availability (Muir et al., 2015).
For introduction to new areas, a key proximate goal is to enhance stress tolerance of the local reef assemblage by adding species at the recipient location. While this proximate goal is focused on maintaining reef-dominated states, introduction to new areas can contribute to the goal of preserving a threatened species if the potential source locations are also threatened by climate warming and largely isolated from other populations (as is the case for the Persian Gulf; Coles and Riegl, 2013).
How to Do It
Engaging in managed relocation is a multifaceted process with a variety of approaches to determining (1) whether to translocate, (2) how and at what stage to move individuals, (3) which species and individuals to move, (4) what is the best source or target location, and (5) when, how often, and for how long to translocate (Pérez et al., 2012; Schwartz and Martin, 2013; see Figure 4.2). Tools including vulnerability assessment, risk-benefit analysis, and feasibility assessment have been developed to
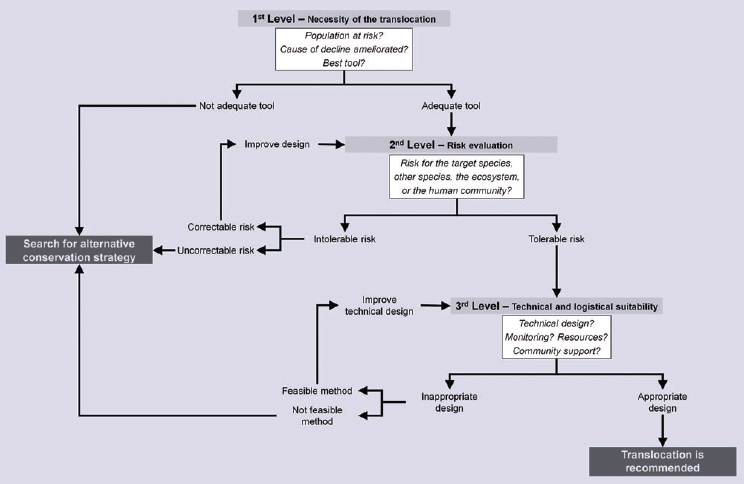
guide the relocation process, with existing decision analysis tools for managed relocation particularly focused on (1) and (3) (Hoegh-Guldberg et al., 2008; Pérez et al., 2012; Schwartz and Martin, 2013; Weeks et al., 2011; see Figure 4.2).
The Decision to Translocate
Managed relocation is relevant when limited dispersal, slow population growth, small populations, or fragmented habitat distribution impede natural movement responses to climate change or other such stressors (Hewitt et al., 2011). As sessile, benthic organisms with only limited movement potential associated with growth and fragmentation, any coral dispersal beyond a few meters can normally only occur through gametes and larvae. Specifically, while tissue covered fragments and sloughed tissue may travel short distances from the parent colony (Sammarco, 1982), coral larvae typically disperse distances of hundreds of meters to several kilometers, and on occasion, can travel over hundreds of kilometers (Graham et al., 2008). Natural dispersal may also be limited
by arrival in insufficient numbers or diversity to establish. If the populations are small, establishment might fail due to processes such as demographic stochasticity (random variation in individual survival, growth, and reproduction, where extreme outcomes such as few survivors are more likely in small populations), environmental stochasticity (random variation in environmental conditions, including extreme events such as hurricane disturbances), demographic Allee effects (threshold population sizes necessary for population growth), genetic drift (random loss of genetic diversity at low population sizes), and inbreeding (exposure of deleterious recessive alleles in small populations) (Gilpin and Soule, 1986; Lande, 1998). An additional limitation to successful dispersal for some corals arises due to obligate outcrossing, where low genetic diversity can lead to recruitment failure (Baums, 2008; Miller et al., 2018), essentially a diversity-dependent Allee effect. Obligate outcrossers comprise the major reef-building corals of the Caribbean (Miller et al., 2018), where low diversity might help explain the decline in observed recruitment (Miller et al., 2018; Williams et al., 2008).
How and at What Stage to Move
The typical coral relocation process involves collecting colony fragments, growing fragments in nursery settings, and replanting nursery clones in different reef areas. The options are broad, and moving corals could occur at different life stages (e.g., larvae, juveniles, or adults), with or without use of an intermediate facility. Bringing larvae, colonies, or fragments collected in the field into laboratory or nursery facilities can allow for acclimation to different conditions, including those expected at the recipient site, as well as a quarantine period to reduce the risk of spreading diseases and invasive species (Edwards, 2010). Coral transplantation has been a feature of basic coral biological research for decades (e.g., Edwards and Clark, 1999; Harriott and Fisk, 1988; Yap, 1994; Yap et al., 1992) especially in the field of morphological plasticity (e.g., Foster, 1979). Transplant survival can be low in some settings (Edwards and Clark, 1999) but can be enhanced through careful handling, feeding, or other protocols (Barton et al., 2017; Lirman and Schopmeyer, 2016; Toh et al., 2014).
The first step in any relocation is to remove individuals from the source location. It is important to minimize damage to the source population, particularly if the goal is to move a species already under threat. The least damaging extraction method may be at odds with the method that would maximize relocation success in the new location. For example, the life stage that is probably least damaging to extract and easiest to move is coral larvae, but larvae may also be the least likely to succeed in the
new location compared to adults (Edwards et al., 2015). An intermediate approach is the most common: removing small fragments of source colonies and growing corals in nurseries prior to outplant (Young et al., 2012). This can reduce the impact on source populations and increase outplant success. An additional approach to mitigating the impact on source populations is the use of “corals of opportunity,” such as those that would be damaged during a dredging project if they were not moved, or fragments resulting from a ship grounding. Collecting coral fragments that have been damaged by storms or ships also has low impact on source populations but generally has low success (Ferse, 2010; Garrison and Ward, 2012).
If the goal is to use managed relocation to re-establish a reef ecosystem in a particular place regardless of coral species composition, implementation becomes more challenging. The continuum of possible goals is broad, ranging from creating a simple three-dimensional coral structure to support fish communities to restoring ecological complexity and ecosystem services (Rinkevich, 2014). The coral reef restoration community can offer insights into how such a task might be approached (Johnson et al., 2011; Ladd et al., 2018; Lirman and Schopmeyer, 2016; Rinkevich, 1995; Young et al., 2012), but the underlying science for how to take this approach is still in its infancy, particularly in regard to large-scale collection and nursery culturing of non-native species for transplantation from outside of the species historical range.
Following removal from the source location, the subsequent steps for relocation are cultivation of coral propagules and reattachment, as described in Box 2.1. Long-term relocation success requires the survival, reproduction, and recruitment of transplanted corals (Richmond et al., 2018), which might rely on reducing local stressors or additional restoration efforts (e.g., algal removal, reintroduction of herbivores) as part of the relocation program design. Especially in cases where current reef communities are degraded, relocation is very unlikely to restore populations unless the underlying cause of degradation (e.g., sedimentation, loss of herbivores) is reversed. Relocations to advance the gene flow of adaptive alleles into a healthy population with fewer local stressors, lower algal cover, and more abundant and diverse herbivore communities may be more successful. Best practices for managed relocation, from propagation and outplanting techniques to identification of appropriate abiotic and biotic conditions in outplanted locations, mirror those of coral restoration, reviewed in Ladd et al. (2018), Lirman and Schopmeyer (2016), Meesters et al. (2015), and Young et al. (2012).
One potential consideration in reattachment is the density and composition of relocated corals. In coral restoration projects, densities of outplanted corals vary widely (0.1-25 corals/m2), with little data on how
density affects restoration success despite its expected importance (Ladd et al., 2018). High density of a focal coral species can overcome Allee effects (e.g., increase spawning success), diffuse corallivore predation, and reduce competition from other benthic competitiors (e.g., sponges, algae), but low density can decrease disease spread and intraspecific competition for space (Ladd et al., 2018).
How to Identify Which Species and Individuals to Move
The focal species, or assemblage of species, for relocation depends on the management goal. If the goal is to prevent extinction of climate-vulnerable species, then the focus will be on stress-vulnerable species as indicated by characteristics such as limited dispersal, rarity, low fecundity, long generation times, and susceptibility to thermal stress (Chauvenet et al., 2013; Hewitt et al., 2011; Loss et al., 2011). If the goal is to protect coral-dominated reefs, then the focus might be on stress-tolerant individuals or species as those most likely to persist through future stress (Côté and Darling, 2010). However, a focus on a diverse assemblage might also be the target for a goal of protecting coral-dominated reefs given the role of diversity in adaptive capacity, ecological resilience, and ecosystem function and service provisioning as highlighted in Chapter 1 (Levin and Lubchenco, 2008). One of the strongest results from careful bookkeeping of success of coral fragments is that different genotypes can express different survival, growth, and stress-resistant phenotypes (Morikawa and Palumbi, in press). These variants might be used in outplanting into different locations, might show tradeoffs between different fitness traits (such as growth versus heat resistance), and might provide a buffer against genetic erosion of nursery stocks.
Managed selection (see Chapter 2) is an approach for identifying coral with stress-tolerant genotypes. For assisted gene flow, identification of stress-tolerant genotypes might use emerging molecular tools such as genomics, proteomics, transcriptomics, and metabolomics, which provide insight into the cause-and-effect relationships between stressor exposure and response at the levels from cells to ecosystems (Downs et al., 2005, 2012). While promising, these tools are likely to be species-specific and, because they are typically identified under short-term heat-stress experiments, they may not be indicative of long-term heat tolerance (Louis et al., 2017). Instead, direct physiological testing of reef corals collected from native environments has been shown to be effective, and provides high predictive ability in comparisons of coral clones in nurseries (Morikawa and Palumbi, in press). For assisted migration, coral morphology can also be an indicator of major coral taxa that generally show high stress tolerance or susceptibility. For example, massive corals, with perforate
skeletons with tissues sequestered deeper in the skeleton, are generally more resistant to particular stressors including bleaching, than other, branching, nonperforate types (Hoegh-Guldberg and Salvat, 1995; Loya et al., 2001; van Woesik et al., 2011).
For introduction to new areas, in addition to stress-tolerance, a focus may be on growth forms that provide fish habitat, reef structure, and associated coastal protection. A diversity of coral morphologies typically predicts fish diversity—coral height predicts fish abundance, and larger-bodied fish disproportionately use tabular corals as habitat—where fish diversity, abundance, and body size contribute to different aspects of ecosystem function and services (Gratwicke and Speight, 2005b; Kerry and Bellwood, 2012).
Regarding species composition, a consideration for relocation success is the potential for interspecific competition between corals. Arborescent (tree-like) and table corals can grow quickly above massive and encrusting species, while massive and encrusting forms can outcompete some branching species through mechanisms such as chemical defense, sweeper tentacles, and mesenterial filaments that digest the tissues of nearby neighbors (Chadwick and Morrow, 2011; Connell et al., 2004; Lang and Chornesky, 1990; Rinkevich and Loya, 1985). Despite competition, diversity might increase outplant success if it enhances ecosystem function through a diversity of functional types (e.g., massive corals buffering against currents and wave action while foliose and arborescent forms provide reef rugosity and herbivore habitat), reduces competition with other benthic species, or reduces corallivory through a mix of palatable and nonpalatable species (Ladd et al., 2018).
How to Identify Source and Target Locations
The target location depends on the relocation type and goal. For example, a goal of building reef structure at vulnerable locations will target high-stress locations, while a goal of promoting intact reef structure where future persistence is most likely will focus on lower-stress locations. Likewise, goals of promoting higher frequencies of adaptive alleles in otherwise established populations will tend to target populations predicted to experience high stress in the future. A management focus on low-stress locations can more effectively achieve a goal of promoting coral cover in a higher-disturbance future, as can high-stress locations in a lower-disturbance future (Game et al., 2008), where the magnitude of disturbance might be more relevant than the frequency in defining “high disturbance” (Fabina et al., 2015). However, in some ocean basins, such as the Caribbean, low-stress locations may be rare. For an example of identification of low-stress locations as a target, Beyer et al. (2018) identify
reef locations globally that may be prime locations for restoration in the absence of local disturbances, based on both connectivity and a number of climate variables.
Using a combination of meteorological, oceanographic, and in situ coral reef monitoring data, it is possible to identify specific areas that have been or are likely to be affected by temperature-induced heat stress (Hughes et al., 2017a). Such areas can be source locations from which corals might be removed for transplantation to areas less likely to be affected (McClanahan et al., 2012). Such heat-tolerant populations might be large in extent, for example if they exist across broad latitudinal gradients such as along the Great Barrier Reef (Berkelmans and van Oppen, 2006; Dixon et al., 2015; Howells et al., 2013), but they can also be small in extent when the environmental mosaic is over short distances such as fore reef versus patch reef or reef crest areas (Morikawa and Palumbi, in press; Oliver and Palumbi, 2011). A global search for generalized areas likely to house heat-resistant coral colonies has yet to be done, but might include areas under heat stress during daytime low tides (back reefs, patch reefs) (Oliver and Palumbi, 2011), equatorial reefs (Jokiel and Coles, 1990), corals near power plants (Keshavmurthy et al., 2012), or regions of high-frequency temperature variability (Safaie et al., 2018). At the local level, monitoring and mapping of coral reefs can also identify those areas most affected by conditions contributing to losses. For environmental conditions besides the focal stressor, analogous conditions between source and target locations (e.g., in depth, illumination, turbidity, and salinity) might increase relocation success as corals from a source area with similar conditions to the explant site are more likely to survive, grow, and reproduce.
A further consideration is connectivity between sites. Promoting range shifts through assisted gene flow or assisted migration relies on connectivity between reefs and therefore might focus on target locations that confer connectivity to downstream reefs (e.g., across environmental gradients). Alternately, promoting population or reef persistence in vulnerable locations may lead to a focus on isolated locations where natural or anthropogenic barriers impede dispersal. Corals do show variability in their degree of isolation across locations (Baums et al., 2005), where identification of isolated locations relies on genetic data, oceanographic models of larval dispersal, or a combination (Foster et al., 2012). Considered alone, genetic isolation might indicate limits to connectivity, or it might indicate post-settlement barriers to establishment. In the latter case, relocation is either not necessary (if thermal regime historically served as a post-settlement barrier) or will be unlikely to succeed (if other environmental factors serve as the post-settlement barrier). Therefore, a combination of oceanographic-based connectivity and genetic data holds the greatest promise to identify locations with physical barriers to dispersal.
One tool for identifying source and target locations in managed relocation is to use species distribution modeling (SDM) to match historic (source) and future (target) climatic conditions between locations (Kreyling et al., 2011). Either combining SDMs with the analyses of connectivity described above or extending the basic SDM framework to directly incorporate dispersal (reviewed in Elith and Leathwick, 2009) would be necessary to identify relevant locations not already connected by natural dispersal. SDMs have been used to identify suitable climate envelopes for managed relocation under future climate change for trees, plants, and reptiles (Fordham et al., 2012; Gray et al., 2011; Regan et al., 2012). Freeman et al. (2013) used a Maxent global bioclimate modeling approach to show that shallow tropical corals within the Indian Ocean currently experience physiochemical conditions most similar to future worldwide conditions under climate change. These authors suggest that this region might be a good source of corals for future managed relocation efforts based on climate matching.
Much like source populations from marginal habitats, identification of recipient locations might capitalize on corals of opportunity. Specifically, new restoration projects taking place at highly degraded sites, which are increasing in the Caribbean (Young et al., 2012), might present unique opportunities to engage in all types of managed relocation by focusing on sources from either more stress-tolerant populations or with a diversity of stress tolerances or stress histories (Broadhurst et al., 2008; Rice and Emery, 2003; Sgrò et al., 2011). Implementing relocation through existing restoration projects can reduce costs and the risks to existing degraded recipient communities described below. However, success would likely be lower than if relocation targeted less degraded sites and could require reduction or elimination of stressors that originally led to coral reef degradation. If the goal were to choose locations with the highest likelihood of relocation success, coral restoration practitioners identify herbivore populations, substrate availability (e.g., low cover of coral competitors such as algae and sponges), and coral cover (as an indicator of habitat quality and to diffuse corallivory) as key factors for selecting target restoration sites (although few studies document the effect of these factors on restoration success) (Ladd et al., 2018).
How to Identify When to Translocate
The decisions for the timing of a relocation project might take a proactive approach of translocating in anticipation of future change or a reactive approach of translocating in response to declines. The proactive approach can rely on SDMs of environmental drivers of coral distributions under present conditions and projected suitable habitat under future conditions
(Chauvenet, 2013; Gallagher et al., 2015; Gray et al., 2011). Combining SDMs with population dynamic models will provide added information on anticipated persistence under different management approaches (Bonebrake et al., 2014; Chauvenet, 2013; Fordham et al., 2012; Regan et al., 2012). The reactive approach relies on monitoring of coral cover through time to detect declines, where temperature recorders (currently available) can guide identification of at-risk locations to monitor.
Current Feasibility
On a technical level, many aspects of collection, transportation, and outplanting are feasible. However, a much greater feasibility challenge to managed relocation is in the information necessary to make the multifaceted decision of whether, what, how many, when, and where to translocate as well as to assess the risks described below.
Moving Individuals at Different Stages
The practice of “coral gardening” is increasing in the Caribbean and elsewhere (Young et al., 2012), applied to a variety of species (Rinkevich, 2014), with well-developed propagation techniques (Barton et al., 2017). While outplanting is occurring at ecologically relevant scales (Lirman and Schopmeyer, 2016), cost can remain a barrier to scaling up in regional restoration projects (Young et al., 2012). In addition, while the drivers of nursery and outplanting success are well studied, how the overall project design and ecological processes affect project success is less well known (Ladd et al., 2018). The cost and complexity will inevitably increase with the project scale in terms of distance transport, number of colonies moved, and number of repeated transports, such that we expect the feasibility of assisted gene flow and assisted migration to be significantly less than that of introduction to new areas (as depicted in Figure 4.1).
While technically feasible, relocation practices are far from perfected. Previous efforts have shown that direct translocation often results in mortality of transplants, generally in the range of 30% (Piniak and Brown, 2008). Losses may occur due to stress associated with handling and increase with both distance and time from collection to transplantation (Naughton and Jokiel, 2001). Problems also result from environmental differences between the donor and receiving sites. Site characteristics such as wave exposure, turbidity, water quality, and substrate stability affect the survival of transplants (Jokiel and Naughton, 2001). Corals taken from deeper depths placed into shallower waters may bleach due to increases in temperatures and light intensities (Lenihan et al., 2008).
While moving and propagating fragments is feasible now, the techniques for fertilizing and moving eggs in captivity are still under development. This approach requires freezing gametes (see Coral Cryopreservation in Chapter 3) or in-tank spawning (see Gamete and Larval Capture and Seeding in Chapter 3). Recent advances (e.g., SECORE and CORALZOO projects) include successful fertilization and settlement of larvae that are now part of active restoration projects, with ongoing research into survival likelihood and its drivers (Lirman and Schopmeyer, 2016; Meesters et al., 2015).
Identifying Whether and What Species and Individuals to Move
The feasibility of identifying the level of stress-tolerance or stress-susceptibility depends on the approach (see comparison of proxy-based and direct approaches in Morikawa and Palumbi, in press). Using environmental proxies will require information on past environmental conditions, which is attainable at coarse spatial scales but potentially more difficult to attain at the finer spatial scales relevant to local acclimation and adaptation. Using biotic characteristics or direct measurements of stress tolerance might require more information gathering. Therefore, environmental proxies might be more feasible to use but will also confer greater uncertainty as they provide indirect rather than direct measures of expected stress tolerance.
For the goal of preventing extinction of climate-vulnerable species, identification of such species can rely on direct or indirect indicators of vulnerability. Direct indicators such as mortality under past stress, population declines, range contractions, and range shifts slower than other species in the community require ongoing monitoring of coral systems, which is feasible but costly. Where long-term monitoring data on population sizes are not present, population trends can be inferred from genetic markers (e.g., A. Chan et al., 2018). In the absence of such direct indicators, indirect indicators of stress susceptibility such as coral morphology are readily available but confer greater uncertainty. Identification of dispersal-limited species, an indicator of both climate vulnerability and the relevance of managed relocation, through genetic data and/or oceanographic models of larval dispersal is feasible but requires investment. For identifying species with low thermal tolerance, dispersal limitation, or other such indicators of vulnerability, the Coral Traits Database (coraltraits.org; Madin et al., 2016) includes scoring of relevant traits such as bleaching susceptibility, fecundity, generation time, spawning date, and egg size.
Beyond the level of stress tolerance or vulnerability, a challenge to identifying a target species is the difficulty in distinguishing coral species in the field due to a high degree of plasticity and polymorphism as well as a high degree of hybridization in corals (Stat et al., 2012). High phenotypic plasticity, including in skeletal morphology, has long been recognized as a challenge to coral taxonomy (Bernard, 1902; Tisthammer and Richmond, 2018). Genetic analyses in the laboratory can differentiate coral species that are visibly similar and reveal cryptic species within existing species delineations (e.g., Knowlton et al., 1992; Ladner and Palumbi, 2012; Warner et al., 2015), hybridization between delineated species (e.g., Forsman et al., 2017; Szmant et al., 1997), and that delineated species are themselves hybrids of other species (e.g., Vollmer and Palumbi, 2002). Cryptic species and hybridization lead to conflicting conclusions regarding species delineation for well-studied genuses (e.g., Acropora, Orbicella [formerly Montastraea], Pocillopora), with ongoing debate over the appropriate molecular markers to distinguish species (Stat et al., 2012). This is especially true when closely related species share many polymorphisms (e.g., Ladner and Palumbi, 2012), resulting in good species resolution only when large numbers of polymorphic markers are used. New approaches with genome-wide data tend to show clearer distinctions among even closely related coral species (e.g., Rose et al., 2018), but are costly and slow. In some cases, identification of cryptic species complexes led eventually to morphological methods that could identify them (e.g., Orbicella; Knowlton et al., 1992). In other cases, some morphologically identical colonies are in different species but some morphologically divergent colonies are not (e.g., Pocillopora damicornis; Pinzón and LaJeunesse, 2011; Torda et al., 2013).
Given stress-tolerant target individuals or species, whether the stress tolerance observed in the source location will be analogous to that in the target location will depend on three key unknowns. First, while both genetics and plasticity contribute to stress tolerance (Bay and Palumbi, 2014, 2015; Liew et al., 2018b), their relative roles are unresolved for most corals (with exceptions; Palumbi et al., 2014). Second, while stress tolerances such as thermal tolerance and disease resistance are holobiont properties that arise from a combination of the coral host, symbiotic zooxanthellae, and microbiome, their relative contributions are typically unresolved. Third, how much transplanted corals adopt local symbiotic zooxanthellae and microbiomes, as compared to maintaining source-location symbionts, is uncertain (in Smith et al., 2009, aquaria corals typically maintain native symbionts, but whether this carries over to outplanted corals is unknown) and will inevitably depend on the managed relocation approach, stage at relocation, and coral life history.
Identifying Where and When to Move
Information necessary to parameterize the species distribution and demographic models that can inform decisions of when and where to move is attainable but might require significant investment. This includes existing species distributions and local-scale environmental conditions such as temperature for SDMs (Elith and Leathwick, 2009), and size- and environment-dependent survival, reproduction, and growth for demographic models (Edmunds et al., 2014). A challenge to applying SDMs to managed relocation is uncertainty and variability in their ability to accurately predict future suitable habitat (Dobrowski et al., 2011). In addition, most predictive models do not mechanistically incorporate the capacity for species to acclimatize or adapt to their environments (Sgrò et al., 2011), although recent efforts have attempted to do so for corals at the global scale (Logan et al., 2014) and at regional scales (e.g., Baskett et al., 2009; Bay et al., 2017; Matz et al., 2018).
Managed relocation decisions will further rely on additional information regarding population and community characteristics. For example, the decision of how many to relocate will rely on knowledge of threshold population sizes for expected increase (as it might depend on Allee effects or demographic stochasticity; Gilpin and Soule, 1986) or coral densities for expected reef persistence (as it might rely on interactions with macroalgae; Mumby et al., 2007). Quantitatively precise information on these thresholds is unlikely to be available, which does not impede project implementation but does introduce uncertainty. In addition, the decision for where to translocate might depend on the degree of site isolation as described above, where attaining the relevant genetic and/or oceanographic data on connectivity is feasible (and exists for some species; e.g., Acropera palmata in Baums et al., 2005, and Orbicella annularis in Foster et al., 2012) but may require investment.
Potential Scale
The distance between a source population and a target location ranges from within a population’s range to across ocean basins, depending on the relocation type (see Figure 4.1). Typically the distinction between relocation types is based on the spatial scale of a species range, but many coral species’ boundaries span entire (and sometimes multiple) ocean basins (e.g., many Caribbean species are basin-wide). Therefore, the appropriate boundaries depend on the scale of genetic differentiation.
The practical scale of implementation at a new location is on the scale of meters to kilometers, similar to the scale of coral restoration efforts.
The temporal scale in terms of both duration and frequency can range from a one-off relocation to a sustained program over several
years, depending on the management approach, goals, risk perception, resources, and success. More frequent and longer relocations will buffer against the risk of a catastrophic event negating the project and increase success likelihood through repeated trials, but it will also increase the risk relocation described below. In addition, the duration of a relocation program will depend on the trajectory of continued greenhouse emissions or other such stressor, with the potential for “conservation reliance” (i.e., requiring continued management intervention) (Scott et al., 2010) unless relocations or other management actions can establish reefs along connectivity gradients that eventually allow natural dispersal to occur.
Scaling up to multiple species might include considerations of whether to translocate additional functional groups that are important to the maintenance of coral-dominated systems. Two such functional groups are coral exosymbionts (e.g., crustaceans that clean and guard corals) (McKeon et al., 2012; Stier et al., 2012) and herbivorous invertebrates and fish. Exosymbiont and herbivore managed relocation is a consideration if factors that limit natural coral dispersal to the recipient location also limit those of the target functional group. For assisted migration or introduction to new areas, moving exosymbionts or herbivores might be relevant if the communities of these functional groups in the recipient location cannot associate with the translocated coral(s) due to a high degree of host specialization. The value of active relocation is also dependent on the stress tolerance (for assisted gene flow) and natural dispersal capabilities of these organisms. Exosymbionts do show host specificity (Stella et al., 2010) and local-scale variation (Rouzé et al., 2017), but information on the drivers of their distribution is limited. Expected scales of herbivorous fish dispersal are on the order of 10-100 kilometers (Cowen et al., 2006), and the impacts of climate change on their growth, survival, and reproduction vary from negative to positive, with the impact of habitat loss from coral bleaching likely outweighing any direct thermal stress (Munday et al., 2008). Herbivorous fish can also be impacted by thermal stress independent of coral mortality, as observed in the most recent bleaching event on the Great Barrier Reef (Stuart-Smith et al., 2018). Therefore, while a potential consideration, whether relocation of exosymbionts and herbivores is necessary for coral reef ecosystem response to climate change is uncertain.
Risk
A key risk for all managed relocation types is the introduction of nonnative pathogens, parasites, algae, microbes, commensal invertebrates, and corallivores (e.g., gastropods) that might overwhelm local controls on their abundance. Such an outcome poses a risk to both the translocated type or species and the entire recipient community. In addition,
the translocated type itself might become “invasive” or predominant, especially if relocation releases it from a natural enemy or predator. The resulting reductions in diversity caused by dominance of an invasive translocated type could reduce adaptive capacity or resilience (Levin and Lubchenco, 2008) of the community and, in extreme cases, newly predominant types can alter ecosystem structure and function.
The risk of invasion likely increases with the distance of relocation: Intercontinental invasions are more frequent than intracontinental invasions (Mueller and Hellmann, 2008), and invasive species have greater invasiveness and greater impact with lower relatedness (measured as phylogenetic distance) to the native community (Strauss et al., 2006). The degree of invasive impact also depends on the novelty of the traits and therefore the ability for the introduced species or type to alter ecosystem processes (Wardle et al., 2011). Invasiveness increases with number of individuals released and number of introductions (Kolar and Lodge, 2001), two factors that also drive relocation success (Fischer and Lindenmayer, 2000), such that a manager will face a tradeoff between maximizing success likelihood and minimizing invasive risk. In addition, invasiveness has a greater impact on historically isolated communities (Richardson and Pyšek, 2006), which can be particularly relevant to managed relocation, depending on the goal. The most dominant taxa in a database of marine invasive species are crustaceans, mollusks, algae, fish, and annelids (Molnar et al., 2008). Tropical marine systems generally experience lower rates of introduction than temperate systems, but invasive algae can still be particularly harmful (Coles and Eldredge, 2002; Padilla and Williams, 2004).
Accidentally introduced diseases pose a particular risk to coral reefs given that diseases can be a major cause of coral mortality with ecosystem-wide effects, especially in the Caribbean. Additionally, the stress caused both by the environmental conditions being addressed by the relocation, as well as the stress on the coral during relocation, may promote disease transmission. Many coral diseases are not host-specific (Green and Bruckner, 2000) such that relocation could lead to spread to new hosts in a recipient community. The regional heterogeneity in disease incidence, likely due to a mix of environmental variability in thermal anomalies and historic exposure-dependent disease resistance (Rosenberg et al., 2007; Ruiz-Moreno et al., 2012), means that local-scale movement has the potential to enhance disease spread.
Compared to invasiveness of associated organisms and diseases, invasiveness of the translocated coral type or species might pose less of a risk for coral reefs. While invasiveness is difficult to predict from traits (Kolar and Lodge, 2001; Richardson and Pyšek, 2006), it is associated with widespread species (Richardson and Pyšek, 2006) and related characteristics
such as dispersal ability (Sakai et al., 2001). In contrast, managed relocation for persistence under climate change typically focuses on range- and dispersal-limited species. There are at least six known species of hard corals that have been unintentionally introduced across oceanic regions: Tubastrea coccina, Tubastrea micranthus, Tubastrea tagusensis, Fungia scutaria, Oculina patagonica, and Siderastrea glynni (Coles and Riegl, 2013; Glynn et al., 2016). The three Tubastrea congeners (orange cup corals) that are now considered to be invasive are nonzooxanthellate corals, and the remaining zooxanthellate corals have not spread. The lack of invasive zooxanthellate corals might be due to a lack of propagule pressure; while the majority of marine invasives are from ballast water and aquaculture (Molnar et al., 2008), the majority of tropical marine invasives are from aquaria release (Padilla and Williams, 2004).
Beyond the coral host, the symbiotic zooxanthellae and microbiomes that comprise the coral holobiont also pose a potential to become invasive. Relocation of zooxanthellae and the microbiome associated with a coral host might be a component of managed relocation goals given that stress tolerance and disease resistance are holobiont properties (Baker, 2003; Baker et al., 2004; Berkelmans and van Oppen, 2006; Teplitski and Ritchie, 2009; Ziegler et al., 2017), but spread of these associated organisms to other corals in the target location might alter the co-evolutionary relationships between corals and their symbionts. Genetic evidence suggests the potential for invasive zooxanthellae across ocean basins (LaJeunesse et al., 2016; Pettay et al., 2015).
A risk associated with any translocation is potential damage to reef habitats when corals are collected or when they are placed on new reefs. Collecting can create problems for very rare coral species. An example is the rare pillar coral Dendrogyra cylindrus, which has low genetic diversity in the Caribbean, and has seldom been observed to recruit sexually (Marhaver et al., 2015). Collecting a range of genotypes for nursery propagation entails potential risk to the very small natural populations. Another risk might be seen if reef habitats were converted to dedicated coral nurseries at large scales, potentially removing natural populations.
A final risk to ecosystem function is tradeoffs in tolerance to multiple stressors or between stress tolerance and other traits (e.g., growth and reproduction), which can then reduce the performance of the translocated types. On the species level, stress-tolerant morphologies (e.g., massive corals) often have lower growth and reproduction than stress-susceptible morphologies (e.g., branching corals; Darling et al., 2012). As noted above, an unknown is whether increased vigor for one stressor in translocated colonies might lead to decreased vigor for other traits (e.g., potential tradeoff between heat resistance and disease resistance).
Additional risks for each relocation type follow:
Assisted gene flow incurs three additional risks (Aitken and Whitlock, 2013; Weeks et al., 2011). First, assisted gene flow to the wrong place or at the wrong time might incur “gene swamping,” where the input of translocated maladapted genes may dominate over existing better-adapted genes, with a decline in total genetic variation across locations. While gene swamping only occurs above a critical value of migration (i.e., relocation input) relative to selection strength, exceeding this value is more likely with small populations (Lenormand, 2002). Second, assisted gene flow might disrupt local adaptation to nonclimatic factors. For example, relocations might lead to corals spawning at suboptimal times, or a mismatch in spawning between relocated and local corals, which could reduce fitness, fertilization success, or the likelihood of introgression of stress-tolerant genes into local populations. The potential for this mismatch on the spawning time cues and degree of variation in synchrony across locations, varies by species and region (Baird et al., 2009b). Third, assisted gene flow might incur outbreeding depression for hybrids between translocated and native colonies. One transplant experiment of local, nonlocal, and local/nonlocal hybrids does show the potential for reduced survival of hybrid and nonlocal types and therefore either outbreeding depression or disruption of local adaptation in corals on scales of hundreds of kilometers (van Oppen et al., 2014).
Assisted migration incurs the additional risk of interspecific hybridization and loss of a species’ identity. Hybridization between introduced species and natives can be a source of invasiveness and a mechanism by which invasives drive biodiversity loss, especially for small, historically isolated populations (Rhymer and Simberloff, 1996; Sakai et al., 2001). This risk is clearly relevant to corals, where hybridization readily occurs (Forsman et al., 2017; Szmant et al., 1997; Vollmer and Palumbi, 2002). How one considers the impacts of hybrids for assisted migration depends on the goal: Hybridization may result in loss of a species, but it may aid in maintaining coral-dominated reefs where species is not a concern. See the Managed Breeding section in Chapter 2 for further discussion.
Introduction to new areas does not incur any unique risk not already described above, but it does have an elevated risk of disease and invasive species spread, especially as all known invasive corals involve cross-basin invasions (Coles and Riegl, 2013).
Limitations
A number of limitations might drive failure of relocations to establish ecologically meaningful populations in the new location (i.e., poor survivorship, growth, or reproductive success). Failure of managed relocation might occur due to moving the target organism(s) between the wrong places or at the wrong time, whether due to stochasticity (e.g., a catastrophic event, such as the 2014-2015 back-to-back bleaching events that disrupted a Florida Keys coral restoration project; Lirman and Schopmeyer, 2016), a knowledge gap, and moving without key mutualists (Hewitt et al., 2011). Key knowledge gaps for relocations generally concern what drives species distributions (including the role of species interactions), species responses to novel environmental conditions, local-scale impacts of climate change, natural scales of long-distance dispersal, and the scale of local adaptation (Chauvenet et al., 2013; Gallagher et al., 2015; Hewitt et al., 2011; Kreyling et al., 2011; McLachlan et al., 2007; Rice and Emery, 2003).
The life stage used for relocation (e.g., larval versus fragment release) can influence the likelihood of success and trades off with potential risks. Earlier relocation might increase the likelihood of failure due to higher mortality at earlier life stages, while later relocation might increase the risk of invasiveness or gene swamping due to increased establishment likelihood. For corals, earlier life stages can also have greater flexibility in symbiosis (Little et al., 2004), such that earlier relocation might increase the likelihood of shifting to local symbionts (Quigley et al., 2017, 2018a). Shifting to local symbionts might decrease expected stress tolerance if symbionts have different stress tolerance between source and target locations (Ulstrup et al., 2006), but it might also increase relocation success if local symbionts confer adaptation to local conditions. Given that thermal tolerance arises from a combination of coral host, symbiont, and microbiome characteristics (Baker, 2003; Baker et al., 2004; Bay and Palumbi, 2014; Berkelmans and van Oppen, 2006; Loya et al., 2001; Ziegler et al., 2017), uncertainty in how relocation affects the symbiont and microbiome contributes to uncertainty in the expected stress tolerance of the translocated type in the target location. The earliest possible relocation, not yet feasible but in development, is at the gamete stage with spawning in captivity and then release of fertilized eggs (Craggs et al., 2017). Using local eggs with nonlocal sperm in assisted gene flow would eliminate the risk of accidentally introducing diseases or invasive species from associated organisms, as well as reduce the risks of gene swamping and disruption of local adaptation to nonclimatic factors. The translocated hybrids would likely be more adapted to nonclimatic conditions, but might also confer lower stress tolerance, as compared to translocating fully nonlocal types.
In terrestrial translocations for general conservation purposes (e.g., reintroductions within historical ranges), about half of the cases with
sufficient data to evaluate efficacy are successful (Dalrymple et al., 2012; Dodd and Seigel, 1991; Fischer and Lindenmayer, 2000), and coral reintroductions exhibit analogous success rates (Young et al., 2012). Therefore, both irreducible (present regardless of knowledge) and reducible (knowledge-dependent) uncertainties may limit translocation success (Ladd et al., 2018). Ongoing climate change can magnify such uncertainties. Drivers of translocation success in terrestrial systems include using wild (rather than captive) sources, large releases, and removal of the original causes of decline (Fischer and Lindenmayer, 2000). In corals, removal of causes of decline might include reduction in nutrient, sediment, and other pollutant loads as well as restoration or protection of ecologically functional herbivorous fish and invertebrate populations (Ban et al., 2014; Pandolfi et al., 2003; Wilkinson, 1999; Zaneveld et al., 2016). Similarly, translocation success may benefit from interventions that decrease exposure to climate stresses. Reduction of such local stressors might also reduce the risk of disease spread (Green and Bruckner, 2000; Rosenberg et al., 2007).
In summary, a number of challenges can limit the success of managed relocation, including the risks and infrastructure needs described in other sections of the chapter (see Table 4.1). In addition to the cost of a failed project, failure could represent loss to the source population, although nursery grow-out techniques that develop larger populations from small fragments mitigate such costs (Lirman and Schopmeyer, 2016). Many of these limitations depend on the management approach (e.g., quarantining of fragments or relocation of gametes reduce risks of non-native species and pathogens) or knowledge availability (e.g., increased knowledge of relative plastic and genetic, and relative coral, zooxanthellae, and microbiome contribution to thermal tolerance can reduce the likelihood of no conference of stress tolerance between source and target locations), while others are irreducible (e.g., storm events following relocation).
Infrastructure
Managed relocation efforts and activities are time and labor intensive. Extensive infrastructure including boats, seawater facilities, and recipient site preparation are critical to success. The collection and transportation of corals can result in colony mortality and require careful planning to reduce handling, transit time, and physical damage. Containers with ample water volumes, temperature control, and circulation/aeration are essential for the survival of colonies and fragments collected from field sites (Precht, 2006). When moved to and maintained in land-based facilities, flowthrough or recirculating seawater systems are needed to allow collected corals to recover from the stress of collecting, and to support
TABLE 4.1 Summary of Limitations to Managed Relocation
| Limitation Category | Limitations |
|---|---|
| Knowledge requirements |
|
| Technical requirements |
|
| Sources of failed relocation |
|
| Risks |
|
continued growth and in the case of translocating gametes, undergo successful gametogenesis and spawning or planulation. Such facilities require dedicated technical staff and redundancy in pumps and the electrical supply to ensure against losses. In situ collecting and outplanting activities are also labor intensive and require numerous scuba divers and all of the associated operational and safety equipment. Volunteers can be used to supplement professional staff, but they require substantial training and supervision.
Beyond the technical infrastructure, additional requirements arise from gathering the data necessary for the managed relocation decision-making process and monitoring. Aspects of the decision-making process that require site, system, or species-specific data include identification of target species, locations, and timing of relocation as well as assessment of risks such as accidentally invasive species and pathogens.
























