18
Fluid Metabolism at High Altitudes
Inder S. Anand1 and Y. Chandrashekhar
INTRODUCTION
Ascent to high altitude is invariably associated with changes in fluid and electrolyte composition in humans and animals. Although these changes are detectable even in those who do not exhibit any of the ill effects of high altitudes, the most florid alterations occur in those who develop high altitude-related illness (Anand et al., 1990; Bärtsch et al., 1991a; Hackett et al., 1981; Hecht et al., 1959; Levine et al., 1989). There is also evidence that subjects who develop acute mountain sickness at high altitudes may be identified prior to the onset of symptoms by distinct changes in fluid balance and in hormones regulating salt and water metabolism (Bärtsch et al., 1991a; Singh et al., 1969). One of the seminal observations in this field was made in Indian soldiers serving at high altitudes (Singh et al., 1969). They found that soldiers predisposed to develop high-altitude pulmonary edema had a reduction in urine output before they developed respiratory symptoms. This finding led to an intense study of fluid metabolism at high altitudes.
A number of high altitude-related syndromes are associated with abnormal salt and water retention. The first such syndrome was described in cattle grazing at high altitudes. This condition, called brisket disease because of fluid retention in the brisket area, is characterized by severe congestive heart failure due to pulmonary arterial hypertension (Hecht et al., 1959). Some degree of peripheral edema is often seen in temporary visitors to high altitudes. Hackett et al. (1981) noted that fluid retention is common to all forms of acute mountain sickness and postulated a spectrum of derangements in fluid metabolism in these conditions. Fluid retention also occurs in two high altitude-related syndromes recently described by these authors: Infants born at low altitude in mainland China and taken to reside at high altitudes in Tibet develop a fatal condition called subacute infantile mountain sickness (Sui et al., 1988). This condition is characterized by severe hypoxic pulmonary hypertension leading to congestive heart failure. The second condition, adult subacute mountain sickness, occurs in adults exposed to extreme altitude and presents with severe salt and water retention without significant pulmonary hypertension (Anand et al., 1990). Therefore, changes in fluid metabolism at high altitudes appear to have important pathogenetic implications and merit detailed study.
PROBLEMS WITH AVAILABLE DATA
One of the major difficulties in making a meaningful interpretation of fluid metabolism at high altitudes is the lack of reliable data. Because experiments at high altitudes are difficult to organize, various approaches have been used to predict the response at high altitudes. These include acute and chronic experiments in hypobaric hypoxic chambers and use of low fraction of inspired oxygen (FIO2) gas mixtures at sea level. Studies during ascent (trekking, flight, etc.) have been made at different altitudes and after varying periods of time at high altitudes. Therefore, the lack of any uniformity in the degree or type of hypoxia makes the comparison of these studies very difficult. Moreover, serial measurements of body fluid compartments on the same subjects have seldom been made despite the fact that changes in fluid metabolism appear to depend on the duration of stay at high altitudes (Hannon and Rogers, 1975; Jain et al., 1980, 1981). Furthermore, some of the older studies were carried out with inadequate methodology, which makes interpretation of data difficult. Thus it is not uncommon to find the same group of investigators reporting different conclusions in successive studies because of methodological differences.
Confounding Variables
Exercise and increased physical activity soon after ascent to high altitudes can significantly alter fluid and hormonal responses (Milledge, 1992). While acute exposure to high altitudes may be associated with dehydration, increased physical activity soon after ascent to high altitudes causes significant salt and water retention (Withey et al., 1983). Therefore, studies that did not control for physical activity may have tested the effects of exercise plus hypoxia, rather than hypoxia alone. Likewise, it is unclear which studies in the literature excluded subjects who might have had early features of acute mountain sickness (AMS). Like exercise, AMS has been shown to have totally different effects on salt and water metabolism as compared to hypoxia alone (Milledge, 1992). Finally there is evidence that fluid responses at high altitudes may be species dependent (Ashack et al., 1985). Thus much of the data in the literature is confusing, controversial, and inconclusive. This chapter identifies some of the major studies on this subject and tries to present a consensus view of the effects of high altitudes on fluid metabolism.
FACTORS INFLUENCING RESPONSES AT HIGH ALTITUDES
A number of factors influence fluid metabolism at high altitudes. The most important ones are the degree of hypoxia, cold, hypobaria, and physical activity. Most workers feel that hypobaria by itself has little effect on fluid metabolism (Epstein and Saruta, 1972; Heyes et al., 1982). The effect of cold is discussed elsewhere (see Freund and Sawka, Chapter 9 in this volume) and will not be considered here. However, details of the interaction between cold and hypoxia remain to be determined.
FLUID METABOLISM AT HIGH ALTITUDES
Fluid metabolism at high altitudes has been studied in several ways. A frequent approach has been the use of simple intake-output data. Some studies have inferred changes in body fluid from the change in weight and energy balance. The best data, however, come from studies of body fluid compartments using isotope dilution techniques. Table 18-1 summarizes the findings of major published studies where body fluid compartments were measured at high altitudes. Most of these studies only report the effects of acute exposure, that is, changes occurring during the first 2 to 3 weeks at high altitudes (Frayser et al., 1975; Hannon et al., 1969; Hoyt et al., 1992; Jain et al., 1980, 1981; Krzywicki et al., 1971; Singh et al., 1990; Surks et al., 1966). Very few studies followed the effect of prolonged stay at high altitudes (Anand et al., 1993; Hoyt et al., 1991; Phillips et al., 1969; Singh et al., 1990). Human studies
TABLE 18-1 Some Major Studies Detailing Alterations in Body Fluid Compartments During Ascent or Stay at High Altitudes
|
Authors |
Study Details |
Comments |
|
Acute and subacute responses at moderate altitudes (< 4,500 m [14,764 ft]) |
||
|
Surks et al., 1966 |
Five males, 19–23 yr, studied at SL and on days 4 and 8 at 4,267 m (14,000 ft). Ascent was rapid. Used ISA, Evan's Blue, D2O. |
Body weight and PV decreased. TBW data unreliable. Fat accounted for most of weight loss. |
|
Hannon et al., 1969 |
Nine males, 20–24 yr, studied at SL and on days 3, 7, and 14 at 4,300 m (14,110 ft). Ascent was rapid. TBW: BD, 4-AA, and D2O. |
TBW increased at 2 weeks, PV and ECV reduced, redistribution from ECV to ICV. D2O data unreliable. Weight loss primarily due to loss of fat. |
|
Kryzwicki et al., 1971 |
Two groups of six males studied at SL, day 6 at 4,300 m, and SL again. Ascent was rapid. TBW: D2O. ECV: thiocynate. |
TBW and ICV reduced, ECV increase insignificant. Hypohydration even with good intake. |
|
Frayser et al., 1975 |
Eight males, 14–37 yr, studied at SL, on day 5 at 2,926 m (9,600 ft), and on days 5 and 10 at 5,334 m (17,500 ft). Ascent was rapid with halt at 2,926 m (9,600 ft) for nonacetazolamide group. TBW: 4-AA. ECV: thiocynate, PV: Evan's Blue. |
All compartments uniformly reduced. |
|
Jain et al., 1980 |
Eighteen male soldiers, lowlanders, mean age 27 yr. Studied at SL and on days 3 and 12 at 3,500 m (11,483 ft). No exercise at high altitudes. TBW: 3H2O. ECV: sulphate. PV: ISA. |
All compartments reduced including ECV. 80% weight loss explained by water loss. |
were made mainly at moderate altitudes of 3,500 to 4,500 m (11,483 to 14,764 ft). Only one study investigated extreme altitudes (over 6,000 m [19,685 ft]) (Anand et al., 1993), and one reported the effect of descent from high altitudes (Jain et al., 1981).
Moderate Altitudes
Probably the most comprehensive human data on body fluid compartments at high altitudes come from studies on Indian soldiers (Jain et al., 1980, 1981; Singh et al., 1986, 1988, 1990). These investigators had the unique advantage of a captive population that they could study at sea level and then again, under strict conditions of complete rest, for a period of up to 12 days after being flown to 3,500 m (11,483 ft). The soldiers lost about 3 percent of body weight during the first 3 days, with a further decrease in weight of about 1 percent during the next 10 days. Total body water (TBW) decreased by about 3.5 percent during the first 3 days but showed no further significant decrease until day 12. Extracellular volume (ECV) fell by a much smaller amount. The most significant change was seen in the plasma volume (PV), which was reduced by about 8 percent on day 3 with a further decrease over the next 10 days. When these changes in body fluid compartment are expressed as percent of body weight, no change in the calculated TBW is noted (Figure 18-1), suggesting that the decrease in TBW is in proportion to the decrease in weight.
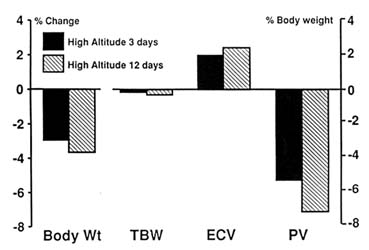
FIGURE 18-1 Changes in body fluid compartments expressed as percent body weight in soldiers taken to 3,500 m (11,483 ft). TBW, total body water; ECV, extracellular volume; PV, plasma volume. SOURCE: Adapted from Jain et al. (1980).
The ECV increases but not significantly, and PV remains reduced even when expressed in this manner. The problem with interpreting such data is that it is not known exactly what happens to body composition as subjects lose weight at high altitudes. If weight loss is entirely due to loss of fat, then the TBW should remain unchanged but show an increase when expressed as a percent body weight. The finding of normal TBW as percent body weight would, therefore, suggest hypohydration. However, if weight loss was only due to loss of muscle mass, then TBW should fall roughly in proportion to body weight. Unfortunately, body composition data on humans at high altitudes are confusing and not reliable (Consolazio et al., 1968; Rose et al., 1988; Surks et al., 1966; Westerterp et al., 1992). Data from Operation Everest II showed that only one-third of the decrease in body weight at simulated altitude was attributable to loss of fat and the remaining two-thirds to loss in fat-free mass (Rose et al., 1988).
Analysis of the carcasses of animals taken to high altitudes is more reliable for assessing changes in body composition (Chinn and Hannon, 1969; Christensen et al., 1975; Hannon and Rogers, 1975; Schnakenberg et al., 1971). Figure 18-2, drawn from data of Hannon and Rogers (1975), shows body composition of mice in Denver (1,600 m [5,249 ft]) and after being kept at a simulated altitude of 4,300 m (14,110 ft) for 3 and 7 days. Carcass analysis showed that mice lost weight during the first 3 days at high altitudes but not thereafter. After 3 and 7 days at high altitudes, TBW as a percent of
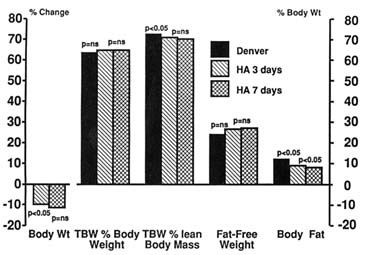
FIGURE 18-2 Carcass data of mice in Denver (1,600 m [5,249 ft]) and after being kept at a simulated altitude of 4,300 m (14,110 ft) for 3 and 7 days. For body weight, p compared to baseline at Denver. ns, not significant; HA, simulated altitude of 4,300 m (14,110 ft); TBW, total body water. SOURCE: Adapted from Hannon and Rogers (1975).
body weight did not change from the Denver value but fell significantly when expressed as a percent of lean body mass. Fat-free mass remained unchanged, but the animals lost fat throughout the stay at high altitudes. These findings, therefore, confirm that although most of the weight loss at high altitudes was due to loss of body fat, the animals also lost body water, became dehydrated, and remained so throughout the 7-d experiment.
In an earlier experiment, the same group (Chinn and Hannon, 1969) found that rats kept at similar altitudes for a longer period of time (26 days) did not show any decrease in TBW even when it was expressed as a percent of lean body mass (Figure 18-3). Weight loss was due to loss of body fat with no change in fat-free mass. Unfortunately, serial measurements were not made on these rats. Therefore, it is not clear whether these animals had initial dehydration from which they had recovered.
In a careful study of goats kept at a higher simulated altitudes (5,500 m [18,045 ft]) for 16 days, Hoyt et al. (1991) found that weight loss was accompanied by a loss of TBW, suggesting dehydration (Figure 18-4). They also found a shift of body fluids so that the ECV increased at the expense of the intracellular volume. This result is in sharp contrast to the findings of other authors (Hannon et al., 1969; Jain et al., 1980, 1981; Singh et al., 1990). Hoyt and coworkers (1991) also found that PV decreased, but the blood volume (BV) did not change because the hematocrit increased.
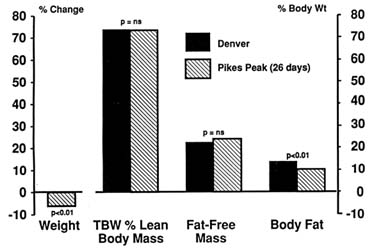
FIGURE 18-3 Carcass data of rats in Denver (1,600 m [5,249 ft]) and after being kept at Pikes Peak (4,300 m [14,110 ft]) for 26 days. ns, not significant; TBW, total body water. SOURCE: Adapted from Chinn and Hannon (1969).
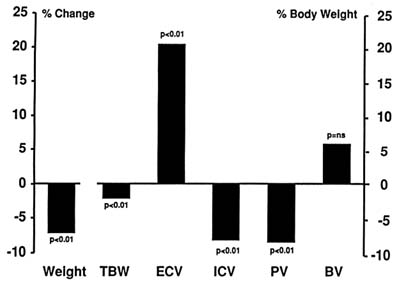
FIGURE 18-4 Changes in body fluid compartments in goats kept at 5,500 m (18,045 ft) for 16 days. ns, not significant; TBW, total body water; ECV, extracellular volume; ICV, intracellular volume; PV, plasma volume; BV, blood volume. SOURCE: Adapted from Hoyt et al. (1991).
Phillips et al. (1969) carried out one of the few studies that tested the effect of prolonged stay at simulated extreme altitudes. They found that sheep kept at 6,200 m (20,341 ft) for up to 32 days had an initial weight loss that later stabilized (Figure 18-5). At 10 days, sheep were hypohydrated with a significant decrease in TBW, but by 32 days, the animals had regained all the lost water. PV, unlike results in most other studies, did not change and BV increased. Therefore, the initial response of the body even at extreme altitudes is hypohydration lasting a few days. Thereafter the body fluids normalize. Whether further stay at that altitude would have caused fluid retention was not tested.
Singh et al. (1990) studied body fluid compartments of soldiers who had stayed at high altitudes (3,500 m [11,483 ft]) for 6 months and compared the findings with those seen in normal subjects at sea level. They found that despite the fact that the weight of these subjects continued to remain low, their TBW and ECV as a percent of body weight were greater than normal. Although this study was not made on the same subjects at sea level and high altitudes, the findings suggest that with chronic exposure to moderate altitudes, subjects are no longer hypohydrated, instead tend to retain fluid, and might eventually become hyperhydrated. However, plasma volume still remains markedly reduced.
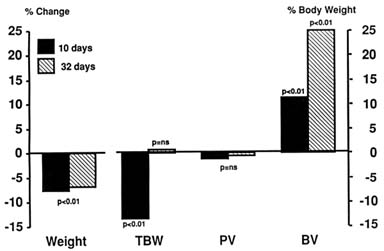
FIGURE 18-5 Changes in body fluid compartments in sheep kept at simulated extreme altitude (6,200 m [20,341 ft]) for 32 days. ns, not significant; TBW, total body water; PV, plasma volume; BV, blood volume. SOURCE: Adapted from Phillips et al. (1969).
Extreme Altitudes
Although it is generally believed that humans are incapable of surviving at extreme altitudes for prolonged periods of time (Pugh, 1962; West, 1984), the recent experience of warfare at altitudes above 6,000 m (19,865 ft) on the Indian subcontinent has belied that impression (Anand and Chandrashekhar, 1992; Anand et al., 1990). Prolonged stay at that altitude causes subacute mountain sickness in some subjects (Anand et al., 1990). This syndrome is characterized by severe edema, ascites, and even generalized anasarca resembling severe congestive heart failure. The pathogenesis of this condition remains unknown, but hypoxic pulmonary hypertension causing congestive heart failure is unlikely to explain the syndrome. This laboratory measured body fluid compartments in a group of asymptomatic soldiers who had been at an altitude of over 6,000 m (19,865 ft) for at least 10 weeks (Anand and Chandrashekhar, 1992). TBW in these soldiers was increased by about 20 percent above normal, PV was 30 percent above normal, hematocrit was 40 percent above normal, and calculated BV was nearly 80 percent above normal (Figure 18-6). These data suggest that although humans can survive at extreme altitudes for prolonged periods of time, many develop fluid retention, and a small subset of them develop subacute mountain sickness. Even those who remain well and asymptomatic tend to retain significant amounts of salt and water.
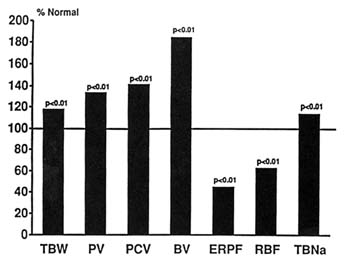
FIGURE 18-6 Body fluid compartments in normal subjects living at extreme altitudes (> 6,000 m) for a prolonged period (> 10 weeks). TBW, total body water; PV, plasma volume; PCV, packed cell volume; BV, blood volume; ERPF, effective renal plasma flow; RBF, renal blood flow; TBNa, total body sodium. SOURCE: Adapted from Anand et al. (1993).
Effect of Exercise
Milledge and his coworkers (Withey et al., 1983) have shown that physical activity soon after ascent to high altitudes has a dramatically opposite effect on fluid metabolism than does ascent alone. Instead of developing hypohydration, subjects retain sodium and water when ascent to high altitudes is combined with hill walking. In contrast to subjects who do not exercise at high altitudes, plasma volume in these subjects increases during the exercise period, causing a fall in hematocrit.
To summarize, acute exposure of humans and animals to moderate altitudes, in the absence of exercise, leads to weight loss and hypohydration. TBW and PV are reduced, and there may be a shift of body fluids between the intra- and extracellular compartments. These changes tend to return towards normal within weeks. Prolonged exposure to high altitudes lasting months may cause fluid retention with an increase in TBW, but the body weight and PV continue to remain low. Physical activity soon after ascent to high altitudes leads to fluid retention. Acute effects of extreme altitudes are not known, but prolonged stay at extreme altitudes may lead to severe salt and water retention.
DETERMINANTS OF FLUID METABOLISM
The major determinants of fluid metabolism at high altitudes include fluid intake and urine output, alterations in neurohormones, and renal function.
Intake-Output Data
Intake-output data at high altitudes are often too crude and unreliable to assess fluid balance accurately. Nevertheless, such data do provide useful information that helps in the understanding of fluid metabolism at high altitudes. A number of studies have shown that intake is reduced at high altitudes whenever fluids are allowed ad libitum but is normal when intake is strictly enforced (Claybaugh and Wade, 1987). Decrease in appetite and thirst, lack of available water, and physical exertion probably all contribute to reduced intake. However, the chief mechanism appears to be a decrease in the sensation of thirst (Jones et al., 1981a, b). Reduced fluid intake can account for approximately 2 to 3 liters of negative fluid balance during the initial few days at high altitudes. The exact mechanism of reduced thirst is also unclear. Changes in antidiuretic hormone (ADH) cannot fully explain it, since thirst is reduced even in patients with diabetes insipidus (a condition characterized by the inability to produce or respond to ADH) (Jones et al., 1981a, b). Similarly, changes in circulating atrial natriuretic peptide (ANP) and angiotensin II do not seem to play a major role in the reduced sensation of thirst (Jones et al., 1981b). Changes in the central release of these hormones may be important, but this has never been proven. The sensation of thirst returns to normal over a period of a few days despite continued stay at high altitudes.
Changes in urine output are also common at high altitudes, but the data are controversial. This laboratory reviewed 57 studies where urine output was measured at high altitudes. The data suggest that there is usually a transient diuresis at high altitudes lasting, on an average, 3 to 4 days. Diuresis is common at moderate altitudes, in subjects who do not exercise soon after transfer to high altitudes, in subjects who do not develop AMS, and in subjects for whom fluid intake is strictly enforced. Diuresis is also seen in acute experiments at sea level when subjects breathe hypoxic gas mixtures (Ashack et al., 1985). In contrast, anti-diuresis commonly accompanies severe hypoxia, at extreme altitudes, in subjects who exercise soon after arriving at high altitudes, and in those who are prone to and later develop AMS.
Other factors that might lead to dehydration at high altitudes include an increase in insensible loss because of low humidity and hyperventilation. The magnitude of this effect is not clear. Malabsorption of various nutrients has also been described at high altitudes (Chinn and Hannon, 1969), but the effect of this phenomenon on fluid metabolism has never been evaluated.
Hormones and High Altitudes
Hormones are important in the control of fluid metabolism both in normal and abnormal conditions. Neurohormonal changes precede the development of salt and water retention. A number of hormones are important in this process (Anand et al., 1989; Packer, 1988), the important ones being renin, aldosterone, catecholamines, ANP, and ADH. Cortisol, prolactin, and growth hormone can also contribute indirectly to salt and water metabolism. A number of studies have investigated the role of these hormones under different conditions of hypoxia, with and without exercise (see Table 18-2).
Renin-Angiotensin-Aldosterone Axis
Aldosterone. At least 20 studies in the literature investigated the effect of hypoxia or high altitudes on aldosterone. High altitudes or hypoxia at rest caused a decrease in aldosterone level in 15 studies (Bouissou et al., 1989; Colice and Ramirez, 1985, 1986; Heyes et al., 1982; Hogan et al., 1973; Maher et al., 1975; Maresh et al., 1985; Milledge et al., 1983; Olsen et al., 1992; Raff et al., 1986; Ramirez et al., 1992; Shigeoka et al., 1985; Slater et al., 1969a, b; Sutton et al., 1977), an increase in 5 studies (Anand et al., 1993; Bärtsch et al., 1991a, b; Frayser et al., 1975; Okazaki et al., 1984), and had no effect in another 3 studies (Ashack et al., 1985; Bärtsch et al., 1991b; Frayser et al., 1975). Most studies also showed that hypoxia attenuated the normal increase in aldosterone seen with exercise at sea level (Maher et al., 1975; Milledge et al., 1983; Olsen et al., 1992; Shigeoka et al., 1985). Thus, although hypoxia acutely reduces aldosterone, it is not clear whether this reduction in aldosterone has a role in the acute diuresis seen at high altitudes. The effect of hypoxia on aldosterone is, however, different in subjects who are prone to develop AMS. Bärtsch et al. (1991a) studied a group of subjects who had never developed AMS and another group who had AMS on at least one occasion and were therefore prone to develop symptoms on re-exposure to high altitudes. At rest both groups showed a similar reduction in aldosterone at high altitudes (Figure 18-7). During exercise at high altitudes, subjects who later developed AMS showed a significantly greater increase in aldosterone, which suggests that aldosterone may be involved in the pathogenesis of this condition.
Renin Activity. A wide variety of changes in plasma renin activity (PRA) has been described at high altitudes. There are at least 16 studies in the literature where PRA was measured during hypobaric hypoxia (Ashack et al., 1985; Bouissou et al., 1989; Colice and Ramirez, 1985; Heyes et al., 1982; Hogan et al., 1973; Keynes et al., 1982; Maher et al., 1975; Milledge et al., 1983; Okazaki et al., 1984; Olsen et al., 1992; Raff et al., 1986; Shigeoka et
TABLE 18-2 Human Studies Detailing Hormonal Alterations During Hypoxia or Ascent or Stay at High Altitudes
|
Authors |
Study Details |
Comments |
|
Acute hypoxia |
||
|
Heyes et al., 1982 |
Eleven males, 18–40 yr, tested with hypoxia, with and without hypobaria. |
ALDO reduced, prolactin rose. AVP increased only with hypotension. Hypobaria not important except for prolactin. |
|
Okazaki et al., 1984 |
Eighteen males, two females, 23–48 yr, studied at 6,000 m (19,685 ft) for 3 hours in chamber. |
No change at 4,000 m (13,123 ft). At 5,000 m (16,404 ft), PV fell, ALDO and ACTH rose. At 6,000 m, ALDO, PRA, and cortisol increased. |
|
Aschak et al., 1985 |
Ten males, 18–24 yr, usual diet. 10.5 and 12 percent FIO2. PO2 35–46 mm Hg. Water loading and replacement of urine volume. |
No change in ALDO, AVP, PRA, NE, bradykinin. No change in urine volume, urine sodium. AVP increased only when subjects had nausea. |
|
Colice and Ramirez, 1985 |
Group 1: hypoxia (SAT 90 percent) for 1 hour. Group 2: hypoxia (SAT 90 percent) for 1 hour, (SAT 80 percent) for 1 hour. Studied twice with normal and low-salt diet. |
ALDO reduced; PRA and ACE unchanged. No further increase in second hour PRA-ALDO uncoupled. No change in hemodynamics. ALDO reduction significant only when both groups pooled. Diet sodium important in degree of response. |
|
Maresh et al., 1985 |
Seven low-altitude and seven high-altitude natives (1,830–2,200 m [6,004–7,218 ft]). Tested in hypobaric chamber (447 mm Hg) for 2 days. |
ALDO reduced; cortisol increased. High-altitude natives have similar but damped responses. ERPF and heart rate increased. |
|
Hypoxia-high altitude studies; no exercise |
||
|
Frayser et al., 1975 |
Nine subjects studied at SL, day 5 at 3,048 m (10,000 ft), days 3 and 7 at 5,334 m (17,500 ft). Eight subjects at SL, day 5 at 2,926 m (9,600 ft), days 5 and 10 at 5,334 m (17,500 ft). Four subjects got acetazolamide before ascent to 5,334 m (17,500 ft). |
At 2,744–3,049 m (9,000–10,000 ft), ALDO, PRA unchanged, cortisol rose. At 5,335 m (17,500 ft), PRA and cortisol rose. Normal by day 5. |
|
Olsen et al., 1992 |
Eight males, 27–42 yr, studied at SL and after 48 hours at 4,350 m (14,272 ft). Measurements at rest and exercise. One-half liter water load. |
Increase in NE, fall in ALDO and PRA. Epi and ANP unchanged. |
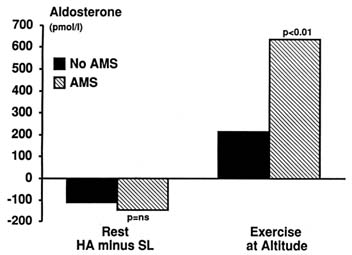
FIGURE 18-7 Effect of exercise at high altitudes (HA) on aldosterone level in subjects susceptible to acute mountain sickness (AMS) compared with nonsusceptible subjects. Ns, not significant. SOURCE: Adapted from Bärtsch et al. (1991a).
al., 1985; Slater et al., 1969a, b; Sutton et al., 1977; Tuffley et al., 1970). At rest, hypoxia decreased PRA in 7 studies (Bouissou et al., 1989; Hogan et al., 1973; Keynes et al., 1982; Milledge et al., 1983; Olsen et al., 1992; Slater et al., 1969a; Sutton et al., 1977), increased it in 3 studies (Frayser et al., 1975; Okazaki et al., 1984; Tuffley et al., 1970), and had no effect in 6 studies (Ashack et al., 1985; Bouissou et al., 1989; Colice and Ramirez, 1985; Heyes et al., 1982; Raff et al., 1986; Tuffley et al., 1970). In contrast, hypoxia caused an attenuation of the increase in PRA normally seen with exercise at sea level.
The overall effect of hypoxia on PRA, therefore, remains unclear. The more important effect of hypoxia appears to be on the renin-angiotensin-aldosterone axis. Normally this axis is tightly coupled in humans and animals. One consistent feature of exposure to high altitudes is the uncoupling of this tight renin-aldosterone relationship, both at rest and during exercise. For any increase in PRA, the increase in aldosterone is markedly reduced during hypoxia (Bouissou et al., 1989; Colice and Ramirez, 1985, 1986; Milledge et al., 1983; Purshottam et al., 1978; Shigeoka et al., 1985; Slater et al., 1969a, b; Sutton et al., 1977). Milledge et al. (1983) showed a brisk and marked increase in aldosterone during exercise at sea level as compared to a blunted response at high altitudes while the PRA rose normally. The cause of this uncoupling is not understood. Adrenal glands are normal or hypertrophied at high altitudes (Gosney, 1985) and respond normally to angiotensin II and adrenocorticotropic hormone (ACTH) infusion (Colice and Ramirez, 1986).
Angiotensin-converting enzyme activity in cultured endothelial cells and in peripheral blood was shown to be reduced during hypoxia (Stalcup et al., 1985), and this was considered to be the cause of reduced aldosterone at high altitudes. There is now some doubt about this finding (Milledge and Catley, 1987). Plasma potassium may be important in regulating aldosterone secretion (Curran-Everett et al., 1988). However, the role of potassium in modulating the acute effects of hypobaric hypoxia are unclear.
Antidiuretic Hormone. Antidiuretic hormone (ADH) is a stress hormone that increases thirst and reduces free water clearance. Both osmolality and volume status modulate its level. Changes in ADH response would be an attractive explanation for the alterations in fluid metabolism seen at high altitudes. Thirteen studies were examined where the effect of hypoxia on ADH was investigated (Ashack et al., 1985; Bärtsch et al., 1991a; Brahmachari et al., 1973; Claybaugh et al., 1982, 1987b; Forsling and Milledge, 1977; Hackett et al., 1978; Harber et al., 1981; Heyes et al., 1982; Okazaki et al., 1984; Porchet et al., 1984; Ramirez et al., 1992; Subramanian et al., 1975). The results are controversial, and there is both evidence for an increase in ADH with hypoxia (Hackett et al., 1978; Singh et al., 1974), no change (Ashack et al., 1985; Forsling and Milledge, 1977; Heyes et al., 1982; Porchet et al., 1984), or a decrease (Brahmachari et al., 1973; Porchet et al., 1984; Subramanian et al., 1975). Part of the explanation for this disagreement may lie in the variety of methods used in these studies and the differences in the nature and degree of hypoxia used (Subramanian et al., 1975). Mild hypoxia causes a reduction in ADH and may be important in the diuresis seen in the early stages of high-altitude ascent (Brahmachari et al., 1973; Porchet et al., 1984). Although ADH is important in mediating thirst, reduced thirst at high altitudes cannot be explained by a change in ADH since a similar response is seen in diabetes insipidus (Jones et al., 1981a, b). Severe hypoxia, extreme altitudes, exercise, and any form of stress, including nausea and vomiting, increase ADH (Ashack et al., 1985; Heyes et al., 1982). ADH is increased in AMS, and subjects prone to develop this condition show an exaggerated increase in ADH during exercise at high altitudes (Figure 18-8).
Atrial Natriuretic Peptide. Atrial natriuretic peptide (ANP) has a number of important physiological effects that can potentially counteract the effects of various salt-retaining hormones. It is natriuretic and diuretic, inhibits aldosterone secretion, and reduces thirst. Despite these interesting effects, the role of ANP at high altitudes appears minimal. More than 10 studies report the effects of hypoxia on ANP (Anand et al., 1993; Bartsch et al., 1986; Bärtsch et al., 1991a, b; Bouissou et al., 1989; du Souich et al., 1987; Hackett et al., 1978; McKenzie et al., 1986; Milledge et al., 1989; Olsen et al., 1992, 1993; Ramirez et al., 1992; Singh et al., 1974). Most of these studies found no change or a mild increase in ANP with hypoxia (Bouissou et al., 1989; Olsen
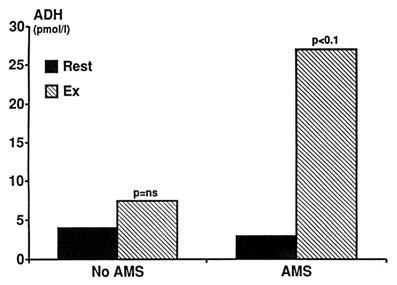
FIGURE 18-8 Effect of exercise at high altitudes on antidiuretic hormone (ADH) level in subjects susceptible to acute mountain sickness (AMS) compared with nonsusceptible subjects. ADH is significantly increased by exercise in AMS-susceptible individuals. ns, not significant. SOURCE: Adapted from Bärtsch et al. (1991a).
et al., 1992; Ramirez et al., 1992). Even in studies where ANP levels were high, no significant protective role against salt and water retention was seen (du Souich et al., 1987). The reason for this is unclear. Although there is evidence that ANP receptor down regulation may occur in conditions with prolonged elevation of circulating ANP, like congestive heart failure, such evidence is lacking in subjects at high altitudes. Like ADH, ANP is high in AMS and in subjects prone to develop this condition even before the onset of symptoms (Figure 18-9).
Catecholamines. Catecholamines help to retain salt and water by modulating renal blood flow (RBF) (Anand et al., 1989; McMurray et al., 1988; Olsen et al., 1993; Packer, 1988). Nearly all studies that measured catecholamines at high altitudes demonstrated an increase in norepinephrine both at rest and during exercise (Anand et al., 1989, 1993; Maher et al., 1975; Olsen et al., 1992, 1993; Ramirez et al., 1992).
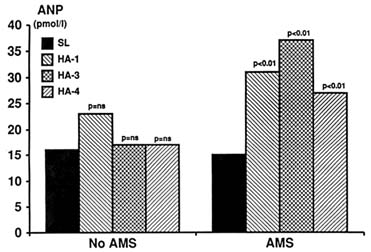
FIGURE 18-9 Effect of exercise at high altitudes on atrial natriuretic peptide (ANP) level in subjects susceptible to acute mountain sickness (AMS) compared with nonsusceptible subjects. ns, not significant; SL, sea level; HA, high altitudes.
SOURCE: Adapted from Bärtsch et al. (1991a).
Renal Function
RBF is an important determinant of salt and water metabolism. Unfortunately, renal functions have not been adequately studied at high altitudes. Eighteen studies were reviewed that reported renal responses to hypoxia or high altitudes (Anand et al., 1993; Ashack et al., 1985; Axelrod and Pitts, 1952; Berger et al., 1949; Castenfors, 1967; Freund et al., 1991; Guiol et al., 1986; Heyes et al., 1982; Maher et al., 1975; McDonald and Kelley, 1948; Monge et al., 1969; Olsen et al., 1993; Ou et al., 1984; Pauli et al., 1968; Rennie et al., 1972; Selkurt, 1953; Subramanian et al., 1975; Ullmann, 1961). As with most high-altitude studies, variation in methodology and experimental design has caused considerable confusion. It appears, however, that moderate acute hypoxia at sea level increases RBF. In contrast, most studies at high altitudes have shown a reduction in effective renal plasma flow (ERPF) with minimal change in glomerular filtration rate (GFR), which suggests a greater efferent than afferent arteriolar vasoconstriction (Olsen et al., 1993). Chronic severe hypoxia, or hypobaria by itself, reduces RBF. ERPF is low even in high-altitude natives, but the RBF is normal because of an increase in hematocrit (Ou et al., 1984). The cause of the decrease in RBF following ascent to high altitudes is unclear, but it is probably due to increased circulating catecholamines and blood viscosity at high altitudes.
EXTREME ALTITUDES
Earlier in this chapter it was shown that prolonged stay at extreme altitudes can lead to considerable salt and water retention in otherwise normal subjects (Figure 18-6). Measurement of renal function in these subjects showed that the ERPF was significantly reduced to around 45 percent normal (Anand et al., 1993). And despite the marked increase in hematocrit, the calculated RBF was still only 60 percent of normal. Total body exchangeable sodium had increased by about 15 percent above normal (Figure 18-6). There was significant increase in norepinephrine, aldosterone, and cortisol (Figure 18-10). Therefore, prolonged stay at extreme altitudes can lead to severe salt and water retention probably because of a reduction in renal blood flow due to high circulating catecholamines. High levels of aldosterone could also contribute to sodium and thereby water retention. These may be the mechanisms responsible for adult subacute mountain sickness reported at extreme altitudes (Anand et al., 1990, 1993).
AUTHORS' CONCLUSIONS AND RECOMMENDATIONS
An extensive review of the literature on fluid metabolism at high altitudes shows that the data are confusing, controversial, and inconclusive. Inadequate
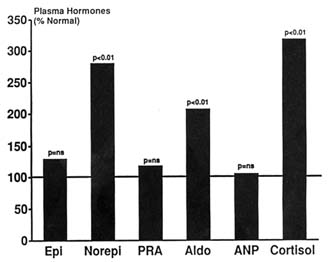
FIGURE 18-10 Neurohormones and renal function in normal subjects living at extreme altitudes (> 6,000 m [19,865 ft]) for a prolonged period (> 10 weeks). ns, not significant; Epi, epinephrine; Norepi, norepinephrine; PRA, plasma renin activity; Aldo, aldosterone; ANP, atrial natriuretic peptide. SOURCE: Adapted from Anand et al. (1993).
methodology, lack of serial measurements on the same subjects, and the presence of confounding variables—like the use of exercise and the presence or absence of AMS in subjects under investigation—are some of the reasons for the dissimilarities in the findings. Moreover, there are virtually no data at extreme altitudes and after prolonged exposure to moderate altitudes. Nevertheless, it appears that acute exposure to moderate altitudes causes transient hypohydration, which is due to increased diuresis and an acute reduction in fluid intake because of decrease in thirst. The meager data available on the effects of chronic hypoxia in sheep and humans suggest that modest fluid retention may occur. Prolonged stay at extreme altitudes may cause severe salt and water retention in otherwise normal subjects. The role of hormones in normal fluid metabolism at high altitudes is unclear, but a number of hormones appear to play a role in the retention of salt and water in pathologic states like acute and subacute mountain sickness. RBF is probably reduced at high altitudes and more so at higher altitudes. Finally, exercise and increased physical activity at high altitudes favor the mechanisms that retain salt and water.
It is recommended that the acute and long-term effects of moderate and extreme altitudes on body fluid compartment and its determinants (neurohormones and renal function) need to be investigated using the same group of subjects and more sophisticated technology. The role of confounding variables like physical activity needs to be better defined. Subjects at high altitudes are almost always involved in some form of physical activity, which at times is fairly strenuous, especially for those engaged in military activity. Only by making serial measurements on the subjects would it be possible to confirm the impression that a prolonged stay may lead to some degree of fluid retention at moderate altitudes and to severe salt and water accumulation at extreme altitudes. Finally, the interaction between cold and altitude also needs to be determined. Such studies would be helpful in identifying subjects prone to high altitude-related illness and in establishing guidelines about the ''safe" length of time humans can spend at various altitudes.
REFERENCES
Anand, I., and Y. Chandrashekhar 1992 Syndromes of subacute mountain sickness: Pathophysiology and manifestations. Pp. 257–275 in The Diagnosis and Treatment of Pulmonary Hypertension, E.K. Weir, S.L. Archer, and J.T. Reeves, eds. Mt. Kisco, N.Y.: Futura Publishing Inc.
Anand, I., R. Ferrari, G. Kalra, P. Wahi, P. Poole-Wilson, and P. Harris 1989 Edema of cardiac origin: Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation 80:299–305.
Anand, I., R. Malhotra, Y. Chandrashekhar, H. Bali, S. Chauhan, R. Bhandari, and P. Wahi 1990 Adult subacute mountain sickness—a syndrome of congestive heart failure in man at very high altitude. Lancet 335:561–565.
Anand, I., Y. Chandrashekhar, S. Rao, R. Malhotra, R. Ferrari, J. Chandana, B. Ramesh, K. Shetty, and M. Boparai 1993 Body fluid compartments, renal blood flow, and hormones at 6,000 m in normal subjects. J. Appl. Physiol. 74:1234–1239.
Ashack, R., M. Farber, M. Weinberger, G. Robertson, N. Fineberg, and F. Manfredi 1985 Renal and hormonal responses to acute hypoxia in normal individuals. J. Lab. Clin. Med. 106:12–16.
Axelrod, D., and R. Pitts 1952 Effects of hypoxia on renal tubular function. J. Appl. Physiol. 4:593–601.
Bartsch, A., C. Hausmaninger, R. Walsh, R. Mentzer, D. Wyatt, and R. Pence 1986 Hypoxia-induced release of atrial natriuretic factor (ANF1) from the isolated rat and rabbit heart. Biochem. Biophys. Res. Comm. 140:427–433.
Bärtsch, P., M. Maggiorini, W. Schobersberger, S. Shaw, W. Rascher, J. Girard, P. Weidmann, and O. Oelz 1991a Enhanced exercise-induced rise of aldosterone and vasopressin preceding mountain sickness. J. Appl. Physiol. 71:136–143.
Bärtsch, P., N. Pfluger, M. Audetat, S. Shaw, P. Weidmann, P. Vock, W. Vetter, D. Rennie, and O. Oelz 1991b Effects of slow ascent to 4,559 m on fluid homeostasis. Aviat. Space Environ. Med. 62:105–110.
Berger, E., M. Gladston, and S. Horwitz 1949 The effect of anoxic anoxia on the human kidney. J. Clin. Invest. 28:648–652.
Bouissou, P., J-P. Richalet, F. Galen, M. Lartigue, P. Larmignat, F. Devaux, C. Dubray, and A. Keromes 1989 Effect of β-adrenoceptor blockade on renin-aldosterone and a-ANF during exercise at altitude. J. Appl. Physiol. 67:141–146.
Brahmachari, H., M. Malhotra, K. Ramachandran, and U. Radhakrishnan 1973 Progressive changes in plasma cortisol, antidiuretic hormone and urinary volume of normal lowlanders during short stay at high altitude. Ind. J. Exp. Biol. 11:454–455.
Castenfors, J. 1967 Renal function during exercise. Acta Physiol. Scand. 293:1–44.
Chinn, K., and J. Hannon 1969 Efficiency of food utilization at high altitude. Fed. Proc. 28:944–947.
Christensen, B., H. Johnson, and A. Ross 1975 Organ fluid changes and electrolyte excretion of rats exposed to high altitude. Aviat. Space Environ. Med. 46:16–20.
Claybaugh, J., and C. Wade 1987 Hormonal regulation of fluid and electrolytes: Environmental effects. Pp. 187–214 in Federation of American Societies for Experimental Biology Conference on Hormonal Regulation of Fluid and Electrolytes: Environmental Effects, J. Claybaugh and C. Wade, eds. Washington, D.C.: Plenum Publishing Corporation.
Claybaugh, J., C. Wade, A. Sato, S. Cucinell, J. Lane, and J. Maher 1982 Antidiuretic hormone responses to eucapnic and hypocapnic hypoxia in humans. J. Appl. Physiol. 53:815–823.
Claybaugh, J., W. Stokes, B. Freund, and G. Bryant 1987 Plasma vasopressin is increased only during the first two of six hours of severe hypoxia in conscious goats [abstract]. Fed. Proc. 46:796.
Colice, G., and G. Ramirez 1985 Effect of hypoxemia on the renin-angiotensin-aldosterone system in humans. J. Appl. Physiol. 58:724–730.
1986 Aldosterone response to angiotensin II during hypoxemia. J. Appl. Physiol. 61:150–154.
Consolazio, C.F., L. Matoush, H. Johnson, and T. Daws 1968 Protein and water balances of young adults during prolonged exposure to high altitude (4,300 meters). Am. J. Clin. Nutr. 21:154–161.
Curran-Everett, D., J. Claybaugh, K. Miki, S. Hong, and J. Krasney 1988 Hormonal and electrolyte responses of conscious sheep to 96 h of hypoxia. Am. J. Physiol. 255:R274–R283.
du Souich, P., C. Saunier, D. Hartman, A. Sautegeau, H. Ong, P. Larose, and R. Babin 1987 Effect of moderate hypoxemia on atrial natriuretic factor and arginine vasopressin in normal man. Biochem. Biophys. Res. Comm. 148:906–912.
Epstein, M., and T. Saruta 1972 Effects of simulated high altitude on renin-aldosterone and Na homeostasis in normal man . J. Appl. Physiol. 33:204–210.
Forsling, M., and J. Milledge 1977 Effect of hypoxia on vasopressin release in man. J. Physiol. 267:22P–23P.
Frayser, R., I. Rennie, G. Gray, and C. Houston 1975 Hormonal and electrolyte response to exposure to 17,500 ft. J. Appl. Physiol. 38:636–642.
Freund, B.J., E. Shizuru, G. Hashiro, and J. Claybaugh 1991 Hormonal, electrolyte and renal responses to exercise are intensity dependent. J. Appl. Physiol. 70:900–906.
Gosney, J.R. 1985 Adrenal cortico medullary hyperplasia in hypobaric hypoxia. J. Pathol. 146:59–64.
Graham T.E., P. Sathasivam, and K.W. McNaughton 1991 Influence of cold, exercise and caffeine on catecholamines and metabolism in men. J. Appl. Physiol. 70(5):2052–2058.
Guiol, C., P. Montastruc, and M-C. Prevost 1986 Renal effect of acute hypobaric pressure breathing in normal and diabetes insipidus rats. J. Physiol. (Paris) 81:41–44.
Hackett, P.H., M. Forsling, J. Milledge, and D. Rennie 1978 Release of vasopressin in man at altitude. Horm. Metab. Res. 10:571–576.
Hackett, P.H., D. Rennie, R. Grover, and J. Reeves 1981 Acute mountain sickness and the edemas of high altitude: A common pathogenesis? Respir. Physiol. 46:383–390.
Hannon, J.P., and G. Rogers 1975 Body composition of mice following exposure to 4,300 and 6,100 meters. Aviat. Space Environ. Med. 46:1232–1235.
Hannon, J.P., K. Chinn, and J. Shields 1969 Effects of acute high-altitude exposure on body fluids. Fed. Proc. 28:1178–1183.
Harber, M., J. Williams, and J. Morton 1981 Antidiuretic hormone excretion at high altitude. Aviat. Space Environ. Med. 52:38–40.
Hecht, H.H., R.L. Lange, W.H. Carnes, H. Kuida, and J.T. Blake 1959 Brisket disease I. General aspects of pulmonary hypertensive heart disease in cattle. Trans. Assoc. Am. Physicians 72:157–172.
Heyes, M., F. Manfredi, D. Robertshaw, M. Weinberger, N. Fineberg, and G. Robertson 1982 Acute effects of hypoxia on renal and endocrine function in normal humans. Am. J. Physiol. 243:R265–R270.
Hogan, R., T. Kotchen, A. Boyd, and L. Hartley 1973 Effect of altitude on renin-aldosterone system and metabolism of water and electrolytes. J. Appl. Physiol. 35:385–390.
Hoyt, R.W., M. Durkot, V. Forte, Jr., L. Hubbard, L. Trad, and A. Cymerman 1991 Hypobaric hypoxia (380 Torr) decreases intracellular and total body water in goats. J. Appl. Physiol. 71:509–513.
Hoyt, R.W., M. Durkot, G. Kamimori, D. Schoeller, and J. Cymerman 1992 Chronic altitude exposure (4,300 m) decreases intracellular and total body water in humans. P. 306 in Hypoxia and Mountain Medicine, J.R. Sutton, G. Coats, and C.S. Houston. Burlington, Vt.: Queen City Printers, Inc.
Jain, S.C., J. Bardhan, Y. Swamy, B. Krishna, and H. Nayar 1980 Body fluid compartments in humans during acute high-altitude exposure. Aviat. Space Environ. Med. 51:234–236.
Jain, S.C., Y. Swamy, A. Grover, and H. Nayar 1981 Body water metabolism in high altitude natives during and after a stay at sea level. Int. J. Biometeorol. 25:47–52.
Jones, R., F. LaRochelle, Jr., and S. Tenney 1981a Role of arginine vasopressin on fluid and electrolyte balance in rats exposed to high altitude. Am. J. Physiol. 240:R182–R186.
Jones, R., C. Terhaard, J. Zullo, and S. Tenney 1981b Mechanism of reduced water intake in rats at high altitude. Am. J. Physiol. 240:R187–R191.
Keynes, R., G. Smith, J. Slater, M. Brown, S. Brown, N. Payne, T. Jowett, and C. Monge 1982 Renin and aldosterone at high altitude in man. J. Endocrinol. 92:131–140.
Krzywicki, H., C. Consolazio, H. Johnson, W. Nielsen, Jr., and R. Barnhart 1971 Water metabolism in humans during acute high-altitude exposure (4,300 m). J. Appl. Physiol. 30:806–809.
Levine, B., K. Yoshimura, T. Kobayashi, M. Fukushima, T. Shibamoto, and G. Ueda 1989 Dexamethasone in the treatment of acute mountain sickness. N. Engl. J. Med. 25:1707–1713.
Maher, J., L. Jones, L. Hartley, G. Williams, and L. Rose 1975 Aldosterone dynamics during graded exercise at sea level and high altitude. J. Appl. Physiol. 39:18–22.
Maresh, C., B. Noble, K. Robertson, and J. Harvey, Jr. 1985 Aldosterone, cortisol, and electrolyte responses to hypobaric hypoxia in moderate-altitude natives. Aviat. Space Environ. Med. 56:1078–1084.
McDonald, R., and V. Kelley 1948 Effects of altitude anoxia on renal function. Am. J. Physiol. 154:193–200.
McKenzie, J., I. Tanaka, T. Inagami, K. Misono, and R. Klein 1986 Alternations in atrial and plasma atrial natriuretic factor (ANF) content during development of hypoxia-induced pulmonary hypertension in the rat. Proc. Soc. Exp. Biol. Med. 181:459–463.
McMurray, J., P. Seidelin, D. Balfour, and A. Struthers 1988 Physiological increase in circulating norepinephrine is antinatriuretic in man. J. Hyperten. 6:757–761.
Milledge, J.S. 1992 Salt and water control at altitude. Int. J. Sports Med. 13:S61–S63.
Milledge, J.S., and D. Catley 1987 Angiotensin converting activity and hypoxia. Clin. Sci. 72:149.
Milledge, J.S., D. Catley, E. Williams, W. Withey, and B. Minty 1983 Effect of prolonged exercise at altitude on the renin-aldosterone system. J. Appl. Physiol. 55:413–418.
Milledge, J.S., J. Beeley, S. McArthur, and A. Morice 1989 Atrial natriuretic peptide, altitude and acute mountain sickness. Clin. Sci. 77:509–514.
Monge, C., R. Lozano, C. Marchena, J. Whittembury, and C. Torres 1969 Kidney function in the high altitude native. Fed. Proc. 28:1199–1203.
Okazaki, S., Y. Tamura, T. Hatano, and N. Matsui 1984 Hormonal disturbances of fluid-electrolyte metabolism under altitude exposure in man. Aviat. Space Environ. Med. 55:200–205.
Olsen, N., I-L. Kanstrup, J-P. Richalet, J. Hansen, G. Plazen, and F-X. Galen 1992 Effects of acute hypoxia on renal and endocrine function at rest and during graded exercise in hydrated subjects. J. Appl. Physiol. 73:2036–2043.
Olsen, N., J. Hansen, I-L. Kanstrup, J-P. Richalet, and P. Leyssac 1993 Renal hemodynamics, tubular function, and response to low-dose dopamine during acute hypoxia in humans. J. Appl. Physiol. 74:2166–2173.
Ou, L., J. Silverstein, and B. Edwards 1984 Renal function in rats chronically exposed to high altitude. Am. J. Physiol. 247:F45–F49.
Packer, M. 1988 Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77:721–730.
Pauli, H., B. Truniger, J. Larsen, and R. Mulhausen 1968 Renal function during prolonged exposure to hypoxia and carbon monoxide. Scand. J. Clin. Lab. Invest. 103:55–60.
Phillips, R., K. Knox, W. House, and H. Jordan 1969 Metabolic responses in sheep chronically exposed to 6,200 m simulated altitude. Fed. Proc. 28:974–977.
Porchet, M., H. Contat, B. Waeber, J. Nussberger, and H. Brunner 1984 Response of plasma arginine vasopressin levels to rapid changes in altitude. Clin. Physiol. 4:435–438.
Pugh, L.C.G.E. 1962 Physiological and medical aspects of Himalayan scientific and mountaineering expedition. Br. Med. J. 2:621–627.
Purshottam, T., M. Pahwa, and H. Brahmachari 1978 Effects of 6 hours hypoxic and cold exposure on urinary electrolyte and catecholamine excretion. Aviat. Space Environ. Med. 49:62–65.
Raff, H., R. Sandri, and T. Segerson 1986 Renin, ACTH, and adrenocortical function during hypoxia and hemorrhage in conscious rats. Am. J. Physiol. 250:R240–R244.
Ramirez, G., M. Hammond, S. Agosti, P. Bittle, J. Dietz, and G. Colice 1992 Effects of hypoxemia at sea level and high altitude on sodium excretion and hormonal levels. Aviat. Space Environ. Med. 63:891–898.
Rennie, I., R. Frayser, G. Gray, and C. Houston 1972 Urine and plasma proteins in men at 5,400 m. J. Appl. Physiol. 32:369–373.
Rose, M.S., C. Houston, C. Fulco, G. Coates, J. Sutton, and A. Cymerman 1988 Operation Everest II: Nutrition and body composition. J. Appl. Physiol. 65:2545–2551.
Schnakenberg, D.D., L. Krabill, and P. Weiser 1971 The anorexic effect of high altitude on weight gain, nitrogen retention and body composition of rats. J. Nutr. 101:787–796.
Selkurtt, E. 1953 Influence of hypoxia on renal circulation and on excretion of electrolytes and water. Am. J. Physiol. 172:700–708.
Shigeoka, J., G. Colice, and G. Ramirez 1985 Effect of normoxemic and hypoxemic exercise on renin and aldosterone. J. Appl. Physiol. 59:142–148.
Singh, I., P. Khanna, M. Srivastava, M. Lal, S. Roy, and C. Subramanyam 1969 Acute mountain sickness. N. Engl. J. Med. 280:175–184.
Singh, I., M. Malhotra, P. Khanna, R. Nanda, T. Purshottam, T. Upadhyay, U. Radhakrishnan, and H. Brahmachari 1974 Changes in plasma cortisol, blood antidiuretic hormone and urinary catecholamines in high altitude pulmonary edema. Int. J. Biometeorol. 18:211–221.
Singh, M., S. Jain, S. Rawal, H. Divekar, R. Parshad, A. Tyagi, and K. Sinha 1986 Comparative study of acetazolamide and spironolactone on body fluid compartments on induction to high altitude. Int. J. Biometeorol. 30:33–41.
Singh, M., S. Rawal, A. Tyagi, M. J. Bhagat, R. Parshad, and H. Divekar 1988 Changes in body fluid compartments on re-induction to high altitude and effect of diuretics. Int. J. Biometeorol. 32:36–40.
Singh, M., S. Rawal, and A. Tyagi 1990 Body fluid status on induction, reinduction and prolonged stay at high altitude of human volunteers. Int. J. Biometeorol. 34:93–97.
Slater, J., R. Tuffley, E. Williams, C. Beresford, P. Sonksen, R. Edwards, R. Ekins, and M. McLaughlin 1969a Control of aldosterone secretion during acclimatization to hypoxia in man. Clin. Sci. 37:327–341.
Slater, J., E. Williams, R. Edwards, R. Ekins, P. Sonksen, C. Beresford, and M. McLaughlin 1969b Potassium retention during the respiratory alkalosis of mild hypoxia in man: Its relationship to aldosterone secretion and other metabolic changes. Clin. Sci. 37:311–326.
Stalcup, S., J. Lipset, J. Woan, P. Leuenberger, and R. Mellins 1985 Inhibition of angiotensin converting enzyme activity in cultured endothelial cells by hypoxia. J. Clin. Invest. 75:1745.
Subramanian, R., B. Bhatia, and H. Siddiqui 1975 Urine output and blood ADH in rats under different grades of hypoxia. P. 126 in Selected Topics in Environmental Biology, B. Bhatia, G.S. Chinna, and B. Singh, eds. New Delhi: Interprint Publications.
Sui, G., Y. Liu, X. Cheng, I.S. Anand, E. Harris, P. Harris, and D. Heath 1988 Subacute infantile mountain sickness. J. Pathol. 155:161–170.
Surks, M., K. Chinn, and L. Matoush 1966 Alterations in body composition in man after acute exposure to high altitude. J. Appl. Physiol. 21:1741–1746.
Sutton, J., G. Viol, G. Gray, M. McFadden, and P. Keane 1977 Renin, aldosterone, electrolyte, and cortisol responses to hypoxic decompression. J. Appl. Physiol. 43:421–424.
Tuffley, R., D. Rubenstein, J. Slater, and E. Williams 1970 Serum renin activity during exposure to hypoxia. J. Endocrinol. 48:497–510.
Ullmann, E. 1961 Acute anoxia and the excretion of water and electrolytes. J. Physiol. (London) 155:417–437.
West, J. 1984 Highest inhabitants in the world. Nature (London) 324:517.
Westerterp, K.R., B. Kayser, F. Brouns, J. Herry, and W. Saris 1992 Energy expenditure climbing Mt. Everest. J. Appl. Physiol. 73:1815–1819.
Withey, W., J. Milledge, E. Williams, B. Minty, E. Bryson, N. Luff, M. Older, and J. Beeley 1983 Fluid and electrolyte homeostasis during prolonged exercise at altitude. J. Appl. Physiol. 55:409–412.


























