4
Nicotine
Electronic cigarettes are designed to deliver a nicotine-containing aerosol to the user. According to the 1988 Surgeon General’s report The Health Consequences of Smoking: Nicotine Addiction, “Nicotine is the drug in tobacco that causes addiction” (HHS, 1988, p. 9). Because dependence on tobacco is produced primarily through the pharmacological effects of nicotine (Benowitz, 2009), an understanding of the pharmacology (i.e., disposition kinetics, metabolism, and pharmacodynamics) of nicotine, concentration of nicotine in commercial e-cigarette liquids and aerosols, systemic nicotine exposure among users, and factors that may affect nicotine exposure are essential to understanding the potential addictiveness of e-cigarettes. In addition, although most of the harm caused by tobacco smoking is attributed to combustion products, nicotine contributes to health outcomes such as cardiovascular disease in smokers (HHS, 2014). Therefore it is important to understand mechanisms of action of nicotine to understand its role in the overall health effects of e-cigarettes.
CONCENTRATION OF NICOTINE IN COMMERCIAL E-CIGARETTES
Although some e-cigarettes/e-liquids do not contain nicotine, most do, and the nicotine contents of e-cigarettes are variable. Based on vaping machine studies, higher nicotine concentration of e-liquids results in higher nicotine yield of any given e-cigarette (Talih et al., 2015). As with combustible tobacco cigarettes, machine-derived nicotine yield of
e-cigarettes is not necessarily predictive of users’ systemic exposures to nicotine. Other factors such as power of the e-cigarette and user behavior and use patterns are also critical. Nevertheless, e-liquid nicotine concentration may be a determinant of systemic nicotine exposure. Here, the committee reviews current evidence related to the range of nicotine concentrations in commercially available e-cigarettes, whether cartridges of first-generation and closed-tank e-cigarettes or refill liquids used in other open-system e-cigarettes. The committee also discusses labeling accuracy of nicotine content.
There is no consensus in the way nicotine strength is reported on labels of products or in studies. The nicotine strength on the label of some products is qualitative (e.g., zero, low, medium, high, super high) or quantitative on others. The unit of quantitative measure of nicotine strength is often reported on labels or in studies as amount per cartridge (mg), percentage per volume (e.g., 2.4 percent nicotine), concentration (mg/ml), or amount of nicotine per amount of e-liquid (µg/mg or mg/g).
A previous systematic review of the evidence evaluating chemicals in refill solutions and cartridges included studies published between January 2007 and September 2013 (Cheng, 2014). Based on 10 of the 29 studies included in this review, which reported on nicotine concentration of e-liquids, the review found that nicotine levels in e-liquids varied considerably, with a range of 0–87.2 mg/ml. For example, one study assessed the level of nicotine in popular brands of refill liquids from the United States and Western Europe (Etter et al., 2013). Among the 20 samples from 10 different brands, the range of nicotine on the labels was 6–30 mg. The range of measured nicotine concentration was 6–29.0 mg/ml; the measured concentration ranged from 85 to 107 percent of the labeled nicotine content. Another study assessed the nicotine content of 16 e-cigarette brands (20 cartridges and 15 refill liquids) based on high popularity in markets in Poland, the United Kingdom, and the United States (Goniewicz et al., 2013). Measured nicotine in cartridges ranged from 0.3 to 19 mg (per cartridge) and 0 to 25 mg in refill liquids. In another study, nicotine concentration was measured in a convenience sample of seven e-cigarette refill liquids (Cameron et al., 2014). Measured mean nicotine concentration across the seven brands ranged from 8.5 to 22.2 mg/ml, and were equivalent to or lower than labeled concentrations.
A number of studies have assessed nicotine concentration in e-liquids since the 2014 review by Cheng. Goniewicz and colleagues (2015) measured nicotine in 32, 29, and 30 popular brands of e-liquids purchased between 2013 and 2014 in the United States, South Korea, and Poland, respectively. In samples from the United States, nicotine in the e-liquid ranged from below limit of quantitation (BLQ) to 36.6 mg/ml. Of 32 samples, 9 (28 percent) had measured nicotine levels that deviated from
the labeled nicotine strength by more than 20 percent. In South Korea, two-thirds of the products tested did not have detectable levels of nicotine while the higher concentration was 150 mg/ml (this product was labeled “Pure Nicotine”). The range of nicotine strength in Polish samples was BLQ to 24.7 mg/ml. Ten percent of the Polish products tested showed deviations from the label of greater than 20 percent, while none of the products labeled nicotine-free contained detectable amounts of nicotine. Lisko and colleagues (2015) measured nicotine concentration in 36 cartridge and refill e-liquids in the U.S. market that had favorable online reviews. Nicotine content ranged from undetected to 20.5 mg/g. The measured nicotine concentrations were 5.8–41.7 percent lower than the labeled nicotine content. Tierney and colleagues (2016) reported a range of 6 to 24 mg/ml in a sample of 30 cartridge and refill e-liquids.
Etter and Bugey (2017) assessed the agreement between labeled and measured nicotine content across brands and across batches within the same brand. Eighteen e-liquids from 11 frequently used brands in the United States, the United Kingdom, France, and Switzerland were purchased in 2013. Nicotine on the labels ranged from 16 to 48 mg/ml. The measured nicotine concentrations ranged from 15.5 to 52.0 mg/ml. A majority of the sample, 82 percent, had measured nicotine concentration within 10 percent of the labeled content. Differences across batches within the same brands were small (0.5 percent). By contrast, Goniewicz and colleagues (2014), in a study that measured nicotine content of e-liquids from six popular products in the United Kingdom that were purchased 4 weeks apart, found the mean difference between batches of the same brand ranged from 1 to 31 percent.
Some clinical studies have reported the nicotine content of their participants’ usual brands of e-cigarettes. St.Helen and colleagues (2016a,b) characterized nicotine delivery and e-cigarette nicotine pharmacokinetic profiles among experienced e-cigarette users. Among the 13 enrolled participants, the labeled nicotine content of their usual e-liquids ranged from 6 to 24 mg/ml. The measured nicotine content ranged from 5.0 to 15.3 mg/g (note the difference in units). In another study of experienced e-cigarette users by St.Helen and colleagues (2017), the average nicotine on the label of the participants’ usual e-liquids was 7.9 mg/ml (range = 3–18 mg/ml). The measured nicotine concentration averaged 7.4 mg/ml (range = 1.6–19.9 mg/ml).
The preferred nicotine strength may differ across types of e-cigarettes used, particularly based on the power of the e-cigarettes. Users of high-powered e-cigarettes tend to use e-liquids with lower nicotine concentrations. Wagener and colleagues (2017) enrolled 9 second-generation and 11 third-generation e-cigarette users in a clinical study. The average power of the second-generation e-cigarettes was 8.6 W compared with 71.6 W of the
third-generation e-cigarettes. The average nicotine concentration of users of second-generation e-cigarettes was 22.3 mg/ml (range = 11–36 mg/ml) compared with 4.1 mg/ml (range = 1.5–6 mg/ml).
In summary, these studies show that nicotine content varies widely among products. Some studies show agreement between the nicotine content on the label and what was chemically measured while other studies show greater deviation of measured nicotine content from labeled content. One study showed that nicotine content is similar across batches of the same brand while another showed wider variability. Finally, the choice of preferred nicotine strength may be influenced, in part, by the characteristics of the e-cigarette used, including the power of the device.
NICOTINE CONCENTRATION IN E-CIGARETTE EMISSIONS
Nicotine concentration in e-cigarette emissions is an important determinant of systemic exposure to nicotine, and likely directly affects the abuse liability of e-cigarettes. E-cigarettes are designed to deliver nicotine to the user. Device characteristics that alter nicotine concentration in the aerosol are expected to also affect the abuse liability of e-cigarettes.
The systematic review by Cheng (2014) also included a review of nicotine delivery. As discussed above, Cheng identified five studies between January 2007 and September 2013 that reported amounts of nicotine in e-cigarette aerosol (Cobb et al., 2010; Goniewicz et al., 2013; Pellegrino et al., 2012; Trehy et al., 2011; Westenberger, 2009). The unit of measurement of nicotine in e-cigarette aerosol varied among the studies and included amount in a certain number of puffs (e.g., 100 or 150 puffs) and amount per volume of air (e.g., μg/100 ml puff, mg/m3). One major finding was that delivery of nicotine is not consistent across products.
For example, Goniewicz and colleagues (2013) assessed nicotine in aerosol in a study described previously. Sixteen popular e-cigarettes, including 20 cartridges, were obtained from Poland, the United Kingdom, and the United States based on popularity. Aerosol was generated from 300 puffs from each e-cigarette in 20 series of 15 puffs. Puffing conditions were based on average puff topography from 10 experienced e-cigarette users (70-ml puff volume, 1.8-second puff duration, and 10-second interpuff interval). As mentioned before, nicotine in the cartridges ranged from 0.3 to 19 mg. Nicotine in the aerosol varied by brand and ranged from 0.5 to 15.4 mg per 300 puffs. Also, nicotine in the aerosol varied from 21 percent to 85 percent of the nicotine present in the cartridge.
Adamson and colleagues (2016) compared nicotine delivery from a commercially available e-cigarette (Vype ePen) with 3R4F reference cigarettes (University of Kentucky) using the Health Canada Intense smoking regime (2-second puff duration, 55-ml puff volume, 30-second
interpuff interval). The e-cigarette was used at 4.0 V (5.7 W) and contained e-liquid with nicotine concentration of 18 mg/ml. Two different smoking machines were used, namely, Borgwaldt RM20S and Vitrocell VC10. Mean nicotine per puff from the 3R4F combustible tobacco cigarette was 0.171 (SD = 0.055) mg and 0.193 (SD = 0.055) mg on the RM20S and VC10, respectively. In comparison, mean amount of nicotine per puff from the e-cigarette was 0.049 (SD = 0.006) mg and 0.053 (SD = 0.012) mg. Interestingly, the nicotine concentration per puff increased from puff to puff when generating the combustible tobacco cigarette smoke. This is because tar and nicotine deposit down the cigarette rod on burning, enriching the distillable material in the rod for later puffs (Adamson et al., 2016). By contrast, the e-cigarette nicotine concentration was found to be highly consistent from puff to puff. The implications for variation or lack thereof in nicotine concentration per puff between combustible tobacco cigarette use versus e-cigarette use are not clear. However, this study shows that at a power of 5.7 W, e-cigarettes deliver less nicotine per puff than combustible tobacco cigarettes. Nicotine delivery per puff is expected to increase with power. The study mentioned that delivery at a power of 4.6 W was 0.032 mg of nicotine per puff. This was based on another study by the same research group, which compared nicotine delivery from Vype ePen at low voltage with 3R4F reference combustible tobacco cigarettes (Margham et al., 2016).
Talih and colleagues (2015) examined the influence of puff duration and puff velocity (or flow rate), as well as device power and nicotine concentration, on vaping machine-derived emissions from e-cigarettes. One type of e-cigarette cartridge, V4L CoolCart, was used in the study, and aerosols were generated by a machine designed and manufactured by the American University of Beirut. Five distinct puff profiles representing a combustible tobacco cigarette smoker and four types of e-cigarette user profiles (different puff duration and puff velocity) were examined. Power and e-liquid nicotine concentration were varied. The study found that nicotine yield ranged by more than 50-fold across conditions, from 0.11 mg to 4.70 mg in 15 puffs. Nicotine yield in 15 puffs was positively related to puff duration, power (voltage), and nicotine concentration of the e-liquid. Interestingly, puff velocity was not related to nicotine yields. This study showed that the concentration of nicotine in e-cigarette aerosols is determined both by e-cigarette characteristics and user behavior.
In summary, nicotine concentration in e-cigarette aerosol is variable among e-cigarettes. In the conditions tested, nicotine yield from an e-cigarette was lower than that of a reference combustible tobacco cigarette. However, the concentration of nicotine in e-cigarette aerosol is a product of device characteristics and user behavior. Nicotine yield
increases with e-cigarette power and e-liquid nicotine concentration, and with increasing puff duration.
pH OF E-LIQUIDS
Nicotine is a weak base with a pKa of 8.5. The absorption and renal excretion of nicotine is highly pH dependent (IOM, 2001). In acidic environments, nicotine is in its protonated, charged state and does not cross membranes rapidly. For example, the smoke of flue-cured cigarettes (the most common form) has pH ranging from 5.5 to 6.0, resulting in nicotine existing primarily in the protonated form (Benowitz et al., 2009). Studies have shown little buccal absorption of nicotine from flue-cured cigarette smoke (Gori et al., 1986). On the other hand, smoke from air-cured tobacco, the dominant form used in pipes and cigars, has a pH of 6.5 or greater, results in a higher fraction of unprotonated (free-base) nicotine, and is absorbed in the mouth (Armitage et al., 1978).
The proportion of free-based (unprotonated) nicotine, which is the more volatile and readily absorbed form, increases with pH (Pankow, 2001; Pankow et al., 1997). Given its relatively high volatility, more free-base nicotine in combustible tobacco cigarette smoke is thought to lead to greater deposition of free-base nicotine in the mouth and throat (Henningfield et al., 2004). Although free-base nicotine is absorbed in the mouth and upper respiratory tract, the rate of such absorption into the blood is slower than in the lungs (Bergstrom et al., 1995). On the other hand, deposition of free-base nicotine in the mouth and throat leads to greater sensory effects due to possible activation of peripheral nerves (Henningfield et al., 2004).
The pH of e-liquids and its implications for nicotine absorption and pharmacological effects of e-cigarettes have not been extensively studied. By definition, pH is relevant to aqueous solutions (water as the solvent). To measure the apparent pH of e-liquids, which have propylene glycol (PG) and/or glycerol as the solvent, the e-liquid is first dissolved in a known amount of deionized water and pH measured over a time period (El-Hellani et al., 2015). Using this method, Lisko and colleagues (2015) found that the pH of a sample of 36 cartridges and refill liquids ranged from 5.1 to 9.1. The pH was positively correlated with the nicotine concentration of the e-liquid. Interestingly, this relationship was stronger in laboratory-prepared e-liquids than commercial e-liquids, indicating a potential effect of flavor additives on pH. El-Hellani and colleagues (2015) reported that cartridges from three brands with various nicotine concentrations and refill liquids had pH ranging from 7.4 to 9.7. This study found wide variability in nicotine partitioning between the unprotonated and protonated states of nicotine in the e-liquid and aerosols. Unproton-
ated nicotine was found to account for 18–95 percent of the total nicotine, depending on the product in question and the pH. Based on high agreement between measured and predicted amounts of protonated nicotine in laboratory-prepared e-liquids and poorer agreement in commercial e-liquids, the authors inferred, similar to Lisko and colleagues, that flavor additives in commercial e-liquids likely affect e-liquid pH (El-Hellani et al., 2015). Etter and Bugey (2017) reported pH of 18 e-liquids ranging from 8.1 to 9.9 (average = 9.1). The pH of 14 usual brand e-liquids of participants in a clinical study by St.Helen and colleagues (2017) ranged from 4.33 to 9.10 (average = 6.80).
Given that nicotine partitioning in the protonated and unprotonated forms in e-liquid and aerosol varies widely among products (El-Hellani et al., 2015), it is important to understand how such variation impacts nicotine deposition in the airways, rates of absorption, systemic exposure, and sensory effects. A pilot study by St.Helen and colleagues (2017) found elevated rates of nicotine absorption and maximum plasma nicotine concentration when participants used a strawberry e-liquid (18 mg/ml nicotine, 50/50 glycerol/PG, pH 8.29) compared with a tobacco e-liquid (18 mg/ml nicotine, 50/50 glycerol/PG, pH 9.10). After 15 puffs (30-second interpuff) with the same e-cigarette on separate days, 5-, 15-, and 30-minute areas under the plasma nicotine concentration-time curve (AUC) were 17–23 percent higher and maximum plasma nicotine concentration was 22 percent higher with the less basic strawberry e-liquid compared with the tobacco. This study was not a systematic study of the effect of pH, but suggests that a potential effect of flavorants is through pH. Systematic studies of the effect of e-liquid and aerosol pH on e-cigarette pharmacology are needed for more definitive answers. The pH of e-liquids is one e-liquid characteristic that the Food and Drug Administration may consider regulating, but more research is needed.
NICOTINE SALTS
Nearly all e-cigarettes use solvents such as PG and glycerol as the carrier compounds in the aerosol. However, novel e-cigarettes are being developed that do not contain glycerol or PG, but contain nicotine base and a weak organic acid that forms a nicotine salt. These devices are patterned after technology described by Rose and colleagues (2008). One example is JUULTM by JUUL Labs. Chemical analysis of the liquid in JUULTM pods, which are prefilled cartridges, found benzoic acid and nicotine in a 0.97–1 molar concentration ratio (44.8 ± 0.6 and 61.6 ± 1.5 mg/ml, respectively) (Pankow et al., 2017), indicating that benzoic acid is a major ingredient of this device. The nicotine salt, nicotine benzoate, likely forms when the device is activated, and is delivered to the user in an aerosol
form. Furthermore, Philip Morris Products S.A. recently developed a novel e-cigarette called P3L (Teichert et al., 2017). The device consists of a cartridge containing nicotine base and lactic acid in separate cavities. On activation and controlled heating, the nicotine salt (nicotine lactate) is released as an aerosol. In a clinical study, maximum plasma nicotine concentrations from use of three formulations of P3L, namely, 50, 80, and 150 µg/puff P3L, were 9.7, 11.2, and 9.8 ng/ml, respectively (Teichert et al., 2017). JUUL™ and new products such as P3L show the potential use of nicotine salts to deliver nicotine in electronic nicotine delivery systems.
TOXICOLOGY AND MODES OF ACTION
In this section, the pharmacology of nicotine is summarized, but it is not intended to be a systematic review of the topic. Several authoritative reviews have been published on nicotine and were identified as the primary sources for this summary. They include the 1988 Surgeon General’s report on smoking, The Health Consequences of Smoking: Nicotine Addiction (HHS, 1988); the 2001 Institute of Medicine report Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction (IOM, 2001); the 2010 Surgeon General’s report How Tobacco Smoke Causes Disease (HHS, 2010b); and reviews on nicotine chemistry, metabolism, disposition kinetics, and pharmacology (e.g., England et al., 2017). Individual adverse outcomes of nicotine are covered in greater detail in the specific health outcomes sections.
General Pharmacology of Nicotine
Nicotine, 3-(1-methyl-2-pyrrolidinyl) pyridine, consists of a pyridine and a pyrrolidine ring, is volatile, and has a molecular weight of 162.23 (Benowitz, 2009). It is the most abundant tobacco alkaloid, making up about 95 percent of the alkaloid content of combustible tobacco cigarettes and 1.5 percent by weight in cigarette tobacco (Benowitz et al., 2009). The nicotine content of commercially available e-liquids varies from low to high (commonly 0.3–5 percent by volume) (Cameron et al., 2014; Cheng, 2014; Etter and Bugey, 2017; Etter et al., 2013; Goniewicz et al., 2015). Most of the nicotine in tobacco is the levorotary (S)-isomer; (R)-nicotine is found in much smaller quantities (0.1–0.6 percent) (Benowitz et al., 2009).
On activation of the e-cigarette, nicotine is released from the e-liquid on aerosol particles or volatilized to gas-phase nicotine, which are then inhaled. Nicotine bound to particles can be deposited into the lungs, where it is expected to be rapidly absorbed into the pulmonary venous circulation, or to evaporate from particles on impact in the mouth and upper airways and absorbed into the circulation, but slower than in the lungs.
As with tobacco smoke, gas-phase nicotine is expected to be absorbed in the mouth and upper airways, which may contribute to the sensory effects of nicotine in the mouth and throat. Once nicotine enters the pulmonary venous circulation, it then enters the arterial circulation and rapidly moves across the blood–brain barrier into the brain (Benowitz, 2009). Nicotine then diffuses readily in brain tissue and (S)-nicotine, the predominant form, binds stereoselectively to nicotine cholinergic receptors (nAChRs) (Benowitz, 2009). nAChRs are ligand-gated ion channels, which open when a cholinergic agonist binds to the outside of the channel. When the channels open, they allow the entry of cations such as calcium and sodium, which activates signal transduction pathways, including activation of voltage-dependent calcium channels that allow further entry of calcium (Benowitz, 2009).
Nicotine-induced stimulation of central nervous system nAChRs results in the release of multiple neurotransmitters in the brain, dopamine being dominant, which have been related to nicotine’s pharmacodynamic effects. The action of nicotine leads to the release of dopamine, which is associated with pleasure and appetite suppression, in the mesolimbic area, the frontal cortex, and the corpus striatum (Benowitz, 2009). Dopamine release in the shell of the nucleus accumbens and the dopaminergic neurons in the ventral tegmental area of the midbrain are especially important because this pathway is involved in drug-induced reward (HHS, 2014). The pleasurable experience from dopamine release plays a critical role in the reinforcing effects of nicotine. When dopamine neurons in rat brain are chemically or anatomically lesioned, self-administration of nicotine is prevented (Benowitz, 2009; IOM, 2001). Other nicotine-induced behaviors are mediated by a variety of neurotransmitters that are also released, including norepinephrine (arousal, appetite suppression), acetylcholine (arousal, cognitive enhancement), serotonin (mood modulation, appetite suppression), γ-aminobutyric acid (reduction of anxiety and tension), glutamate (learning, memory enhancement), and endorphins (reduction of anxiety and tension) (Benowitz, 2008).
Nicotine addiction develops as a neurobiological adaptation to chronic nicotine exposure (HHS, 2014). An important characteristic of nicotine dependence is the emergence of withdrawal symptoms on abrupt cessation of nicotine administration (compulsory nicotine administration is the other characteristic of nicotine dependence). Tolerance (neuroadaptation) to nicotine develops for some nicotinic effects on repeated exposure to nicotine. The number of nAChR binding sites in the brain increases, which is thought to represent upregulation in the response of nicotine-mediated desensitization of receptors (Benowitz, 2009). During periods of abstinence in chronic smokers, such as during nighttime sleep, previously desensitized α4β2 nAChRs become unoccupied and recover to
a responsive state. Abstinence symptoms are believed to develop when these nAChRs revert to this unoccupied and responsive state. Craving and withdrawal symptoms are alleviated through nicotine binding and desensitization of the receptors.
nAChRs are also located at the interganglionic junctions of the autonomic nervous system and on organs throughout the body as part of the parasympathetic autonomic nervous system (HHS, 2010b, 2014). Stimulation of these globally expressed nAChRs causes wide-ranging physiological effects such as nicotine intoxication syndrome. Symptoms of nicotine intoxication syndrome include nausea and vomiting. More severe poisoning can progress to diarrhea, increased salivation and respiratory secretions, bradycardia, seizures, and respiratory depression. The rapid development of tolerance to nicotine with repeated administration helps counter the development of acute nicotine toxicity (HHS, 2014).
Nicotine Receptor Pharmacology
The nAChR complex, a pentamer, includes combinations of α, β, γ, and δ subunits (IOM, 2001), and is found in the peripheral and central nervous systems (Benowitz, 2009; Gotti et al., 2006). nAChRs have been located in the brain, neuromuscular junctions, autonomic ganglia, and adrenal medulla (Gundisch, 2000; IOM, 2001). The varied effects of nicotine in both the peripheral and central nervous systems are mediated by the specific configurations of the subunits. While nicotine exerts diverse pharmacological effects in the peripheral nervous system (e.g., stimulation in the trachea that may enhance the reinforcing effect of self-administration), it is generally believed that the actions of nicotine in the central nervous system are pivotal to reinforcing tobacco use (HHS, 1988). Neuronal subunits that are thought to be attributed to the effects of nicotine contain α3,4,7 and β2,4 subunits (IOM, 2001). The mammalian brain contains up to nine α subunits (α2 to α10) and three β subunits (β2 to β4). In the human brain, α4β2, α3β4, and α7 (homomeric) are the most abundant receptor subtypes; α4β2, with or without the presence of other subunits, is predominant and is thought to be the primary receptor mediating nicotine dependence in humans (Benowitz, 2009). The α4β2 receptor may also include subunits such as α5, α6, and/or β3. The additional subunits on the α4β2 receptor can modulate the sensitivity and function of the receptor. Furthermore, it appears that the β2 subunit is particularly important in reinforcing effects of nicotine. β2 subunit gene knockout mice did not show the behavioral effects of nicotine (Benowitz, 2009; Picciotto, 1998). The behavioral effects of nicotine were restored on reinsertion of the β2 subunit into the ventral tegmental area of the β2 knockout mice (Benowitz, 2009; Maskos et al., 2005). The α4 subunit seems to play a role
in determining sensitivity to nicotine while the α7 subunit appears to play an important role in withdrawal, learning, and sensory gating, and is involved in rapid synaptic transmission (Benowitz, 2009; IOM, 2001). In addition, the cardiovascular effects of nicotine are thought to be mediated by the α3β4 nAChR.
Pharmacokinetics and Pharmacodynamics of Nicotine
The amount of nicotine delivered and the way in which it is delivered influences the addictiveness of a tobacco product (HHS, 2010b). The abuse liability of tobacco products increases with greater delivery, faster rate of absorption, and higher blood nicotine concentrations. Furthermore, the route of administration and dose of nicotine influence the time course of nicotine in the brain and the resulting pharmacological effects (Hukkanen et al., 2005). Nicotine in tobacco smoke, once it reaches the small airways and alveolar region of the lungs, is rapidly absorbed into the pulmonary venous circulation. From there, nicotine moves quickly to the left ventricle of the heart, then to the systemic arterial circulation, and then to the brain (Hukkanen et al., 2005). High levels of nicotine reach the brain in about 15 seconds after a puff on a combustible tobacco cigarette (Berridge et al., 2010). This rapid increase in nicotine levels in the brain, faster than with intravenous administration, leads to activation of the dopaminergic reward system, as discussed before, and produces rapid behavioral reinforcement (Hukkanen et al., 2005). Given the rapid rise of nicotine and associated psychoactive effects, smoking allows the smoker to titrate the level of nicotine and related effects during smoking. This makes smoking the most reinforcing and dependence-producing form of nicotine administration.
Nicotine is delivered from e-cigarettes through the pulmonary route in a manner that is very similar to that of combustible tobacco cigarettes. As discussed above and in detail later in this chapter under Exposure to Nicotine and Nicotine Derivatives from E-Cigarettes, e-cigarettes can deliver nicotine levels comparable to combustible tobacco cigarettes (St.Helen et al., 2016a), and the plasma nicotine profile can resemble that of combustible tobacco cigarette smokers (Dawkins et al., 2016; Ramoa et al., 2016; St.Helen et al., 2016a; Wagener et al., 2017). With the potential for high and rapid delivery of nicotine to the user, e-cigarettes are expected to produce nicotine-related psychoactive effects that can cause or maintain nicotine dependence. Whether or not e-cigarettes are as reinforcing and dependence producing as combustible tobacco cigarettes is an important question, with implications for both smoking cessation and transitioning from e-cigarettes to combustible tobacco cigarettes. The abuse liability of
e-cigarettes relative to tobacco cigarettes is discussed in greater detail in Chapter 8.
Nicotine from chewing tobacco and snuff are rapidly absorbed because these products are buffered to alkaline pH. However, blood nicotine concentration rises gradually and plateaus at about 30 minutes, with levels remaining elevated and slowly decreasing over a nicotine half-life (2 hours) or more (Hukkanen et al., 2005). The rise in brain nicotine concentrations is slower than with smoking. Formulations of nicotine replacement therapy (NRT) are also buffered to alkaline pH to facilitate oral absorption. A considerable amount of nicotine administered orally is swallowed and undergoes first-pass metabolism. Because gastric fluid is acidic, nicotine is poorly absorbed in the stomach. On the other hand, nicotine is absorbed more efficiently in the small intestine due to the alkaline pH there and the large surface area. Given the slower rate of increase of nicotine levels in the blood and the brain from NRT administered orally, the abuse liability of NRT is considered to be low (Hukkanen et al., 2005). The gradual rise of nicotine levels in the brain allows for the development of tolerance to the pharmacological effects of nicotine, resulting in less intense central nervous system stimulation.
Nicotine base absorbs readily through the skin. This is the basis for nicotine transdermal systems, such as nicotine patches, and is also the reason for some nicotine toxicities in the occupational setting (green tobacco sickness). Nicotine-containing e-liquids can potentially make contact with the skin of users or non-users, such as children and infants. Therefore, dermal contact with nicotine-containing e-liquids can lead to systemic nicotine exposure.
Once absorbed into the circulation, nicotine is distributed extensively to body tissues with average steady-state volume of distribution ranging from 2.2 to 3.3 L/kg (see Table 4-1). Less than 5 percent of nicotine dose binds to plasma proteins (Hukkanen et al., 2005). Nicotine has low affinity for adipose tissue and high affinity for liver, kidney, spleen, lung tissues, and the brain. The receptor-binding capacity of nicotine in the brain is higher in smokers compared with non-smokers because of the upregulation of nicotinic cholinergic receptors in the brain of smokers (Hukkanen et al., 2005). Due to ion-trapping, nicotine accumulates in gastric juice and saliva. Nicotine also accumulates in breast milk as well as in fetal serum and amniotic fluids (once it crosses the placental barrier) in slightly higher concentrations than maternal serum.
Peak-to-trough blood nicotine levels oscillate considerably from cigarette to cigarette (Benowitz, 2009). During daily smoking, typical peak blood nicotine concentrations range from 19 to 50 ng/ml, while typical trough concentrations range from 10 to 37 ng/ml; depending on how the cigarette is smoked, each cigarette increases blood nicotine concentra-
| Clearance (ml/minute) | Renal Clearance (ml/minute) | Non-Renal Clearance (ml/minute) | Volume of Distribution Elimination (Steady State) Half-Life (L/kg) (minute) | |
|---|---|---|---|---|
| Nicotine | 1,110–1,500 | 35–90 | 1,050–1,460 | 2.2–3.3 100–150 |
| Cotinine | 42–55 | 3–9 | 36–52 | 0.69–0.93 770–1,130 |
| Trans-3´hydroxycotinine | 82 | 50 | 32 | 0.66 396 |
SOURCE: Hukkanen et al., 2005.
tions by 5–30 ng/ml (Benowitz et al., 2009). Given the rapid delivery and absorption of nicotine from smoking, blood nicotine concentration rises while smoking and peaks at the end of smoking. Blood nicotine levels then decline rapidly over the next 20 minutes as nicotine distributes to tissue, with a distribution half-life of 8 minutes (Hukkanen et al., 2005). The elimination half-life of nicotine is about 2 hours. Consistent with this half-life, nicotine from regular smoking over 6–9 hours accumulates in the body. Smoking, therefore, results in exposure to nicotine in an intermittent and transient manner, but importantly, exposure to nicotine lasts 24 hours per day (Benowitz, 2009). The persistent systemic exposure to nicotine leads to persistent presence of nicotine in the brain throughout the day and night and results in structural and functional changes in nicotinic receptors and in intracellular processes of neuroadaptation (Benowitz, 2009). There is wide variability in patterns of e-cigarette use during the day. Nonetheless, as discussed later, e-cigarette users also administer nicotine throughout the day, likely leading to persistent systemic exposure to nicotine and associated neuroadaptation and tolerance to pharmacological effects of nicotine observed in combustible tobacco cigarette smokers.
Biotransformation of Nicotine
The metabolism of nicotine has been reviewed in depth elsewhere (Benowitz et al., 2009; Hukkanen et al., 2005) and is summarized in this section (see also Figure 4-1). The main site of nicotine metabolism is the liver, where it is extensively metabolized. Nicotine contains both aromatic and aliphatic carbon and nitrogen atoms, which can be sites for metabolic oxidation and subsequent conjugation reactions (IOM, 2001). Cotinine is
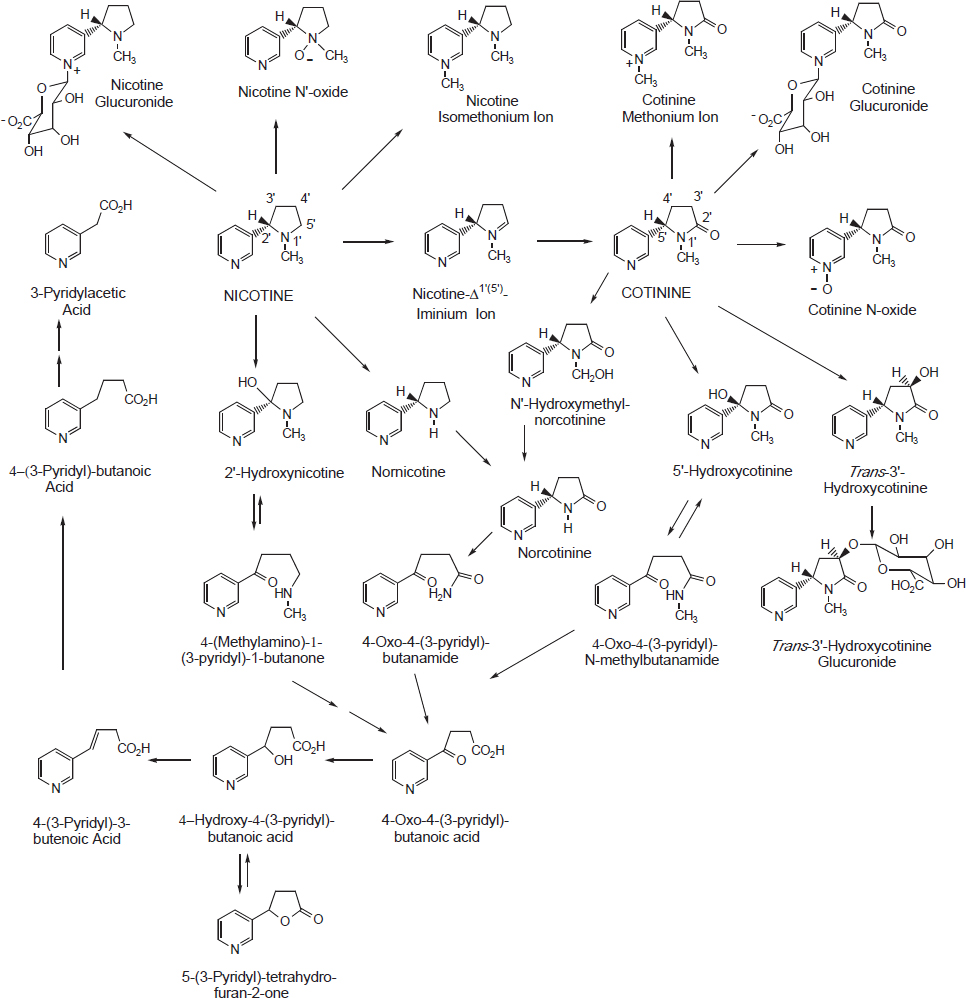
SOURCE: Hukkanen et al., 2005.
quantitatively the most important nicotine metabolite in mammals. About 70–80 percent of nicotine is metabolized through the cotinine pathway. Nicotine is converted to cotinine via a two-step metabolic process, consisting of a cytochrome P450–mediated reaction (CPY2A6) to produce nicotine-Δ1´(5´)-iminium ion followed by a cytoplasmic aldehyde oxidase reaction (Hukkanen et al., 2005). Cotinine is further metabolized to a number of metabolites. About 4 to 7 percent of nicotine absorbed in smokers is converted to nicotine N´-oxide through the action of flavin-containing monooxygenase 3 (FMO3). Nicotine N´-oxide is not metabolized further and is excreted in this form or reduced back to nicotine. Cotinine and nicotine N´-oxide are formed through oxidation of the pyrrolidine ring.
Nonoxidative methylation of the pyridine nitrogen and glucuronidation of nicotine are two additional metabolic pathways. The methylation pathway is catalyzed by N-methyltransferase, forming the nicotine isomethonium ion in small amounts in smokers. Formation of (S)-nicotine-N-β-glucuronide, which constitutes about 3–5 percent of nicotine metabolites excreted in urine, is catalyzed by the uridine diphosphate-glucuronosyltransferase (UGT) enzyme (Hukkanen et al., 2005).
Nornicotine, which is also a constituent of tobacco leaves, is formed from absorbed nicotine through oxidative N-demethylation through the CYP450 system. About 0.41 and 0.65 percent of nicotine is excreted as nornicotine in users of transdermal nicotine and smokers, respectively (Hukkanen et al., 2005). Finally, nicotine undergoes 2´-hydroxylation through CYP450 activity to produce 2´-hydroxynicotine as an intermediate. 2-Hydroxynicotine yields 4-(methylamino)-1-(3-pyridyl)-1-butanone and nicotine-Δ1´(2´)-iminium ion. 4-Oxo-4-(3-pyridyl)butanoic acid and 4-hydroxy-4-(3-pyridyl)-butanoic acid are derived from 4-(methylamino)-1-(3-pyridyl)-1-butanone and form about 10–15 percent of excreted nicotine and metabolites (Hukkanen et al., 2005).
Despite the cotinine pathway being the predominant metabolic route of nicotine, only 10–15 percent of nicotine absorbed by smokers is excreted as unchanged cotinine. Cotinine has six primary metabolites in humans: 3´-hydroxycotinine, 5´-hydroxycotinine, cotinine-N-oxide, cotinine methonium ion, cotinine glucuronide, and norcotinine (Benowitz et al., 2009). 3´-Hydroxycotinine, the most abundant nicotine metabolite in smokers’ urine, and its O-glucuronide conjugate account for 40–60 percent of the nicotine dose in urine. Conversion of cotinine to cotinine N-oxide is formed by CYP450 enzymes, unlike formation of nicotine N-oxide, and accounts for 2–5 percent of the excreted nicotine and metabolites in urine. Norcotinine, making up about 1 percent of excreted nicotine and metabolites in urine, is formed either through demethylation of cotinine or oxidation of nornicotine (Hukkanen et al., 2005).
Nicotine and metabolites measured in urine, referred to as the total nicotine equivalents, account for approximately 90 percent of the systemic dose of nicotine (Benowitz et al., 2009). To summarize quantitatively the pattern of nicotine metabolism in humans, nicotine and metabolites are excreted in urine as nicotine N-oxide (4–7 percent), nicotine glucuronide (3–5 percent), cotinine (10–15 percent), trans-3´-hydroxycotinine (33–40 percent), cotinine glucuronide (12–17 percent), and trans-3´-hydroxycotinine glucuronide (7–9 percent).
Based on measurement of blood nicotine levels after administration of a known dose, average total clearance of nicotine is about 1,200 ml/minute. Given that nonrenal clearance makes up about 70 percent of liver blood flow, about 70 percent of the nicotine dose is removed from the
blood in each pass through the liver (Benowitz et al., 2009). On the other hand, clearances of cotinine and trans-3´-hydroxycotinine are slower and average about 45 ml/minute and 82 ml/minute, respectively (Hukkanen et al., 2005). The ratio of plasma or saliva 3´-hydroxycotinine to cotinine (3HC/cotinine), which is highly correlated with oral clearances of nicotine and cotinine and half-life of cotinine, is a validated non-invasive proxy of CYP2A6 metabolism of nicotine (Dempsey et al., 2004). The ratio of 3HC/cotinine in urine is also used as a proxy of CYP2A6 nicotine metabolism, with forms including unconjugated, glucuronidated, or total (unconjugated + glucuronidated) 3´-hydroxycotinine and cotinine. The validity of the ratio of 3´-hydroxycotinine to cotinine in urine as a proxy of CYP2A6 nicotine metabolism when using glucuronidated 3´-hydroxy-cotinine and/or cotinine may be influenced by observed differences in rates of glucuronidation of nicotine and cotinine among individuals or groups (Berg et al., 2010a,b).
CYP2A6 is the major enzyme involved in oxidation of nicotine to cotinine and cotinine to 3´-hydroxycotinine. CYP2A6 is also involved in 2´-hydroxylation of nicotine and in the formation of 5´-hydroxycotinine and norcotinine from cotinine (Hukkanen et al., 2005). Other enzymes involved in nicotine oxidation include CYP2B6 (second most active), CYP2D6, CYP2E1, and CYP2A13. CYP2A13 is a close relative of CYP2A6, is highly expressed in the respiratory tract, and includes shared substrates with CYP2A6 such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (Hukkanen et al., 2005). Aldehyde oxidase is the enzyme involved in the conversion of nicotine-Δ1´(5´)-iminium ion to cotinine. FMO3 catalyzes the formation of nicotine N´-oxide. Amine N-methyltransferase, whose expression is highest in human thyroid, adrenal gland, and lung, catalyzes N-methylation of nicotine. UDP-glucuronosyltransferase (UGT) catalyzes the phase II N-glucuronidation of nicotine and cotinine and N- and O-glucuronidation of 3´-hydroxycotinine. UGT2B10 and UGT1A4 are the main enzymes involved in N-glucuronidation of nicotine and cotinine, while UGT1A9 plays a minor role; UGT2B10 is thought to be a more efficient catalyst of N-glucuronidation (Benowitz et al., 2009; Berg et al., 2010a; Chen et al., 2007; Ehmer et al., 2004; Hukkanen et al., 2005; Kaivosaari et al., 2007). It is yet unknown which enzyme(s) catalyzes O-glucuronidation of 3´-hydroxycotinine but evidence suggests that UGT1A9 and UGT2B7 are involved given their action in NNAL O-glucuronidation and the high correlation between 3´-hydroxycotinine O-glucuronide and NNAL-O-glucuronide. Evidence suggests that UGT2B17 plays a major role in O-glucuronidation of 3´-hydroxycotinine while UGT2B10 and UGT1A4 are involved in its N-glucuronidation (Chen et al., 2012).
Gender and Racial Differences in Nicotine Metabolism and Genetic Polymorphisms
The rate of elimination of nicotine and cotinine varies considerably in humans and across species. A number of factors contribute to this observed interindividual variation, including physiological factors such as diet, age, gender, pathological conditions, medications, smoking, and racial and ethnic differences. These factors and known polymorphisms in genes encoding nicotine-metabolizing enzymes have been discussed in detail elsewhere (Benowitz et al., 2009).
Given that most nicotine is cleared through hepatic extraction, factors that change liver blood flow such as meals, exercise, and other physiological events can influence nicotine clearance. After eating a meal, hepatic blood flow increases by an estimated 30 percent and nicotine clearance increases by about 40 percent (Hukkanen et al., 2005). Some food constituents and additives are also known to mediate enzymes involved in nicotine metabolism. Menthol, a flavorant used in foods, toothpaste, combustible tobacco cigarettes, and e-cigarettes, moderately inhibits CYP2A6. Metabolism of nicotine to cotinine and glucuronidation of nicotine were inhibited after smoking mentholated cigarettes compared with after smoking non-mentholated cigarettes (Benowitz et al., 2004; Hukkanen et al., 2005). Although their effects on nicotine metabolism have not been studied, grapefruit and wheatgrass juice inhibit metabolism of coumarin, a CYP2A6 substrate, indicating that these foods likely inhibit nicotine metabolism.
Age is another physiological influence on the rate of nicotine metabolism. Clearance of nicotine decreases with age among adults. Compared with young adults, total clearance was 23 percent lower and renal clearance was 49 percent lower in the elderly (Hukkanen et al., 2005). Hepatic blood flow is lower in the elderly, leading to reduction in hepatic extraction of nicotine. At the other end, the half-life of nicotine in neonates has been shown to be three to four times longer than in adults, indicative of much slower rates of nicotine metabolism.
Hepatic clearance of nicotine slows during sleep as blood flow to the liver declines. The combination of this variation in hepatic blood flow and effect of meals on hepatic blood flow and nicotine clearance results in circadian variations in blood nicotine levels even during constant nicotine dosing (Hukkanen et al., 2005).
Gender-related differences in nicotine metabolism have been noted, with some studies reporting alternative conclusions. However, studies support that nicotine and cotinine clearances are higher in women compared with men; oral contraceptives further induce nicotine metabolism; and pregnancy markedly increases nicotine metabolism (Hukkanen et al., 2005). These gender and pregnancy differences are attributed to sex hor-
mones, given that estrogens and progesterone are higher in women than men, higher in women using oral contraceptives compared those who are not, and even higher during pregnancy (Hukkanen et al., 2005). Compared with men, nicotine and cotinine clearances were 13 percent and 16 percent higher in women not using oral contraceptives. Nicotine and cotinine clearances were induced by 30 percent and 33 percent, respectively, in women using oral contraceptives compared with women not using oral contraceptives (Hukkanen et al., 2005). Differences in coumarin metabolism have also been reported between women and men, supporting the idea that these gender differences are associated with CYP2A6 activity (Hukkanen et al., 2005). Pregnancy increases clearance of nicotine and cotinine by 60 percent and 140 percent, respectively, through increased induction of CYP2A6. Gender differences in nicotine glucuronidation has not been found in human studies, but studies using human liver microsomes suggest slower glucuronidation in women (Ghosheh and Hawes, 2002; Pulvers et al., 2016).
Significant racial differences in nicotine and cotinine metabolism have been noted. These differences may be a result of genetic variations in nicotine-metabolizing enzymes as well as other external factors, such as predominant types of cigarettes smoked by a racial/ethnic group (e.g., menthol versus non-menthol). The fractional clearance of nicotine to cotinine, metabolic clearance of nicotine to cotinine, and total and non-renal clearance of cotinine were significantly lower in blacks compared with whites (Benowitz et al., 1999; Perez-Stable et al., 1998). Nicotine and cotinine glucuronidation, although polymorphic in blacks (i.e., presence of both people who formed N-glucuronide fast and those who formed it slowly), were lower compared with whites, who showed unimodal distribution of glucuronidation (Berg et al., 2010a; Hukkanen et al., 2005). In comparisons among Chinese Americans, Latinos, and whites, total and non-renal clearance of nicotine and cotinine, and metabolic clearance of nicotine via the cotinine pathway were lowest among Chinese Americans (Benowitz et al., 2002; Hukkanen et al., 2005). Chinese are known to have higher frequencies of reduced function or dysfunctional CYP2A6 alleles compared with whites (Hukkanen et al., 2005; Pitarque et al., 2001; Wang et al., 2003). Japanese are also known to have higher frequencies of null and reduced activity CYP2A6 alleles, resulting in slower nicotine metabolism.
Polymorphisms in genes encoding nicotine-metabolizing enzymes are important determinants of the rate of nicotine metabolism in individuals and across racial groups, and have been discussed in detail elsewhere (Hukkanen et al., 2005). The rate of nicotine metabolism is associated with the likelihood of being an adult smoker (Schoedel et al., 2004), number of cigarettes smoked per day (Benowitz et al., 2003; Schoedel et al., 2004),
exposure to tobacco-related toxicants (Derby et al., 2008), and efficacy of smoking cessation with NRT (Lerman et al., 2006, 2010; Schnoll et al., 2009). Several polymorphisms have been noted in CYP2A6. The wild-type allele is denoted by CYP2A6*1A. Fully inactive CYP2A6 alleles are associated with substantial reduction in CYP2A6 activity. CYP2A6 whole gene deletion alleles include CYP2A6*4A, CYP2A6*4B, and CYP2A6*4D. Reduced activity also comes from alleles containing a single nucleotide change such as CYP2A6*2 and CYP2A6*5. Slow nicotine metabolizers include those with alleles such as CYP2A6*6, CYP2A6*7, CYP2A6*8, and CYP2A6*9, which produce functional enzymes with reduced metabolic capacities. Other alleles such as CYP2A6*1XN produce enzymes with increased metabolic activity. Polymorphisms have also been noted in other genes that encode enzymes involved in nicotine metabolism, such as in CYP2B6, CYP2D6, CYP2E1, and CYP2A13. Polymorphisms in the genes for aldehyde oxidase have not been reported while several polymorphisms have been detected in the human FMO3 gene.
Other factors that lead to variation in nicotine metabolism include pathological conditions, medications, and tobacco smoke itself. Hepatic pathologies impact nicotine metabolism. Based on coumarin metabolism as a proxy for CYP2A6 activity, hepatitis A and alcoholic liver disease are expected to slow hepatic extraction of nicotine while liver fluke parasite infection induces nicotine metabolism. Kidney failure decreases both renal clearance of nicotine and also hepatic clearance due to inhibition of CYP2A6 activity or downregulation of hepatic CYP2A6 expression by accumulated uremic toxins (Benowitz et al., 2009). Drugs such as rifampicin, dexamethasone, phenobarbital, and other anticonvulsant drugs are known to induce CYP2A6. Other compounds such as pilocarpine, metyrapone, methoxsalen, naphthalene, rifampicin, and others that are known to reduce coumarin metabolism through inhibition of CYP2A6 are expected to inhibit nicotine metabolism (Hukkanen et al., 2005). Studies with smokers as well as those with coumarin support that tobacco smoke inhibits CYP2A6-mediated metabolism of nicotine. While the exact CYP2A6 inhibitor(s) in tobacco smoke have not been identified, β-nicotyrine, a minor tobacco alkaloid, inhibits CYP2A6 in vitro (Benowitz et al., 2009; Denton et al., 2004). Downregulation of CYP2A6 expression, but not CYP2A6 inhibition, is another explanation for smoking-induced reduction of nicotine clearance. While smoking reduces nicotine C-oxidation, it appears that it induces 3′-hydroxycotinine O-glucuronidation. Rates of N-glucuronidation of nicotine and cotinine have not been shown to be affected by smoking.
Species Differences in Nicotine Metabolism
The highest total metabolism of nicotine has been seen in guinea pig and hamster hepatocytes followed by those of mice and humans, indicating cross-species differences in nicotine metabolism. All mammal species produce cotinine and 3′-hydroxycotinine as the major metabolites of nicotine. However, guinea pigs and rats form as much nicotine N′-oxide as cotinine and 3′-hydroxycotinine. Other differences exist across species, including rates of nicotine metabolism, relative amounts of metabolites produced, as well as differences in the major CYP enzymes involved in nicotine metabolism. For example, CYP2A is inactive in nicotine metabolism in rats while CYP2B is the main active enzyme. In nonhuman primates, nicotine metabolism resembles that of humans. Nicotine N′-glucuronidation also differs across species, with highest activity in the human liver and no activity in rats, mice, dogs, and rabbits. Cotinine glucuronidation has only been detected in humans (Hukkanen et al., 2005).
Other Effects of Nicotine
Carcinogenesis
Concerns about the potential carcinogenic risk of nicotine is important due to the growing prevalence of use of alternative forms of nicotine delivery such as e-cigarettes and other non-combustible tobacco products, as well as smokers who attempt to quit through extended use of NRT. Carcinogenesis consists of initiation, promotion, and progression. A complete carcinogen is an agent (physical, chemical, or biological, e.g., viruses) that can, by itself, induce tumors, usually with initiating, promoting, and progressing properties (Haussmann and Fariss, 2016). Initiation, the first stage of the cancer process, consists of genetic alterations such as mutations and deletions made by the initiating agent. Promotion involves the selective clonal expansion of initiated cells to produce preneoplastic lesions; both endogenous and exogenous agents that stimulate cell growth can act as tumor promoters. Importantly, repeated applications of or continuous exposure to agents that promote tumors is required for continued growth of preneoplastic lesions (Klaassen and Watkins, 2015). Progression entails conversion of benign preneoplastic lesions into invasive cancer.
Current evidence does not support the idea that nicotine is a human carcinogen, let alone a complete carcinogen. Specifically with respect to initiation, the 2014 Surgeon General’s report found mixed data for a genotoxic effect of nicotine; most studies were negative (HHS, 2014). The Lung Health Study, a 5-year randomized trial to assess the effects of smoking cessation on chronic lung disease and lung function, investigated the cancer risk from using NRT products (Murray et al., 2009). This
has been the only study to provide information on long-term NRT users. It found no evidence for an effect of NRT use on overall cancer risk or specifically for lung or gastrointestinal tract cancers. One study reported no additional mutagenic potential from increasing nicotine yield in cigarette smoke (Chen et al., 2008). In fact, the only exception was an animal study which found sarcomas in the muscle and uterus of A/J mice exposed to nicotine; no other tumors were found (Galitovskiy et al., 2012). In this study, A/J mice were subcutaneously injected with a nicotine dose of 3 mg/kg five times per week for 24 months (equivalent to 2.1 mg/kg/day of nicotine) (Grando, 2014), a dose comparable to that from consuming regular Scandinavian snus (Wickholm et al., 2012). The Surgeon General’s report (HHS, 2014, p. 114) found that the current body of evidence from animal and human studies on this topic failed to support the hypothesis that nicotine is a human carcinogen, concluding that “there is insufficient data to conclude that nicotine causes or contributes to cancer in humans.”
The Surgeon General’s report (HHS, 2014, p. 114) went on to conclude that “there is evidence showing possible oral, esophageal, or pancreatic cancer risks” (HHS, 2014, p. 114); the risks are indirect evidence based on some evidence of endogenous formation of the carcinogenic tobacco-specific nitrosamine (TSNA), N′-nitrosonornicotine (NNN), in users of NRT (Carmella et al., 1997; Knezevich et al., 2013; Stepanov et al., 2009a,b) and elevated risk of these cancers in users of smokeless tobacco products (IARC, 2012). NNN is a potent carcinogen that has been shown to induce tumors locally and systemically (Hecht, 1998), and is associated with increased risk of esophageal cancer in smokers (Yuan et al., 2011). Although the Surgeon General’s report did not find evidence to conclude that nicotine causes cancer, the report also stated that “there is some biological basis for proposing that nicotine may promote cancer based on experimental studies that have limitations in replicating human exposure and on mechanistic studies, but human evidence is lacking” (HHS, 2014, p. 113). Of importance to the potential tumor-promoting properties of nicotine are nAChRs located in organs such as the lungs, which can be involved in triggering signaling pathways in lung cells. As discussed in the 2014 Surgeon General’s report, nicotine’s effects on carcinogenic pathways include (1) inhibition of apoptosis; (2) stimulation of the release of epidermal growth factor and activation of Ras-Raf-ERK cascade, which affects cell proliferation; (3) activation of ERK, PI3-K, and mTOR and the expression of PPAR-β/δ by stimulating fibroblast production; and (4) possible promotion of metastases because nicotine stimulates cell motility and migration, loss of cell adhesion, and induction of the transition of well-differentiated epithelial cells to highly invasive carcinoma via epithelial-mesenchymal transition (HHS, 2014). The potential for nicotine to promote and spread tumors through its effects on cancer cell survival
and protection from apoptosis, nAChR mediation of nicotine-dependent upregulation of proliferative and survival genes, effects on metastasis, and nicotine-related induction of pathological angiogenesis that facilitates tumor survival and spreading have been discussed extensively in a review by Grando (2014).
In addition to the 2014 Surgeon General’s report, other studies also found that there is insufficient evidence to determine whether nicotine is a human carcinogen. A systematic review was conducted to determine the potential carcinogenic effect of nicotine at levels found in users of nicotine delivery systems (Haussmann and Fariss, 2016). The only epidemiological study included was the study on long-term NRT use after smoking cessation, same as the 2014 Surgeon General’s report. The review concluded that “for human studies (NRT use), there appears to be inadequate evidence for an association between nicotine exposure and the presence of or lack of a carcinogenic effect due to a limited number of studies” (Haussmann and Fariss, 2016, p. 709). Based on animal studies, the review concluded that “limited evidence suggests an association between long-term nicotine exposure and a lack of a complete carcinogenic effect” (Haussmann and Fariss, 2016, p. 715). The review of approximately 70 animal studies also concluded that there is inadequate evidence to conclude that nicotine exposure does or does not modulate (stimulate) carcinogenesis in humans.
An additional line of evidence to inform our understanding of whether nicotine can contribute to increased cancer risk is to assess the occurrence of cancer in smokeless tobacco users. Smokeless tobacco products used in Scandinavia have lower levels of TSNAs compared with traditional smokeless tobacco products and combustible tobacco products (HHS, 2014; Stepanov et al., 2006), but deliver as much nicotine as combustible tobacco cigarettes (Digard et al., 2013). In Sweden, the prevalence of smokeless tobacco use is 12.3 percent (20.7 percent in men, 3.5 percent in women) (Leon et al., 2016). In a longitudinal cohort of male Swedish construction workers, use of snus by never-smoking users was independently associated with increased risk of pancreatic cancer (higher risk compared with never users of any tobacco), but was unrelated to incidence of oral and lung cancers (Luo et al., 2007). Exposure to the TSNA NNK is the likely explanation for the observed increased in pancreatic cancer risk among snus users. NNK exposure is known to induce pancreatic cancer in rats when administered orally (Rivenson et al., 1988). Furthermore, a study of smokeless tobacco users enrolled in the National Longitudinal Mortality Study in the United States found that current smokeless tobacco users did not have elevated mortality from all cancers combined, and pancreatic, esophageal, and oral cavity cancers separately, compared with never users of tobacco (Timberlake et al., 2017). These studies provide
additional evidence to suggest that nicotine per se is not contributing to human cancer risk.
When the evidence is viewed in total, while there is a biological rationale for how nicotine could potentially act as a carcinogen in humans, there is no human evidence to support the hypothesis that nicotine is a human carcinogen. While it is biologically plausible that nicotine can act as a tumor promoter, the existing body of evidence indicates this is unlikely to translate into increased risk of human cancer. Studies of NRT users, which show no increase in cancer risk (Murray et al., 2009), and studies of smokeless tobacco users, which show increase in risk of cancers related to TSNA exposure but not an increase in risk of other cancers (Luo et al., 2007; Timberlake et al., 2017), indicate that it is unlikely that nicotine exposure acts as a tumor promoter to increase the risk of cancer in humans. Based on the existing body of evidence, it is reasonable to infer there is likely no significant increase in risk of cancer from exposure to nicotine delivered by e-cigarettes.
Cardiovascular Effects
The cardiovascular effects of nicotine have been reviewed in the 2010 and 2014 Surgeon General’s reports and elsewhere (Benowitz and Burbank, 2016; HHS, 2010b, 2014). Given that epidemiological studies cannot effectively disentangle smoking-related cardiovascular disease caused by nicotine and that caused by other toxic substances in tobacco smoke, analysis of epidemiological studies of long-term NRT or smokeless tobacco users facilitates evaluation of the cardiovascular risk of nicotine. The factors that mediate the effects of nicotine on the cardiovascular system are complex. Many of these effects are thought to be related to activation of nAChRs. As stated before, nAChRs are found in endothelial, immune, neuronal, and muscle cells (HHS, 2014).
Activation of the sympathetic nervous system produces hemodynamic effects manifested as increased heart rate, blood pressure, myocardial contractility, and cutaneous and coronary vasoconstriction (Benowitz and Fraiman, 2017; Bhatnagar, 2016). Stimulation of the sympathetic nervous system by nicotine is thought to be a result of activation of nAChRs in the peripheral nervous system, as well as those in the central nervous system (Benowitz and Burbank, 2016). Nicotine increases adrenal release of epinephrine and adrenergic neuron release of norepinephrine (HHS, 2010a). Heart rate and blood pressure increase regardless of the nicotine source or route of administration. Blood vessels constrict in response to nicotine, including coronary blood vessels and blood vessels in the skin, but those in skeletal muscle dilate (Benowitz and Burbank, 2016). Increased sympathetic activity from acute exposure to nicotine is also
associated with a decrease in heart rate variability in both smokers and nicotine-naïve healthy human subjects (Bhatnagar, 2016).
Nicotine also impacts coronary blood flow, but the net effect is a balance of two actions with opposite effects (Benowitz and Burbank, 2016). Through its action on α1-adrenergic receptors in vascular smooth muscle, nicotine can constrict coronary arteries and decrease blood flow. On the other hand, nicotine-induced accelerated heart rate increases cardiac output, which causes flow-mediated dilation (FMD). FMD directly stimulates β2 receptors in the coronary artery for vasodilation. While the pathophysiological significance of the sympathomimetic-driven hemodynamic effects of nicotine are unclear, increases in heart rate, reduction in heart rate variability, and endothelial dysfunction can lead to reduced myocardial blood flow, coronary occlusion, and increased myocardial demand for oxygen and nutrients, all of which are known to be associated with increased risks of myocardial ischemia/infarction and sudden death (Bhatnagar, 2016).
Other effects of nicotine on the cardiovascular system are believed to include myocardial remodeling, arrhythmogenesis, thrombogenesis, endothelial dysfunction, inflammation, and angiogenesis (Benowitz and Burbank, 2016). Persistent sympathetic stimulation by nicotine, particularly through β-adrenergic activation, can enhance myocardial tissue remodeling. Tissue remodeling (hypertrophy and fibrosis) creates heart failure. The arrhythmogenic effect of nicotine is mediated through catecholamine release, which can contribute to ventricular tachycardia and fibrillation. The thrombogenic effect of nicotine varies. Some animal studies have reported increased platelet activation from acute exposure to nicotine, whereas long-term exposure in rodents leads to reduced platelet activation. Studies of NRT and smokeless tobacco do not show increased platelet activation following nicotine intake. Endothelial dysfunction, which consists of impaired FMD (the vasodilatory response to increased local blood flow), is mediated primarily by oxidative stress and chronic inflammation. It is not clear what additional effect nicotine has on endothelial dysfunction above that of the effects of powerful oxidants and pro-inflammatory agents. Nevertheless, impaired endothelial function has been observed in people following local infusion of nicotine and use of a nicotine inhaler (Bhatnagar, 2016; Neunteufl et al., 2002). Inflammation plays an important role in several mechanisms that lead to cardiovascular diseases, namely atherogenesis and acute ischemic events. However, nicotine appears to have both anti-inflammatory and pro-inflammatory effects. Nicotine can act on the immune system directly by activating nAChRs that modulate immune function or indirectly by activating the sympathetic nervous system. Nicotine can also act on the cholinergic immune system by activating non-neuronal α7
nAChRs, which has an anti-inflammatory effect. On the other hand, by acting as a chemotactic agent, nicotine can contribute to inflammation by facilitating migration of neutrophils (HHS, 2010a). In other studies, nicotine enhanced leukocyte–endothelium interactions, resulting in greater leukocyte rolling and adhesion in mice; nicotine stimulated an inflammatory response by acting on human monocyte-derived dendritic cells; and nicotine increased secretion of pro-inflammatory cytokines in cultured dendritic cells (HHS, 2010a). Nevertheless, based on studies showing significant decline in inflammatory markers after switching from smoking to transdermal nicotine and similar levels of inflammatory markers between smokeless tobacco users and non-tobacco users, nicotine is not believed to be the main determinant of an inflammatory response in smokers (HHS, 2010a). Similarly, acute exposure to nicotine enhanced angiogenesis through its action on α7 nAChRs, but chronic exposure to nicotine in rodents led to impairment of angiogenesis, which indicates that nicotine is not an important driver of tobacco smoke-related angiogenesis (Benowitz and Burbank, 2016).
Smoking is associated with a more atherogenic lipid profile, progression of chronic hypertension to accelerated or malignant hypertension, and type 2 diabetes, which raises questions about the role of nicotine. While nicotine is known to induce lipolysis via catecholamine action at β-adrenoreceptors, and increasing plasma-free fatty acid concentrations, which possibly results in enhanced synthesis of low-density lipoproteins (LDLs) and lowering of high-density lipoproteins (HDLs), cessation studies using NRT and nicotine nasal sprays report improvement in HDL/LDL ratios and reduced dyslipidemia (Benowitz and Burbank, 2016; HHS, 2010a; Murray et al., 1996). Smoking causes transient increases in blood pressure, but is not associated with high blood pressure. A majority of smokeless tobacco studies have also not reported an increased incidence or prevalence of hypertension in users. However, smoking is likely associated with progression of chronic hypertension to accelerated or malignant hypertension; nicotine-induced vasoconstriction can play a role in this escalation. Finally, smokers have increased insulin resistance compared with non-smokers and cigarette smoking is recognized as an important risk factor for type 2 diabetes. It appears that nicotine is the main constituent in tobacco smoke responsible for increased insulin resistance in people. This is based on studies showing a dose–response association between hyperinsulinemia and insulin resistance in people with long-term use of nicotine gum (HHS, 2010a). Nicotine-induced release of hormones such catecholamine, cortisol, and growth hormone, which are insulin antagonists, can enhance insulin resistance. In addition, nicotine produces insulin resistance by directly activating AMP-activated protein
kinase via α7 nAChR effects in adipose tissue (Benowitz and Burbank, 2016).
Because e-cigarettes are designed to deliver nicotine to the user, the cardiovascular effects of nicotine must be considered when assessing the overall potential cardiovascular effects of e-cigarettes. The evidence related to the cardiovascular effects of e-cigarettes is reviewed in Chapter 9. However, based on known cardiovascular effects of nicotine (Benowitz and Burbank, 2016; HHS, 2010b), exposure to nicotine from e-cigarettes likely elevates the risk in people with preexisting cardiovascular disease(s), but the risk in people without cardiovascular disease(s) is uncertain.
EXPOSURE TO NICOTINE AND NICOTINE DERIVATIVES FROM E-CIGARETTES
The abuse liability of e-cigarettes and their potential to help combustible tobacco cigarette smokers quit smoking and/or sustain dual use of combustible tobacco cigarette and e-cigarettes depend to a great extent on the amount of nicotine delivered and how it is delivered. E-cigarettes, which deliver more nicotine and facilitate faster nicotine absorption and higher blood nicotine concentrations, are expected to be more satisfying and addictive.
This section primarily addresses the question: What is the nicotine exposure profile of e-cigarettes? In short, how fast is nicotine from e-cigarettes absorbed, and what is the systemic exposure to nicotine? These questions can be answered through clinical studies that measure biomarkers of nicotine exposure after e-cigarette use, including pharmacokinetic parameters such as the maximum blood nicotine concentration (Cmax) and time to maximum concentration (Tmax). Studies that assess nicotine exposure biomarkers in smokers who switch to e-cigarettes over a study period are also useful in describing nicotine exposure from e-cigarettes. Furthermore, other studies measure biomarkers of nicotine exposure longitudinally in long-term e-cigarette users, thus providing information on the stability or progression of nicotine intake in e-cigarette users.
The committee identified 27 clinical studies that investigated acute nicotine exposure from e-cigarette use. Details of each study, including product used, nicotine content of e-cigarettes, sample size, puffing protocol, and biomarker concentrations or pharmacokinetic parameters are presented in Table 4-2. The studies entailed nicotine administration during either a controlled session (bout of fixed number of puffs), during ad lib use over a period of time, or both. The studies enrolled either combustible tobacco cigarette smokers who had not used e-cigarettes before or were infrequent users (often referred to as inexperienced users
or e-cigarette–naïve smokers) or current e-cigarette users (often referred to as experienced users). Two studies enrolled both experienced users and e-cigarette–naïve smokers (Farsalinos et al., 2015; Fearon et al., 2017).
Comparisons of nicotine exposure from e-cigarettes with other inhaled forms of nicotine such as combustible tobacco cigarettes or nicotine inhalers can inform questions of the relative addictiveness of e-cigarettes or their ability to serve as a substitute for combustible tobacco cigarettes among smokers who want to quit (Benowitz et al., 2009). Some studies included combustible tobacco cigarettes or inhalers as comparators. For general reference, combustible tobacco cigarette smokers absorb about 1 mg (range = 0.3–2 mg) of nicotine systemically from smoking, which represents about 80 to 90 percent of the amount of nicotine inhaled (Armitage et al., 1975). Average venous blood nicotine Cmax ranges from 15 to 30 ng/ml and Tmax ranges from 5 to 8 minutes from the first puff (Benowitz et al., 2009). Typical average venous plasma nicotine Cmax from a 1-mg nicotine spray ranges from 5 to 8 ng/ml, and Tmax ranges from 11 to 18 minutes from the start of administration.
Clinical Studies with E-Cigarette–Naïve Smokers (Inexperienced Users)
Seventeen studies, including the ones by Fearon and colleagues (2017) and Farsalinos and colleagues (2015), enrolled smokers with no or little experience with e-cigarettes. The study by Bullen and colleagues (2010) was the first such study. Study participants were randomized to use an e-cigarette (Ruyan V8) with or without nicotine (16-mg nicotine cartridge), nicotine inhaler (Nicorette) or their usual combustible tobacco cigarette over 4 study days. A subset (n = 8) gave venous blood samples for nicotine pharmacokinetic analysis. Participants used the e-cigarette and combustible tobacco cigarette ad lib over 5 minutes and the inhaler over 20 minutes. Use of the e-cigarette with nicotine (16 mg cartridge) resulted in only a small increase in plasma nicotine (Cmax = 1.3 ng/ml). By comparison, average Cmax for the combustible tobacco cigarette and inhaler were 13.4 ng/ml and 2.1 ng/ml, respectively. The fastest Tmax was achieved with the combustible tobacco cigarette (14.3 minutes after first puff) followed by the nicotine e-cigarette (19.6 minutes after first puff) and the inhaler (32 minutes after first administration). While the authors concluded that the pharmacokinetic profile of the e-cigarette was similar to the inhaler, they also suggested that the shorter Tmax with the e-cigarette compared with the inhaler may be due to absorption of nicotine from e-cigarette aerosol in the respiratory tract while nicotine from the inhaler is absorbed buccally.
Eissenberg (2010) presented preliminary findings of a within-sub-
TABLE 4-2 Summary of Clinical Studies Examining Nicotine Exposure from E-Cigarette Use
| Reference | Study Characteristics | ||
|---|---|---|---|
| Study Product | Nicotine Content | Sample Size | |
| Clinical Studies with E-Cigarette–Naïve Smokers | |||
| Bullen et al., 2010 | Ruyan V8 | 16-mg cartridge | 8 |
| Eissenberg, 2010 | NPRO by NJOY or Hydro by Crown Seven | 16-mg cartridge (both brands) | 16 |
| Vansickel et al., 2010 | NPRO by NJOY or Hydro by Crown Seven | NPRO: 18-mg cartridge; Hydro, 16-mg cartridge | 32 |
| Vansickel et al., 2012 | Vapor King | 18-mg/ml cartridge | 20 |
| Flouris et al., 2013 | Giant by Nobacco G.P. | 11-mg/ml cartridge | 15 |
| Farsalinos et al., 2015 | eVic by Joyetech (2nd generation) | 18-mg/ml | 23 |
| Results | ||
|---|---|---|
| Puffing Protocol | Biomarker | Study Comparison |
| e-cigarette: 5 minutes ad lib; inhaler: 20 minutes ad lib; usual cigarette: 5 minutes ad lib | plasma nicotine: Cmax: 1.3 (0–2.6) ng/ml (mean and 95% CI); Tmax: 19.6 (4.9–34.2) minutes following initial puff | inhaler: Cmax: 2.1 (1.0–3.1) ng/ml, Tmax: 32 (18.7–45.3) minutes; usual cigarette: Cmax: 13.4 (6.5–20.3) ng/ml, Tmax: 14.3 (8.8–19.9) minutes |
| two 10-puff standardized sessions, 30-second interval, sessions were 1 hour apart | plasma nicotine: after first session: NPRO, 3.5 (0.5) ng/ml (mean, SEM); Hydro, 2.5 (0.2) ng/ml | usual brand cigarette: 16.8 (3.4) ng/ml |
| two 10-puff standardized sessions, 30-second interval, sessions were 1 hour apart | no significant change in plasma nicotine | usual brand cigarette: baseline: 2.1 (0.32) ng/ml (mean, SD); 5 minutes after session 18.8 (11.8) ng/ml |
| six 10-puff standardized sessions, 30 seconds between puffs; sessions were 30 minutes apart | plasma nicotine: baseline: 2.2 (0.78) ng/ml (mean, SD); 5 minutes after last session: 7.4 (5.1) ng/ml | N/A |
| median = 11 puffs; puffs varied between participants; took equivalent puffs to be equivalent to two usual brand cigarettes based on a ratio of 1.5 cigarettes to e-cigarette nicotine absorption ratio | plasma cotinine: increased significantly immediately after and 1 hour after e-cigarette use | usual brand cigarette: no significant difference in plasma cotinine between e-cigarette and cigarette use |
| 10 puffs in 5 minutes followed by ad lib use in 60 minutes | plasma nicotine: baseline: 1.6 (0.3) ng/ml (mean, SEM); 5 minutes after first puff: 4.3 (0.7) ng/ml; after 65 minutes: 13.8 (1.6) ng/ml | N/A |
| Reference | Study Characteristics | ||
|---|---|---|---|
| Study Product | Nicotine Content | Sample Size | |
| Nides et al., 2014 | King Bold by NJOY | 26-mg cartridge | 25 |
| Hajek et al., 2015 | Green Smoke | 2.4% cartridge (24 mg/ml) | 6 |
| Oncken et al., 2015 | Joye eGo-C | 18-mg/ml e-liquid with tobacco or tobacco and menthol | 20 |
| Yan and D’Ruiz, 2015 | blu e-cigs | 5 different formulations: 3 with 24 mg/ml and 2 with 16 mg/ml | 23 |
| Results | ||
|---|---|---|
| Puffing Protocol | Biomarker | Study Comparison |
| two 10-puff standardized sessions, 30-second interval, sessions were 1 hour apart | plasma nicotine: 30 seconds after first 10 puffs: 3.5 (0.69) ng/ml (mean, SEM); 10 minutes after 10 puffs of second session: 5.1 (1.1–7.1) ng/ml (mean, range) | N/A |
| two 5-minute ad lib sessions: the first was at baseline and the second 4 weeks later | plasma nicotine: baseline: Cmax: 4.6 (3.0) ng/ml (mean, SD); Tmax: 5.0 (0.0) minutes; week 4: Cmax: 5.7 (3.3) ng/ml; Tmax: 5.0 (0.0) minutes | N/A |
| two 5-minute ad lib sessions; each session was preceded by 7–10 days of e-cigarette use with a different e-liquid | plasma nicotine: session 1: baseline: 4.2 (1.1) ng/ml (mean, SE); 5 minutes after first puff: 8.2 (1.7) ng/ml; session 2: baseline: 4.2 (0.7) ng/ml; 5 minutes after first puff: 9.3 (0.73) ng/ml | N/A |
| 50-puff standardized session, 5-second puff, 30-second interval,1-hour ad lib session | plasma nicotine: baseline: range of mean: 0.01 (0.05)–0.04 (0.13) ng/ml (mean, SD); 5 minutes after first puff: range of mean: 1.99 (1.47)–3.00 (1.38) ng/ml; 30 minutes after first puff: range of mean: 9.96 (3.59)–17.05 (6.64) ng/ml | one Marlboro Gold King Size: baseline: 0.03 (0.12) ng/ml (mean, SD); 5 minutes after first puff: 14.42 (9.42) ng/ml; 30 minutes after first puff: 7.86 (1.99) |
| plasma nicotine: range of mean at end of ad lib session: 13.70 (5.95)–22.42 (7.66) ng/ml | end of ad lib: 29.23 (10.84) ng/ml | |
| Reference | Study Characteristics | ||
|---|---|---|---|
| Study Product | Nicotine Content | Sample Size | |
| D’Ruiz et al., 2015 | Not specified (but same study as Yan and D’Ruiz, 2015) | 5 different formulations: 3 with 24 mg/ml and 2 with 16 mg/ml | 23 |
| Antoniewicz et al., 2016 | eGo XL 3.7-V battery with dual-coil CE5 atomizer | 12 mg/ml e-liquid | 16 |
| Lopez et al., 2016 | eGO 3.3-V battery with 1.5-Ω Smoktech cartomizer | 4 different e-liquids: 0, 8, 18, or 36 mg/ml nicotine | 16 |
| Walele et al., 2016 | e-cigarette prototype | 2 mg/ml nicotine (flavored and unflavored) | 12 |
| 0%, 0.4%, 0.9%, 2.0% nicotine | 12 | ||
| Results | ||
|---|---|---|
| Puffing Protocol | Biomarker | Study Comparison |
| 50-puff standardized session, 5-second puff, 30-second interval | plasma nicotine: Cmax within first 30 minutes: 10.3 (3.7)–18.1 (6.47) (range of mean, SD) | tobacco cigarette: Cmax within first 30 minutes: 15.8 (8.64) ng/ml (mean, SD) |
| 1-hour ad lib session | plasma nicotine: range of mean at end of ad lib session: 13.7 (5.98)–22.4 (7.65) ng/ml | end of ad lib: 29.2 (10.86) ng/ml |
| 10 puffs in 10 minutes | plasma cotinine: 4 hours after 10 puffs: 4.1 ng/ml (median); IQR: 3.5, 4.7 ng/ml | John Silver cigarette (1 mg): plasma cotinine using similar protocol: 7.8 ng/ml (median), IQR: 4.6, 14.2 |
| two 10-puff standardized sessions, 30-second interval, sessions were 1 hour apart | plasma nicotine: 5 minutes after 1 puff: 3.8 (3.30) ng/ml for 0 mg/ml e-liquid (mean, SD); 8.8 (6.3) ng/ml for 8 mg/ml; 13.2 (13.2) ng/ml for 18 mg/ml: 17.0 (17.9) ng/ml for 36 mg/ml e-liquid | N/A |
| 10-puff standardized session, 4-second puff, 30-second interval (e-cigarette and inhaler), 2-second puff for tobacco cigarette | plasma nicotine: unflavored: Cmax: 3.6 (33.9) ng/ml (mean, CV%); Tmax: 9 (range, 1–15) minutes; flavored: Cmax 2.5 (41.6) ng/ml; Tmax: 10 (3–45) minutes | JPS Silver King Size cigarette: Cmax: 21.2 (43.1) ng/ml; Tmax: 3.0 (1–6) minutes; inhaler: Cmax: 2.5 (45.2); Tmax: 13 (5–15) minutes |
| 10-puff standardized session, 4-second puff, 30-second interval | plasma nicotine: 0%: Cmax: 0.6 (346.4) ng/ml (mean, CV%); Tmax: 60 minutes (median); 0.4%: Cmax 1.0 (41) ng/ml; Tmax: 5 (1–60) minutes; 0.9%: Cmax 1.9 (33) ng/ml; Tmax: 7 (1–15) minutes; 2.0% Cmax 3.6 (20.9) ng/ml; Tmax: 7 (3–30) minutes | N/A |
| Reference | Study Characteristics | ||
|---|---|---|---|
| Study Product | Nicotine Content | Sample Size | |
| 0%, 0.4%, 0.9%, 2.0% nicotine | 12 | ||
| Fearon et al., 2017 | Vype vPro ePen (Nicoventures, Ltd.) | 1.86% | 23 |
| Papaseit et al., 2016 | Nhoss (tank) | 16 mg/ml | 9 |
| Stiles et al., 2017 | Vuse Solo | 14-, 29-, and 36-mg cartridge | 45 |
| Clinical Studies with Experienced E-Cigarette Users | |||
| Vansickel and Eissenberg, 2013 | usual brands | average of e-liquid: 17.6 mg/ml; range: 9–24 mg/ml | 8 |
| Dawkins and Corcoran, 2014 | SKYCIG | 18-mg cartridge | 14 |
| Results | ||
|---|---|---|
| Puffing Protocol | Biomarker | Study Comparison |
| 10-puff standardized session, 30 seconds between puffs; followed by 15–60 minutes ad lib use | plasma nicotine: standardized: Cmax: 2.5 (0.5–6.9) ng/ml (GM, range); Tmax: 6.0 (median); ad lib: Cmax: 5.9 (1.6–12.5) ng/ml; Tmax: 75 minutes (median) | JPS Blue: plasma nicotine: standardized: Cmax: 13.0 (5.3–35.5) ng/ml; Tmax: 7 minutes (median); ad lib: Cmax: 14.1 (6.9–40.6) ng/ml; Tmax: 75 minutes |
| two 10-puff sessions (30-second interpuff interval); 1 hour apart | plasma nicotine: first bout: Cmax: 5.8 (0.0–14.5) ng/ml (median, range); Tmax: 15 (0–55) minutes; second bout: Cmax: 5.9 (0.0–24.6) ng/ml; Tmax: 75 (55–120) minutes | Marlboro: first bout: Cmax: 7.3 (2.9–16.4) ng/ml (median, range); Tmax: 5 (5–45) minutes; second bout: Cmax: 9.0 (3.7–19.6) ng/ml; Tmax: 90 (65–120) minutes |
| up to 10 minutes of ad lib use of e-cigarette or cigarette; 30 minutes chewing nicotine gum | plasma nicotine: Cmax: 14 mg: 3.01 ng/ml; 29 mg: 4.67 ng/ml; 36 mg: 5.36 ng/ml; Tmax: 14 mg: 27.35 minutes; 29 mg: 21.83 minutes; 36 mg: 24.17 minutes | usual brand cigarette: Cmax: 17.98 ng/ml; Tmax: 8.13 minutes; nicotine gum: Cmax: 5.26; Tmax: 50.88 min |
| 10-puff standardized session, 30 seconds between puffs | plasma nicotine: baseline: 2 (0) ng/ml (mean, SEM); 5 minutes after first puff: 10.3 (2) ng/ml | N/A |
| 1-hour ad lib session | 16.3 (4.5) ng/ml | |
| 10 puffs within 5 minutes; ad lib interval | plasma nicotine: baseline: 0.74 (0.12) ng/ml (mean, SEM); 10 minutes after start of 10 puffs: 6.77 (1.23) ng/ml | N/A |
| 1-hour ad lib session | 13.91 (2.12) ng/ml | |
| Reference | Study Characteristics | ||
|---|---|---|---|
| Study Product | Nicotine Content | Sample Size | |
| Farsalinos et al., 2015 | eVic by Joyetech (2nd generation) | 18 mg/ml | 24 |
| Ramoa et al., 2016 | eGO 3.3-V battery with 1.5-Ω cartomizer | 4 different e-liquids: 0, 8, 18, or 36 mg/ml nicotine | 16 |
| Spindle et al., 2015 | usual battery with 1.5-Ω SmokTech cartomizer | usual e-liquid: 21.7 (3.9) mg/ml (mean, SD); range: 12–24 mg/ml | 13 |
| Dawkins et al., 2016 | eVic Supreme (3.9 V with Nautilus Aspir tank (1.8 Ω) | ) 6 and 24 mg/ml e nicotine | 11 |
| St.Helen et al., 2016a | usual brands | usual e-liquid: 12.5 (7.1) mg/ml (mean, SD); range: 6–24 mg/ml | 13 |
| Wagener et al., 2017 | usual brands | 2nd generation: 22.3 (7.5) mg/ml (mean, SD) (range; 11–36 mg/ml); 3rd generation: 4.1 (2.9) mg/ml (range; 1.5–6 mg/ml) | 20 |
| Results | ||
|---|---|---|
| Puffing Protocol | Biomarker | Study Comparison |
| 10 puffs in 5 minutes followed by ad lib use in 60 minutes | plasma nicotine: baseline: 2.1 (0.3) ng/ml (mean, SEM); 5 minutes after first puff: 7.9 (0.9) ng/ml; after 65 minutes: 24.1 (2.0) ng/ml | N/A |
| two 10-puff standardized sessions, 30-second interval, sessions were 1 hour apart | plasma nicotine: change from baseline: −4.4 (17.9) ng/ml for 0 mg/ml e-liquid (mean, SD); 11.1 (9.4) ng/ml for 8 mg/ml; 18.1 (15.5) ng/ml for 18 mg/ml; and 24.1 (18.2) ng/ml for 36 mg/ml | N/A |
| 10-puff standardized session, 30 seconds between puffs, with or without topography device | plasma nicotine: baseline: 2.4 (0.2) ng/ml (mean, SEM); 5 minutes after first puff: 19.2 (2.3) ng/ml | N/A |
| 60-minute ad lib session | plasma nicotine: 10 minutes after first puff: 8.59 (7.52) ng/ml (mean, SD) for 6 mg/ml e-liquid and 33.77 (34.88) ng/ml for 24 mg/ml; end of session: 22.03 (16.19) for 6 mg/ml and 43.57 (34.78) ng/ml for 24 mg/ml e-liquid | N/A |
| standardized session of 15 puffs, 30-second interval | plasma nicotine: Cmax: 8.4 (5.1) ng/ml (mean, SD) (range: 2.3–19.8 ng/ml); Tmax: 5.1 (7.6) minutes (range: 2–30 minutes) | N/A |
| 10-puff standardized session, 30 seconds between puffs | 5 minutes after first puff: 2nd generation: 7.3 (2.8) ng/ml (mean, SD); 3rd generation: 17.5 (12.9) ng/ml | N/A |
| Reference | Study Characteristics | ||
|---|---|---|---|
| Study Product | Nicotine Content | Sample Size | |
| Fearon et al., 2017 | Vype vPro ePen (Nicoventures, Ltd.) and Nicolites (Nicocigs Ltd.) | Vype (1.86%) and Nicolites (1.33%) | 18 |
| Hajek et al., 2017 | usual brand and 8 other common brands | range: 16–48 mg/ml | 11 |
| Spindle et al., 2017 | usual battery with 1.5-Ω SmokTech cartomizer | usual e-liquid: 18.9 (5.9) mg/ml (mean, SD) | 29 |
| St.Helen et al., 2017 | KangerTech mini Protank 3 | two test e-liquids (18 mg/ml); usual e-liquid: 7.9 (6.0) mg/ml (mean, SD) (range: 3–18 mg/ml) | 14 |
| Results | ||
|---|---|---|
| Puffing Protocol | Biomarker | Study Comparison |
| 115-minute ad lib session | plasma nicotine: after last puff: 2nd generation: 23.5 (12.8) ng/ml; 3rd generation: 24.8 (11.6) ng/ml | |
| 5-minute ad lib use session | plasma nicotine: Vype: standardized: Cmax: 7.8 (0.0–40.2) ng/ml (GM, range); Tmax: 6.0 (median); Nicolites: Cmax: 4.7 (1.2–18.2) ng/ml; Tmax: 9 minutes (median) | Marlboro Ultralights tobacco cigarette: plasma nicotine: Cmax: 7.2 (0.7–37.6) ng/ml; Tmax: 6 minutes (median) |
| 5-minute ad lib session | plasma nicotine: Cmax: 7.5 (5.0)–13.6 (9.7) ng/ml (range of means (SD)); Tmax: 4–6 minutes after first puff (range of means) | usual tobacco cigarette: Cmax: 17.9 (16.0) ng/ml; Tmax: 4 minutes |
| 10-puff standardized session, 30 seconds between puffs, with or without topography device | plasma nicotine: baseline: 4.0 (0.7) ng/ml (mean, SEM); immediately after 10 puffs: 20.6 (2.8) ng/ml | N/A |
| 90-minute ad lib, with or without topography device | plasma nicotine: end of session: 35.0 (4.6) ng/ml | |
| standardized session of 15 puffs, 30-second interval | plasma nicotine: test e-liquids: Cmax: 12.1 (2.0) ng/ml and 9.5 (1.2) ng/ml (mean, SEM); Tmax: 5.4 (1.4) and 4.9 (1.2) minutes after last puff; usual e-liquids: Cmax: 6.2 (1.0) ng/ml; Tmax: 3.1 (0.4) minutes after last puff | N/A |
| 90-minute ad lib | plasma nicotine: test e-liquids: after 90 minutes: 16.5 (3.1) ng/ml and 11.3 (2.3) ng/ml; usual e-liquids: 11.2 (1.7) ng/ml | |
ject study that compared nicotine exposure from two e-cigarette brands (NPRO by NJOY and Hydro by Crown Seven), both 16 mg nicotine. Participants (n = 16) engaged in two 10-puff sessions, with 30-second interpuff intervals, and sessions were 1 hour apart. Other conditions include smoking their usual brand of combustible tobacco cigarettes and sham smoking (puffing an unlit cigarette). The committee presents the results from the first session, which reflected nicotine exposure after a period of abstinence (more than 12 hours). Only the combustible tobacco cigarette significantly increased plasma nicotine levels. Mean plasma nicotine levels immediately after the first session were: NPRO (3.5 ng/ml); Hydro (2.5 ng/ml); and usual brand of combustible tobacco cigarette (16.8 ng/ml).
Vansickel and colleagues (2010) present the results of the full study described above by Eissenberg. The NPRO cartridge used was 18 mg nicotine while the Hydro cartridge was 16 mg nicotine. Participants took 10 puffs at two separate times during each session, as described before. The participants’ usual tobacco cigarette brand significantly increased plasma nicotine concentration 5 minutes after the first puff, while NPRO, Hydro, and sham smoking did not alter blood nicotine levels.
Vansickel and colleagues (2012) conducted another study, this time with the e-cigarette Vapor King (18-mg/ml nicotine cartridge). Participants (n = 20) vaped the e-cigarette in six sessions (or six bouts), each time taking 10 puffs, one puff every 30 seconds and 1 hour between sessions. Average plasma nicotine concentration at baseline was 2.2 ng/ml, was significantly different after the fourth session, and reached 7.4 ng/ml 5 minutes after the final bout. This study indicated that prolonged use was required with these e-cigarettes before significantly elevating plasma nicotine levels. Still, the plasma nicotine levels after six 10-puff sessions were still lower than that from one typical combustible tobacco cigarette.
Flouris and colleagues (2013) examined the impact of e-cigarette use on serum cotinine and lung function in smokers. Fifteen smokers with no history of e-cigarette use crossed over among three conditions: their usual brand of cigarettes, an e-cigarette (Giant, 11-mg/ml nicotine cartridge), and sham smoking. Participants smoked two of their usual cigarettes over a 30-minute period. The number of puffs on the e-cigarette that would lead to equivalent amounts of nicotine delivered from the e-cigarette compared with their usual e-cigarettes was estimated using data from a pilot survey of 141 e-cigarette users who were former smokers. Information on nicotine content of the participants’ combustible tobacco cigarettes, nicotine concentration of e-liquids, and number of puffs required to consume 1 ml of e-liquid were used to obtain a tobacco/e-cigarette absorption ratio of 1.5. Based on this ratio, participants took a median of 11 puffs (mean ± SD = 10.4 ± 2.7 puffs) over a 30-minute period. Serum
cotinine immediately after and 1 hour after active smoking or e-cigarette use was significantly higher than baseline but there was no significant difference in serum cotinine between e-cigarette use and combustible tobacco cigarette use. This study suggests that when e-cigarettes deliver levels of nicotine comparable with combustible tobacco cigarettes, the overall systemic exposure to nicotine from e-cigarettes is similar to that of combustible tobacco cigarettes.
Nides and colleagues (2014) conducted a study of nicotine exposure from NJOY e-cigarettes. Participants (n = 25) were given King Bold by NJOY (26-mg nicotine cartridge) and were involved in two 10-puff standardized sessions (30-second interpuff interval, 1 hour between sessions). Average plasma nicotine concentration 30 seconds after the first session was 3.5 ng/ml, a fraction of that from smoking a typical combustible tobacco cigarette.
In another study, Yan and D’Ruiz (2015) randomized 23 smokers to five different formulations of blu e-cigarettes (three with 24 mg/ml nicotine and two with 16 mg/ml nicotine). For each cigarette, participants took 50 puffs (5-second duration, 30-second interpuff interval) during a standardized session followed by a 1-hour ad lib session. Participants also smoked a Marlboro Gold King Size cigarette (usual puff duration, 30-second interpuff interval). The range of average baseline plasma nicotine concentrations across all e-cigarette formulations ranged from 0.01 to 0.04 ng/ml, which increased to 1.99–3.00 ng/ml after 10 puffs and 7.86–17.05 ng/ml after 50 puffs. By comparison, average baseline plasma nicotine concentration was 0.03 ng/ml and increased to 14.42 ng/ml after smoking one cigarette. At the end of the 1-hour ad lib session, average plasma nicotine concentration ranged from 13.70 to 22.42 ng/ml with the e-cigarettes compared with 29.23 ng/ml with the combustible tobacco cigarettes. This study again showed that prolonged use of this e-cigarette was required before reaching plasma nicotine levels typically obtained after smoking just one combustible tobacco cigarette.
Farsalinos and colleagues (2015) compared plasma nicotine from acute e-cigarette use in 23 e-cigarette–naïve smokers with 24 experienced e-cigarette users. All participants were asked to take 10 puffs from a second-generation e-cigarette with 18 mg/ml e-liquid over 5 minutes followed by 60 minutes of ad lib use. Plasma nicotine levels were significantly higher in the experienced e-cigarette users 5 minutes after initiating puffing and at the end of the ad lib session. This study demonstrates that experience contributes to the ability of e-cigarette users to self-dose with nicotine from e-cigarettes.
Hajek and colleagues (2015) described differences in nicotine exposure among e-cigarette–naïve users who learned how to use the e-cigarette over a 4-week period. Six smokers interested in quitting, who were tak-
ing part in a larger study assessing toxicant exposure and nicotine intake (McRobbie et al., 2015), provided pharmacokinetic data. Participants used a Green Smoke first-generation e-cigarette (2.4 percent nicotine on label or 24 mg/ml) over a 5-minute ad lib period on the targeted quit smoking date (baseline) and 4 weeks later. While average plasma nicotine Cmax increased from baseline (week 1) to week 4 (4.6–5.7 ng/ml), that change was not significant; Tmax stayed the same (5 minutes). However, AUC from 0 to infinity was significantly higher at week 4, indicating higher systemic nicotine exposure. This study suggests that nicotine intake and systemic exposure from e-cigarettes can increase with practice.
Oncken and colleagues (2015) examined nicotine exposure with e-cigarette use in smokers who were not seeking treatment. The e-cigarette used was Joye e-Go-C. Participants crossed over between two e-liquid flavors, tobacco with menthol and tobacco, both 18 mg/ml nicotine. During each arm, participants used the e-cigarette over the previous 7–10 days at home. On the last day of each arm, participants were involved in a 5-minute ad lib session at the laboratory. Twenty participants completed at least one session and 18 completed both. Average plasma nicotine concentration increased significantly from baseline to 5 minutes after the first puff during both laboratory sessions. Average plasma nicotine increased from 4.2–8.2 ng/ml at session one and 4.2–9.3 at session two. There was no effect of laboratory session, flavor, or sex on change in plasma nicotine levels. However, women who were given their preferred flavor of e-liquid had significantly higher increase in plasma nicotine levels. The same effect was not seen among men.
Antoniewicz and colleagues (2016) conducted a study on the effects of e-cigarette aerosol on vascular function, and also reported plasma cotinine levels. Sixteen healthy seldom smokers who were also naïve e-cigarette users crossed over between use of an e-cigarette and a control condition of no e-cigarette use. The e-cigarette used was a second-generation CE5 atomizer with eGo XL 3.7-V battery, and 12 mg/ml nicotine e-liquid. Participants took 10 puffs in 10 minutes and cotinine was measured in plasma collected before and 4 hours after use. Median plasma cotinine at 4 hours was 4.1 ng/ml (participants with baseline cotinine were omitted from the study). By comparison, median plasma cotinine in smokers of John Silver cigarettes (1 mg) following a similar protocol by the same authors was significantly higher (7.8 ng/ml) (Antoniewicz et al., 2016; Mobarrez et al., 2014), suggesting that exposure to nicotine was lower from e-cigarettes compared with combustible tobacco cigarettes among seldom smokers.
Lopez and colleagues (2016) presented preliminary results of a study that examined the influence of e-liquid nicotine concentration on plasma cotinine levels. Sixteen smokers were enrolled and crossed over among e-liquids with 0, 8, 18, and 36 mg/ml nicotine. Participants used
a 3.3-V eGo battery with a 1.5-Ω dual-coil cartomizer (Smoktech) and were involved in two 10-puff standardized sessions (30-second interval, 1 hour between sessions). Five minutes after the first puff, plasma nicotine concentrations for the 0, 8, 18, and 36 mg/ml e-liquids were 3.8 ng/ml, 8.8 ng/ml, 13.2 ng/ml, and 17.0 ng/ml, respectively. This study indicated that for naïve users, plasma nicotine increased with e-liquid nicotine concentration.
Walele and colleagues (2016) described a study examining the nicotine pharmacokinetics of an e-cigarette prototype. The first part of the study entailed testing the effect of flavoring (menthol versus non-menthol) on nicotine exposure (2.0 percent nicotine for flavored and unflavored e-liquid). The second part examined nicotine exposure using e-liquids with 0 percent, 0.4 percent, 0.9 percent, and 2.0 percent nicotine (0, 4 mg/ml, 9 mg/ml, and 20 mg/ml, respectively). Twelve participants were enrolled in each part. In the first part, participants also smoked a JPS Silver King Size cigarette and a nicotine inhaler (15 mg). For the e-cigarette and inhaler, participants took 10 puffs (4-second duration, 30-second interpuff interval) and for the cigarette, 2-second puffs. Mean Cmax was significantly higher for the unflavored compared with the flavored e-cigarette (3.6 versus 2.5 ng/ml). Mean Cmax for the cigarette was 21.2 ng/ml and that of the inhaler was 2.5 ng/ml. Average Tmax for the unflavored and flavored e-liquid was 9 and 10 minutes after the first puff, respectively. The average Tmax for the cigarette and inhaler was 3 and 13 minutes after the first puff, respectively. The plasma nicotine profile was similar between the e-cigarette and the nicotine inhaler. In part two, the average Cmax for the 0 percent, 0.4 percent, 0.9 percent, and 2.0 percent nicotine was 0.6 ng/ml, 1.0 ng/ml, 1.9 ng/ml, and 3.6 ng/ml, respectively, increasing with the nicotine concentration of the e-liquid. Tmax did not vary across e-liquid nicotine concentration. This study was the first to examine the effect of flavors on nicotine pharmacokinetics of e-liquids and suggests that nicotine exposure was higher with the unflavored e-liquid compared with the flavored e-liquid (menthol). The role of e-liquid flavors on nicotine absorption and systemic exposure to nicotine is not well understood and needs further study.
Fearon and colleagues (2017) conducted a study to describe nicotine exposure from use of Vype vPro ePen e-cigarettes (Nicoventures, Ltd.). This study involved two parts; the first part is described here. E-cigarette–naïve smokers (n = 23) were enrolled in the first part and used a cartridge with 1.86 percent nicotine (18.6 mg/ml nicotine). Participants took part in a 10-puff standardized session (30-second interpuff interval) which was followed by 15–60 minutes of ad lib use starting at 15 minutes after the start of the initial standardized puffing session. Mean Cmax after 10 puffs
was significantly lower with the e-cigarette (geometric mean [GM] = 2.5 ng/ml) than with the conventional cigarette (GM = 13.4 ng/ml).
Papaseit and colleagues (2016) conducted a crossover study with nine e-cigarette–naïve users who were randomized to a second-generation e-cigarette (Nhoss) with 16 mg/ml e-liquid or Marlboro cigarette (0.8 mg nicotine per cigarette, USA) on each of 2 days. Participants took 10 puffs from each product (30-second interpuff interval) during two administrations, 1 hour apart. Plasma nicotine Cmax and Tmax did not differ significantly between products at either administration. Plasma AUC from 0 to 55 minutes was significantly higher with the combustible tobacco cigarette during the first administration, indicating higher systemic nicotine exposure from combustible tobacco cigarettes compared with e-cigarettes. It should be noted that plasma nicotine Cmax of 7.3 and 9.0 ng/ml from smoking the Marlboro cigarette are lower than typical Cmax from smoking combustible tobacco cigarettes.
Stiles and colleagues (2017) examined nicotine exposure from use of three different Vuse Solo e-cigarette formulations (14, 29, and 36 mg nicotine/cartridge) in e-cigarette–naïve smokers. Participants used the designated e-cigarette ad lib for up to 10 minutes. The comparators were their usual brand of combustible tobacco cigarettes, which was also smoked during a 10-minute ad lib period, and a nicotine gum (4 mg), which was chewed for 30 minutes. Average Cmax was significantly higher with smoking compared with the e-cigarettes. There were no significant differences in Cmax between the Vuse Solo 29 mg and Vuse Solo 36 mg e-cigarettes compared with the nicotine gum but Cmax was significantly lower with the Vuse Solo 14 mg compared with the nicotine gum. Tmax was shorter for the cigarette (8.1 minutes) compared with e-cigarettes (21.8–27.4 minutes) and nicotine gum (50.9 minutes). Based on the pharmacokinetic profiles of the products tested, the authors concluded that the abuse liability for the Vuse Solo e-cigarettes was lower than that of combustible tobacco cigarettes, but higher than that of nicotine gum.
These studies suggest that e-cigarettes deliver lower levels of nicotine when used by e-cigarette–naïve smokers compared with levels delivered from combustible tobacco cigarettes, which is about 1 mg (Djordjevic and Doran, 2009). Studies that include direct comparisons with combustible tobacco cigarettes show that, among these naïve users, plasma nicotine levels are much lower with e-cigarettes compared with their usual combustible tobacco cigarettes. All but three of the studies examined nicotine exposure from cigalikes (Antoniewicz et al., 2016; Farsalinos et al., 2015; Lopez et al., 2016).
Clinical Studies with Experienced E-Cigarette Users
Twelve studies reported nicotine exposure in experienced or current e-cigarette users. Vansickel and Eissenberg (2013) conducted a study that described systemic nicotine exposure among e-cigarette users who used their usual devices. Participants (n = 8) used their e-cigarettes during a 10-puff standardized session (30-second interpuff interval) followed by a 1-hour ad lib session. Nicotine concentration of the e-liquids ranged from 9 to 24 mg/ml (mean = 17.6 mg/ml). Plasma nicotine increased significantly from 2 ng/ml at baseline to 10.3 ng/ml 5 minutes after the first puff of 10, and increased to 16.3 ng/ml at the end of the 1-hour ad lib session. This study demonstrated significant nicotine delivery among experienced e-cigarette users who use their own e-cigarettes.
Dawkins and Corcoran (2014) enrolled 14 experienced e-cigarette users in a study to examine nicotine delivery from SKYCIG, a cigalike. Participants used the e-cigarette (18-mg cartridge) during a 10-puff session (puffs were taken within 5 minutes but puff interval was not controlled) and during a 1-hour ad lib session. Plasma nicotine increased from 0.74 ng/ml at baseline to 6.77 ng/ml 10 minutes after the start of the 10-puff session and increased to 13.91 ng/ml at the end of the ad lib session. Plasma nicotine concentration from use of this first-generation e-cigarette was consistent with levels measured in some e-cigarette–naïve users (Lopez et al., 2016; Stiles et al., 2017; Vansickel et al., 2012).
Ramoa and colleagues (2016) presented the preliminary results of a study that examined the relationship between e-liquid nicotine concentration and plasma nicotine concentration. Sixteen experienced e-cigarette users were involved in a within-subject comparison of e-liquids containing 0, 8, 18, and 36 mg/ml nicotine. All participants used a 1.5-Ω cartomizer that was powered by an eGO 3.3-V battery. Participants were involved in two 10-puff standardized sessions (30-second interpuff interval, sessions 1 hour apart). Plasma nicotine concentrations were related to the concentration of nicotine in the e-liquid. The change from baseline for the 0, 8, 18, and 36 mg/ml nicotine e-liquids was −4.4 ng/ml, 11.1 ng/ml, 18.1 ng/ml, and 24.1 ng/ml. This study also demonstrated that e-cigarettes can elevate blood nicotine levels in experienced users within the range of that of combustible tobacco cigarettes.
Spindle and colleagues (2015) examined the impact of using a topography device with e-cigarettes on plasma nicotine levels. Thirteen e-cigarette users were given their usual e-cigarette battery and a study cartomizer (1.5-Ω SmokTech) and their usual e-liquid (mean = 21.7 mg/ml nicotine, range = 12–24 mg/ml) and participated in a 10-puff standardized session (30-second interpuff interval) with or without the topography device, on different days. The topography device did not influence plasma nicotine concentration. Plasma nicotine increased from an average of 2.4
ng/ml at baseline to an average of 19.3 ng/ml immediately following e-cigarette use. This study again demonstrated that e-cigarettes can cause blood nicotine concentration to be elevated into the range of that of combustible tobacco cigarettes.
Dawkins and colleagues (2016) conducted another study in which they evaluated blood nicotine levels from using e-cigarettes with different e-liquid nicotine concentrations. In this study, 11 e-cigarette users used a Nautilus Aspire tank (1.8 Ω) with an eVic Supreme (3.9 V) and used e-liquids with 6 and 24 mg/ml. Participants used the e-cigarette over a 60-minute ad lib session. Ten minutes after the first puff, plasma nicotine concentrations for the 6 and 24 mg/ml e-liquids were 8.59 ng/ml and 33.77 ng/ml, respectively. At the end of the session, plasma nicotine concentrations for the 6 and 24 mg/ml e-liquids were 22.03 ng/ml and 43.57 ng/ml, respectively. These results are consistent with other studies which show that for a given e-cigarette, plasma nicotine concentration increases with the concentration of nicotine in the e-liquid, and that e-cigarettes can elevate plasma nicotine concentrations to combustible-tobacco-cigarette-like levels (Ramoa et al., 2016; Vansickel and Eissenberg, 2013).
St.Helen and colleagues (2016a) conducted a study of nicotine delivery, retention, and systemic exposure among experienced e-cigarette users. Participants (n = 13) used their usual e-cigarette during a standardized session of 15 puffs (30-second interpuff interval). Systemic nicotine retention was determined by measuring the amount of nicotine inhaled and the amount exhaled into gas traps. Nicotine concentration of the usual e-liquids used in this study ranged from 6 to 24 mg/ml (average = 12.5 mg/ml). An average of 1.3 mg (range = 0.42–2.64 mg) of nicotine was inhaled and an average of 1.22 mg (range = 0.42–2.34 mg) was systemically retained. This represents an average of 93.8 percent systemic retention of the inhaled dose, similar to combustible tobacco cigarettes (80–90 percent). Average Cmax, having controlled for baseline plasma nicotine, was 8.4 ng/ml (range = 2.3–19.8 ng/ml), and average Tmax was 5.1 minutes (all 2–5 minutes and one at 30 minutes) after the last of 15 puffs. This study demonstrated that the shape of the plasma nicotine concentration-time curve was, on average, similar to that of combustible tobacco cigarettes, albeit with a lower average Cmax compared with combustible tobacco cigarettes. However, there was variation, including some profiles that resembled that of smokeless tobacco, that is, slow rise to peak and sustained plasma nicotine levels, which are indicative of buccal absorption. Nevertheless, consistent with several other studies, the short time to peak for most e-cigarette users indicates rapid absorption of nicotine in the lungs. The study also demonstrated that, on average, e-cigarettes deliver comparable levels of nicotine to combustible tobacco cigarettes among experienced users.
Wagener and colleagues (2017) compared nicotine exposure from use of second- and third-generation e-cigarettes. Twenty participants (9 second-generation and 11 third-generation users) took 10 puffs in a standardized session (30-second puff interval) with their own devices and usual e-liquid brands. The average e-liquid concentrations used were 22.3 (SD = 7.5) mg/ml for the second-generation devices and 4.1 (2.9) mg/ml for the third-generation devices. Average power for the second generation devices was 8.6 (1.9) W and 71.6 (50.0) W for the third-generation devices. Plasma nicotine concentration 5 minutes after the 10th puff was 7.3 ng/ml for the second-generation e-cigarettes and 17.5 ng/ml for the third-generation e-cigarettes. The study showed that third-generation devices are able to mimic the plasma nicotine concentration of combustible tobacco cigarettes, likely due to their high power levels. The study also showed that users of third-generation devices consume significantly higher amounts of e-liquid compared with users of second-generation e-cigarettes. This implies that users of third-generation devices can potentially be exposed to higher levels of toxic substances that may be present in the e-liquid or given off in the aerosol.
Eighteen experienced e-cigarette users were enrolled in the second part of the study by Fearon and colleagues (2017). Participants crossed over between a Vype vPro ePen (Nicoventures, Ltd.) with 1.86 percent nicotine (18.6 mg/ml) and Nicolites (Nicocigs, Ltd.) with 1.33 percent nicotine (13.3 mg/ml). Participants took part in a 5-minute ad lib use session. A comparator was the Marlboro Ultralights tobacco cigarette. Average plasma nicotine Cmax was 7.8 ng/ml (geometric mean) and 4.7 ng/ml for Vype and Nicolites, respectively, compared with 7.2 ng/ml with the tobacco cigarette. Average plasma nicotine Cmax with the Vype e-cigarette was higher among experienced users compared with that of e-cigarette–naïve participants discussed in part 1 of this study, above.
Hajek and colleagues (2017) described the pharmacokinetic profiles of eight common e-cigarette brands in the United Kingdom as well as the participants’ usual brands. Eleven participants were enrolled. The test e-cigarettes had nicotine concentrations ranging from 16 to 48 mg/ml. Participants used each e-cigarette over a 5-minute ad lib session. Average Cmax ranged between 7.5 and 13.6 ng/ml and average Tmax ranged from 4 to 6 minutes from the first puff for the different e-cigarette brands. Average Cmax and Tmax for the cigalike (first-generation) e-cigarette were 8.5 ng/ml and 6 minutes compared with 11.7 ng/ml and 6 minutes for the refillable model e-cigarettes. Average Cmax for the usual combustible tobacco cigarette was 17.9 ng/ml and Tmax was 4 minutes. While the plasma nicotine Cmax for e-cigarettes was lower than for combustible tobacco cigarettes, there was variability among e-cigarettes; plasma nicotine Cmax was higher with use of the refillable e-cigarettes. This is likely
related to higher power of refillable (second-generation e-cigarettes) compared with cigalikes (Wagener et al., 2017).
In a second study by Spindle and colleagues (2017), which presents the full results of the study, experienced e-cigarette users (n = 29) were given a SmokTech cartomizer (1.5 Ω) to use with their usual e-cigarette battery and usual e-liquid (mean = 18.9 mg/ml nicotine). Participants used the e-cigarette in a 10-puff standardized session (30-second interpuff interval) followed by a 90-minute ad lib session, with or without a topography device attached. Consistent with preliminary results, plasma nicotine was not influenced by the topography device. Average baseline plasma nicotine concentration across conditions was 4.0 ng/ml, which increased to 20.6 ng/ml immediately after the 10 puffs. At the end of the 90-minute ad lib session, mean plasma nicotine concentration was 35.0 ng/ml. This study showed that the e-cigarette can be effective nicotine delivery devices.
St.Helen and colleagues (2017) conducted one of the first studies to examine the effect of e-liquid flavors on e-cigarette nicotine pharmacokinetics. Fourteen experienced e-cigarette users participated in the study and crossed over between two test flavors, strawberry and tobacco (both 18 mg/ml nicotine), and the participants’ usual e-liquid flavors (average = 7.9 mg/ml, range = 3–18 mg/ml nicotine). Each e-liquid was administered on a different day. Participants used the e-cigarette during a 15-puff standardized session (30-second interpuff interval) followed by a 90-min-ute ad lib session. Average amount of nicotine delivered ranged between 0.9 and 1.7 mg (depending on nicotine concentration of e-liquid) and average systemic retention of nicotine ranged from 98.6 percent to 99.2 percent (there was no flavor effect on nicotine delivery and systemic retention). Average plasma nicotine Cmax for two test flavors was 12.1 ng/ml and 9.5 ng/ml, respectively (strawberry versus tobacco); Tmax was 5.4 versus 4.9 minutes after the last of 15 puffs. Based on AUCs at various early time points, it appeared as if the rate of absorption of nicotine was faster with the strawberry compared with the tobacco e-liquid. The differences were not statistically significant, but AUCs from 0 minutes to 5, 15, and 30 minutes for the strawberry e-liquid were 23 percent, 20 percent, and 17 percent higher than that of the tobacco e-liquid, respectively. Cmax and Tmax for the usual flavors were 6.2 ng/ml and 3.1 minutes, respectively. Plasma nicotine concentrations after the 90-minute ad lib session were 16.5 ng/ml (strawberry), 11.3 ng/ml (tobacco), and 11.2 ng/ml (usual brand of e-liquids).
In summary, studies of nicotine delivery and systemic retention in experienced users suggest that e-cigarettes can deliver nicotine in the range of a typical combustible tobacco cigarette, and most of the nicotine is systemically retained under experimental conditions. While variability
remains between products and users, clinical studies with experienced users also indicate that e-cigarettes deliver nicotine in a way that resembles the pharmacokinetic profile of combustible tobacco cigarettes. Several studies reported plasma nicotine concentrations after 10 to 15 puffs or ad lib use (60–90 minutes), which were in the range of that of combustible tobacco cigarettes, particularly after use of high-powered third-generation e-cigarettes or high nicotine concentration e-liquids. These studies support the idea that exposure to nicotine from e-cigarettes is dependent, in part, on user experience. The type of e-cigarette, which is associated with the power used, as well as nicotine concentration of the e-liquid, are also important determinants of systemic nicotine exposure. Studies are needed to understand the role of flavors on the rate of nicotine absorption and systemic exposure in e-cigarette users.
Switching Studies
Studies in which tobacco cigarette smokers are given e-cigarettes to use instead of combustible tobacco cigarettes can be used to compare daily nicotine intake from combustible tobacco cigarettes and e-cigarettes, and answer whether e-cigarettes can effectively replace combustible tobacco cigarettes as a source of nicotine. The committee identified eight publications in which biomarkers of nicotine exposure were reported. Of these, two appear to describe the same parent study and present the same nicotine exposure results (D’Ruiz et al., 2016; O’Connell et al., 2016). Thus, seven studies are described. The seven studies measure biomarkers of nicotine exposure (cotinine and/or total nicotine equivalents, which is the molar sum of nicotine and its metabolites in urine) before and after switching to e-cigarettes.
McRobbie and colleagues (2015) assessed exposure to nicotine (as well as other toxic substances) before and after 4 weeks of e-cigarette use in 40 smokers who wanted to quit smoking. The study used a Green Smoke e-cigarette (first generation) with 2.4 percent nicotine (24 mg/ml) on the label. Participants were initially supplied with two cartridges per day, which was adjusted based on use, and were told to use the e-cigarette ad lib. Thirty-three participants were using the e-cigarette 4 weeks after the quit date. Of these, 16 (8 women) did not smoke combustible tobacco cigarettes during the previous week and 17 (8 women) smoked combustible tobacco cigarettes as well as the e-cigarette (dual users). Overall, urinary cotinine decreased 36 percent, from 1,655 ng/mg creatinine at baseline to 1,063 ng/mg creatinine at 4 weeks. Among the abstinent group, urinary cotinine decreased 17 percent, from 1,073 ng/mg creatinine to 889 ng/mg creatinine. Among the dual users, urinary cotinine decreased 44 percent, from 2,203 ng/mg creatinine to 1,227 ng/mg creatinine. This study sug-
gests a decrease in daily nicotine intake when smokers replace combustible tobacco cigarettes fully or partially with a first-generation e-cigarette.
Adriaens and colleagues (2014) investigated the efficacy of second-generation e-cigarettes to reduce craving and reduce combustible tobacco cigarette consumption in an 8-month randomized controlled trial. Forty-eight e-cigarette–naïve smokers (27 women) with no intention to quit combustible tobacco cigarettes were randomized into two e-cigarette groups and a control group. The two e-cigarette groups were assigned to use Joye eGo-C or Kanger T2-CC with 18 mg/ml nicotine e-liquid while the control group smoked their usual combustible tobacco cigarette. Cotinine was measured in saliva samples collected by participants immediately before their visit to the laboratory at week 1, week 4, and week 8. During each of these visits, participants used their e-cigarette or combustible tobacco cigarette over a 5-minute ad lib session based on assigned group. Between visits, participants in the e-cigarette groups could use the assigned e-cigarette or smoke ad lib, whereas those in the control group could only smoke. Nicotine biomarkers were measured before or after laboratory sessions. After 8 weeks, the control group was also given e-cigarettes. Saliva cotinine was measured in samples collected before the final follow-up visit in the eighth month. No differences in saliva cotinine concentrations were found between the e-cigarette groups and the control group. Furthermore, saliva cotinine decreased significantly in the e-cigarette groups as well as the control group over the first 8 weeks (the period in which the control group was not given e-cigarettes) but increased in the e-cigarette groups at the last follow-up visit (month 8) and did not increase in the control group. At the last follow-up visit, no differences in saliva cotinine concentrations were observed between the e-cigarette groups and the control group (the control group had been allowed to use e-cigarettes over the last 6 months of the study). The average cotinine levels across all participants decreased from 663.50 ng/ml (SD = 350.15) at baseline to 449.96 ng/ml (SD = 193.19) at the end of the study. Saliva cotinine concentrations were examined across levels of combustible tobacco cigarette reduction (i.e., no reduction; greater than or equal to 50 percent reduction; greater than or equal to 80 percent reduction; and 100 percent reduction or quitters). No significant differences in saliva cotinine levels were seen between these groups at the times measured. The results of this study indicate that there was no significant difference in daily nicotine intake among smokers who switched completely to e-cigarettes, those who used both e-cigarettes and combustible tobacco cigarettes, and those who only smoked combustible tobacco cigarettes. In addition, the study also showed that e-cigarette–naïve smokers can titrate their nicotine intake with practice.
Cravo and colleagues (2016) conducted a study to evaluate the safety
profile of an e-cigarette prototype (2.0 percent nicotine) in smokers who switch to the e-cigarette. The nicotine pharmacokinetic profile of this e-cigarette was discussed previously (Walele et al., 2016). Participants were randomized to the e-cigarette or usual cigarette and followed for 12 weeks. Of 419 enrolled participants, 408 (182 women) used the product at least once (full analysis set), and 387 completed the study. A subset of the participants (cohort 2) (40 total, 12 women) was confined to a research facility until day 6. Urinary total nicotine equivalents (molar sum of nicotine, cotinine, nicotine-N-glucuronide, cotinine-N-glucuronide, trans 3′-hydroxycotinine, and trans 3′-hydroxycotinine glucuronide) were used to measure daily nicotine intake. Overall, 40.2 percent of participants were compliant during the unconfined phase of the study, that is, self-reported smoking no combustible tobacco cigarettes on 80 percent or more of the study days (e-cigarette compliant), while 59.8 percent were less e-cigarette compliant. Total nicotine equivalents decreased rapidly in the e-cigarette arm and were significantly lower than the combustible tobacco cigarette arm at weeks 4, 8, and 12. Reductions in total nicotine equivalent were observed from day 2 in the confined participants (fully compliant). Participants in the e-cigarette arm saw average reductions in urinary total nicotine equivalents of 33.3 percent, 29.3 percent, and 25.3 percent in weeks 4, 8, and 12, respectively, relative to baseline. Levels of total nicotine equivalents were even lower among compliant participants of the e-cigarette arm. Participants in the combustible tobacco cigarette arm had stable total nicotine equivalents throughout the study, with reductions of 1.0 percent and 5.9 percent in weeks 4 and 12, respectively, and an increase of 3.0 percent in week 8, relative to baseline. A decrease in urine nicotine equivalents coincided with an increase in nicotine withdrawal symptoms. The results of this study suggest that the e-cigarette product could not effectively deliver nicotine to smokers who switch and resulted in significant decrease in daily nicotine intake. This is consistent with the low blood nicotine levels reported from acute use of this product (Walele et al., 2016).
Two publications, one by D’Ruiz and colleagues (2016), and the other by O’Connell and colleagues (2016), seem to describe the same parent study and nicotine exposure results. The authors of both publications reported changes in nicotine exposure among different groups following a 5-day forced switch from usual brand of tobacco cigarette to exclusive use of commercial e-cigarettes; dual use of commercial e-cigarettes and participants’ usual combustible tobacco cigarette; or discontinued use of all tobacco or nicotine products (O’Connell et al., 2016). Three commercially available blu e-cigarettes (all 24 mg/ml nicotine) were used. A total of 105 participants (37 women) were enrolled and clinically confined over the study duration. Total nicotine equivalents decreased significantly
from baseline to day 5 in the e-cigarette group. Average total nicotine equivalents ranged from 14.5–17.6 mg/24 hours at baseline for the three formulations of e-cigarettes to 10.5–12.7 mg/24 hours at day 5. There was no significant change in total nicotine equivalents in the dual-use group: baseline, 15.7–16.6 mg/24 hours to 15.8–18.4 mg/24 hours. Smoking/nicotine cessation resulted in a significant decrease in total nicotine equivalents: 20.0 mg/24 hours at baseline to 0.5 mg/24 hours at day 5. There were significant reductions in blood nicotine and cotinine from baseline to day 5 in the e-cigarette group for all three e-cigarettes. In the dual-use group, plasma cotinine did not change significantly, but plasma nicotine decreased significantly for one e-cigarette. Nicotine cessation resulted in significant reductions in plasma nicotine and cotinine from baseline to day 5. The study showed that use of e-cigarettes alone (blu e-cigarettes) resulted in significant reductions in daily nicotine intake compared with baseline (before switching). Daily nicotine intake of participants in the dual-use group did not change significantly from baseline.
Pulvers and colleagues (2016) described a study of 40 cigarette smokers (73 percent male) enrolled in a 4-week observational study. The enrolled smokers were interested in e-cigarettes, but not necessarily interested in quitting. The study e-cigarette was an e-Go C (second generation, non-variable voltage) and participants had a choice of seven flavor categories, which included tobacco, mint, fruit, candy, sweets, chocolate, and drink/soda, in nicotine strength of 12 or 24 mg/ml. Biomarkers were measured at baseline and at 4 weeks. Thirty-seven of 40 participants provided follow-up and used the e-cigarette. Sixteen participants (40 percent) reported no cigarettes at week 2 and 6 (15 percent) reported no cigarette use at week 4. Urinary cotinine levels were not significantly different at baseline and week 4 (574.8 versus 440.8 ng/mg creatinine). This suggests that the second-generation e-cigarette used in the study provided adequate nicotine replacement from combustible tobacco cigarettes.
In a study by Strasser and colleagues (2016), 28 combustible tobacco cigarette smokers were randomized to use one of 5 popular brands of first-generation e-cigarettes. Participants smoked their usual brand of combustible tobacco cigarette on day 1 and switched to the e-cigarette thereafter, with visits to the lab on days 5 and 10. Saliva cotinine was collected during each visit. Saliva cotinine decreased significantly from day 1 to day 10 for all e-cigarette brands. Relative to baseline, percentage change at day 10 ranged from 23.4–56.3 percent. This indicated significant reduction in nicotine exposure during e-cigarette use compared with combustible tobacco cigarette use. Furthermore, saliva cotinine did not differ between day 5 and day 10, indicating that nicotine exposure during e-cigarette use remained constant, albeit at levels lower than combustible tobacco cigarette use.
Goniewicz and colleagues (2017) enrolled 20 smokers (60 percent female) in a 2-week study and provided them with M201 (first generation, 11-mg nicotine/cartridge). Participants were given 20 tobacco-flavored cartridges per week and were encouraged to substitute their usual cigarettes with the e-cigarettes. Biomarkers were measured at baseline, week 1, and week 2. Total nicotine equivalents did not change from baseline (50 nmol/mg creatinine) to week 1 (45 nmol/mg creatinine) to week 2 (43 nmol/mg creatinine), indicating that the e-cigarettes can be used to sustain daily nicotine intake.
An important limitation of studies conducted in the users’ naturalistic settings (real world) is the potential for noncompliance with the study regimen. Compliance was either assessed with expired carbon monoxide (Adriaens et al., 2014; Goniewicz et al., 2017) and/or self-reported combustible tobacco cigarette or e-cigarette consumption (Cravo et al., 2016; Goniewicz et al., 2017; Strasser et al., 2016). All five studies that measured expired carbon monoxide in participants who switched from combustible tobacco cigarettes to e-cigarettes reported significantly lower expired carbon monoxide after switching, indicating fewer combustible tobacco cigarettes were smoked when assigned to use e-cigarettes. However, complete abstinence from combustible tobacco cigarettes could not be guaranteed. Studies done in research facilities enforced compliance (D’Ruiz et al., 2016; O’Connell et al., 2016). Of the seven longitudinal studies involving smokers switching to e-cigarettes, three reported no significant change in nicotine exposure from baseline to follow-up with complete or partial replacement of combustible tobacco cigarettes with e-cigarettes (Adriaens et al., 2014; Goniewicz et al., 2017; Pulvers et al., 2016). These studies suggest that some smokers are able to completely replace their daily nicotine intake from combustible tobacco cigarettes with e-cigarettes. On the other hand, the other four studies suggest that some e-cigarettes are ineffective nicotine delivery devices compared with combustible tobacco cigarettes.
Other Studies of Nicotine Exposure
A few other studies have measured nicotine exposure in long-term e-cigarette users to address the question of whether nicotine exposure from e-cigarettes matches that of combustible tobacco cigarettes. Shahab and colleagues (2017) compared exposure to nicotine and other compounds between long-term users (n = 181) of a variety of nicotine/tobacco products in a cross-sectional study. Combustible tobacco cigarette smokers (n = 37), dual cigarette and NRT users (n = 36), dual combustible tobacco cigarette and e-cigarette users (n = 36), NRT-only users (n = 36), and e-cigarette–only users (n = 36) in the United Kingdom were purposively recruited into the study. Daily nicotine intake was measured using saliva
nicotine and cotinine and urinary total nicotine equivalents. While there was greater variability in saliva nicotine and cotinine between product groups than urine total nicotine equivalents, none of the nicotine exposure biomarkers showed clear differences among groups. The following values indicate the urine total nicotine equivalent levels as a percentage of the levels from combustible tobacco cigarette–only smokers: dual cigarette + NRT = 104.2, 95% CI = 64.3–168.9; dual cigarette + e-cigarette = 156.8, 95% CI = 105.1–233.8; NRT-only = 121.6, 95% CI = 62.5–236.8, e-cigarette–only, 126.9, 95% CI = 82.1–196.2. This study was the first to suggest that long-term use of e-cigarettes (and also NRT-only use) is associated with roughly similar daily nicotine intake compared with combustible tobacco cigarette–only use.
Some studies have measured cotinine as a biomarker of nicotine intake and exposure in long-term e-cigarette users. A study by Etter and Bullen (2011) reported saliva cotinine in the saliva of experienced e-cigarette users contacted in real-life settings. Participants visiting a smoking cessation website were recruited to complete an online questionnaire and current e-cigarette users provided a saliva sample (31 of 196 posted vials). Median cotinine of e-cigarette users who had not smoked combustible tobacco cigarettes in the previous 48 hours (n = 30) was 322 ng/ml. In another study by Etter (2016), saliva cotinine levels were measured longitudinally in e-cigarette users. Ninety-eight exclusive e-cigarette users were recruited online to provide saliva samples by mail at baseline and 8 months later. The median cotinine level was 307 ng/ml (interquartile range [IQR] = 114–466 ng/ml) at follow-up and was not significantly different from baseline levels, 252 ng/ml (IQR = 124–421 ng/ml). During that same time, the median nicotine concentration of the e-liquid used had decreased from 11 mg/ml to 6 mg/ml and median volume of e-liquid consumed per month increased from 80 to 100 ml. This study indicated that while e-cigarette users decrease the nicotine concentration of their e-liquids over time, they consume more e-liquid and maintain a relatively constant daily nicotine intake. The authors concluded that in experienced e-cigarette users enrolled online, cotinine levels were similar to levels usually observed in combustible tobacco cigarette smokers.
Hecht and colleagues (2015) measured cotinine and nicotine in the urine of e-cigarette users and smokers enrolled in two separate studies. Average urinary cotinine and nicotine in a group of 28 e-cigarette users who had not smoked combustible tobacco cigarettes for at least 2 months were 1,880 ng/ml (95% CI = 1,420–2,480 ng/ml), and 869 ng/ml (95% CI = 604–1,250 ng/ml), respectively, which were significantly lower than urinary cotinine 3,930 ng/ml (95% CI = 3,500–4,400 ng/ml), and nicotine 1,380 ng/ml (95% CI = 1,190–1,600 ng/ml) from a group of 165 smokers. Urinary cotinine and nicotine from the e-cigarette users were not signifi-
cantly different when compared with a second group of smokers (n = 40): 1,930 ng/ml (95% CI = 1,530–2,440 ng/ml) and 1,270 ng/ml (95% CI = 834–1,710 ng/ml), respectively.
Goney and colleagues (2016) also measured cotinine in the urine of combustible tobacco smokers (n = 33), e-cigarette users (n = 32), and nonsmokers exposed to secondhand smoke (n = 33). Mean urinary cotinine (SD) in e-cigarette users was 1,755 (1,848) ng/g; creatinine; it was not significantly different from that of smokers, 1,720 (1,335) ng/g. Urinary cotinine of those exposed to secondhand smoke was much lower, 81.4 (97.9) ng/g creatinine.
In general, these studies which measured nicotine and/or its metabolites in long-term users of e-cigarettes indicate that nicotine intake in these users match that of combustible tobacco cigarette smokers.
RELATIONSHIP BETWEEN E-CIGARETTE TOPOGRAPHY AND NICOTINE EXPOSURE
Vaping machine studies have demonstrated that puffing topography influences e-cigarette nicotine yields (Talih et al., 2015). Namely, longer puff durations are associated with higher nicotine yields. Therefore, it is worthwhile to examine whether e-cigarette puffing topography is associated with systemic exposure to nicotine among users. Three human studies that addressed this question were identified. For a general discussion of e-cigarette puffing topography, see Chapter 3.
Farsalinos and colleagues (2015) assessed the relationship between puff topography and plasma nicotine in a study with 24 experienced e-cigarette users and 23 e-cigarette–naïve users. Participants were involved in a 10-puff bout over 5 minutes followed by 60 minutes of ad lib use. Number of puffs and puff duration were measured by the e-cigarette (eVic). The study found statistically significant but weak positive correlations between puff duration and plasma nicotine levels after 5 minutes and after 65 minutes. These results are consistent with the vaping machine study (Talih et al., 2015).
In another study, St.Helen and colleagues (2016b) characterized puffing behavior in experienced adult e-cigarette users during 90 minutes of ad lib access to their usual e-cigarette in a hospital research ward. Thirteen participants (seven men, six women) were enrolled. Puff topography (puff duration, interpuff interval, and number of puffs taken) were obtained from video analysis. When all participants were considered (i.e., users of all three generations of e-cigarettes), vaping topography parameters were not significantly correlated with the amount of e-liquid consumed, amount of nicotine inhaled, and nicotine pharmacokinetic parameters. However, when only second-generation (tank) device users (eight partici-
pants) were included in the analysis, the number of puffs taken during the session was positively correlated with the amount of nicotine inhaled and plasma nicotine AUC while interpuff interval was negatively correlated with plasma nicotine Cmax. Puff duration was not significantly correlated with systemic nicotine exposure. The findings suggest that the relationship between puff topography and nicotine exposure may be device-specific (correlations were significant only when one type of device was analyzed). This study had a relatively small sample size, which limits the reliability of the observations made.
Dawkins and colleagues (2016) enrolled 11 e-cigarette users into a crossover study in which study participants used e-liquids with low nicotine (6 mg/ml) and high nicotine (24 mg/ml) during a 60-minute ad lib session. The study found that puff number and puff duration were positively correlated with nicotine boost at each time point under both the high and low nicotine conditions. Interestingly, the correlations were larger at the high nicotine condition, suggesting that device characteristics can moderate the relationship between puff topography and systemic exposure to nicotine.
In summary, these three studies are consistent with the vaping machine study, showing that puffing topography is correlated with systemic exposure to nicotine. More research is needed to understand how the relationship between puffing topography and nicotine exposure differs across device characteristics.
SYNTHESIS
This chapter reviews the literature on nicotine content in e-cigarette liquids and aerosols, e-liquid pH, nicotine pharmacokinetics and pharmacology, and nicotine exposure from e-cigarettes. The nicotine content of e-cigarettes varies widely among products, with varying degrees of agreement between nicotine content on the label and what is chemically measured. The choice of nicotine strength is influenced, in part, by e-cigarette characteristics, such as electrical power. Nicotine concentration in e-cigarette aerosol is also variable among e-cigarettes. The concentration of nicotine in e-cigarette aerosol is a product of device characteristics and user behavior. Nicotine yield increases with e-cigarette power and e-liquid nicotine concentration, and with increasing puff duration. The pH of e-liquids is also variable, with a few studies reporting a range of pH from about 4.3 to 9.9. The committee did not find any study that has systematically assessed the effect of e-liquid pH on e-cigarette pharmacology.
The committee summarized the known pharmacokinetics and pharmacodynamics of nicotine based on reports of the Surgeon General and other authoritative reviews. The potential carcinogenicity and cardio-
vascular effects of nicotine were discussed. Other potential effects of nicotine, such as developmental and respiratory effects, are discussed in Section II of this report. It is important to note that this chapter does not make conclusions on the health effects of e-cigarettes per se, as these are reviewed in later chapters. However, the potential carcinogenicity and cardiovascular effects of nicotine have implications for the health effects of e-cigarettes. As discussed, there is no evidence to indicate that nicotine is a carcinogen. While it is biologically plausible that nicotine can act as a tumor promoter, there is no evidence from studies of long-term NRT users and users of smokeless tobacco products that nicotine increases human cancer risks. Given this evidence, nicotine exposure from e-cigarette use will likely pose minimal cancer risk to users. Based on known cardiovascular effects of nicotine, exposure to nicotine from e-cigarettes likely elevates the cardiovascular disease risk in people with preexisting cardiovascular disease(s) but the cardiovascular risks in people without cardiovascular disease(s) is uncertain.
Finally, the committee reviewed human studies to examine the nicotine exposure profile of e-cigarettes. Clinical studies of acute nicotine exposure from e-cigarette use in e-cigarette–naïve smokers and experienced e-cigarette users were reviewed, as well as studies of long-term e-cigarette use in combustible tobacco cigarette smokers who switch to e-cigarettes over a study period.
Conclusion 4-1. There is conclusive evidence that exposure to nicotine from e-cigarettes is highly variable and depends on product characteristics (including device and e-liquid characteristics) and how the device is operated.
Conclusion 4-2. There is substantial evidence that nicotine intake from e-cigarette devices among experienced adult e-cigarette users can be comparable to that from combustible tobacco cigarettes.
REFERENCES
Adamson, J., D. Thorne, B. Zainuddin, A. Baxter, J. McAughey, and M. Gaça. 2016. Application of dosimetry tools for the assessment of e-cigarette aerosol and cigarette smoke generated on two different in vitro exposure systems. Chemistry Central Journal 10(74):1–16.
Adriaens, K., D. Van Gucht, P. Declerck, and F. Baeyens. 2014. Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research & Public Health 11(11):11220–11248.
Antoniewicz, L., J. A. Bosson, J. Kuhl, S. M. Abdel-Halim, A. Kiessling, F. Mobarrez, and M. Lundback. 2016. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255:179–185.
Armitage, A. K., C. T. Dollery, C. F. George, T. H. Houseman, P. J. Lewis, and D. M. Turner. 1975. Absorption and metabolism of nicotine from cigarettes. BMJ 4(5992):313–316.
Armitage, A., C. Dollery, T. Houseman, E. Kohner, P. J. Lewis, and D. Turner. 1978. Absorption of nicotine from small cigars. Clinical Pharmacology & Therapeutics 23(2):143–151.
Benowitz, N. L. 2008. Neurobiology of nicotine addiction: Implications for smoking cessation treatment. American Journal of Medicine 121(4 Supplement 1):S3–S10.
Benowitz, N. L. 2009. Pharmacology of nicotine: Addiction, smoking-induced disease, and therapeutics. Annual Review of Pharmacology and Toxicology 49:57–71.
Benowitz, N. L., and A. D. Burbank. 2016. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends in Cardiovascular Medicine 26(6):515–523.
Benowitz, N. L., and J. B. Fraiman. 2017. Cardiovascular effects of electronic cigarettes. Nature Reviews Cardiology 14(8):447–456.
Benowitz, N. L., E. J. Perez-Stable, I. Fong, G. Modin, B. Herrera, and P. Jacob, 3rd. 1999. Ethnic differences in N-glucuronidation of nicotine and cotinine. Journal of Pharmacology and Experimental Therapeutics 291(3):1196–1203.
Benowitz, N. L., E. J. Perez-Stable, B. Herrera, and P. Jacob, 3rd. 2002. Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. Journal of the National Cancer Institute 94(2):108–115.
Benowitz, N. L., O. F. Pomerleau, C. S. Pomerleau, and P. Jacob, 3rd. 2003. Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine & Tobacco Research 5(5):621–624.
Benowitz, N. L., B. Herrera, and P. Jacob, 3rd. 2004. Mentholated cigarette smoking inhibits nicotine metabolism. Journal of Pharmacology and Experimental Therapeutics 310(3): 1208–1215.
Benowitz, N. L., J. Hukkanen, and P. Jacob, 3rd. 2009. Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of Experimental Pharmacology (192):29–60.
Berg, J. Z., J. Mason, A. J. Boettcher, D. K. Hatsukami, and S. E. Murphy. 2010a. Nicotine metabolism in African Americans and European Americans: Variation in glucuronidation by ethnicity and UGT2B10 haplotype. Journal of Pharmacology and Experimental Therapeutics 332(1):202–209.
Berg, J. Z., L. B. von Weymarn, E. A. Thompson, K. M. Wickham, N. A. Weisensel, D. K. Hatsukami, and S. E. Murphy. 2010b. UGT2B10 genotype influences nicotine glucuronidation, oxidation, and consumption. Cancer Epidemiology, Biomarkers and Prevention 19(6):1423–1431.
Bergstrom, M., A. Nordberg, E. Lunell, G. Antoni, and B. Langstrom. 1995. Regional deposition of inhaled 11C-nicotine vapor in the human airway as visualized by positron emission tomography. Clinical Pharmacology & Therapeutics 57(3):309–317.
Berridge, M. S., S. M. Apana, K. K. Nagano, C. E. Berridge, G. P. Leisure, and M. V. Boswell. 2010. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology (Berl) 209(4):383–394.
Bhatnagar, A. 2016. E-cigarettes and cardiovascular disease risk: Evaluation of evidence, policy implications, and recommendations. Current Cardiovascular Risk Reports 10(7):24.
Bullen, C., H. McRobbie, S. Thornley, M. Glover, R. Lin, and M. Laugesen. 2010. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tobacco Control 19(2):98–103.
Cameron, J. M., D. N. Howell, J. R. White, D. M. Andrenyak, M. E. Layton, and J. M. Roll. 2014. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tobacco Control 23(1):77–78.
Carmella, S. G., A. Borukhova, D. Desai, and S. S. Hecht. 1997. Evidence for endogenous formation of tobacco-specific nitrosamines in rats treated with tobacco alkaloids and sodium nitrite. Carcinogenesis 18(3):587–592.
Chen, G., A. S. Blevins-Primeau, R. W. Dellinger, J. E. Muscat, and P. Lazarus. 2007. Glucuronidation of nicotine and cotinine by UGT2B10: Loss of function by the UGT2B10 codon 67 (Asp>Tyr) polymorphism. Cancer Research 67(19):9024–9029.
Chen, G., N. E. Giambrone, and P. Lazarus. 2012. Glucuronidation of trans-3’-hydroxycotinine by UGT2B17 and UGT2B10. Pharmacogenetics and Genomics 22(3):183–190.
Chen, J., R. Higby, D. Tian, D. Tan, M. D. Johnson, Y. Xiao, K. J. Kellar, S. Feng, and P. G. Shields. 2008. Toxicological analysis of low-nicotine and nicotine-free cigarettes. Toxicology 249(2–3):194–203.
Cheng, T. R. 2014. Chemical evaluation of electronic cigarettes. Tobacco Control 23:11–17.
Cobb, N. K., M. J. Byron, D. B. Abrams, and P. G. Shields. 2010. Novel nicotine delivery systems and public health: The rise of the “e-cigarette.” American Journal of Public Health 100(12):2340–2342.
Cravo, A. S., J. Bush, G. Sharma, R. Savioz, C. Martin, S. Craige, and T. Walele. 2016. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regulatory Toxicology and Pharmacology 81:S1–S14.
Dawkins, L., and O. Corcoran. 2014. Acute electronic cigarette use: Nicotine delivery and subjective effects in regular users. Psychopharmacology 231(2):401–407.
Dawkins, L. E., C. F. Kimber, M. Doig, C. Feyerabend, and O. Corcoran. 2016. Self-titration by experienced e-cigarette users: Blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 233(15–16):2933–2941.
Dempsey, D., P. Tutka, P. Jacob, 3rd, F. Allen, K. Schoedel, R. F. Tyndale, and N. L. Benowitz. 2004. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics 76(1):64–72.
Denton, T. T., X. Zhang, and J. R. Cashman. 2004. Nicotine-related alkaloids and metabolites as inhibitors of human cytochrome P-450 2A6. Biochemical Pharmacology 67(4):751–756.
Derby, K. S., K. Cuthrell, C. Caberto, S. G. Carmella, A. A. Franke, S. S. Hecht, S. E. Murphy, and L. Le Marchand. 2008. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiology, Biomarker & Prevention 17(12):3526–3535.
Digard, H., C. Proctor, A. Kulasekaran, U. Malmqvist, and A. Richter. 2013. Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine & Tobacco Research 15(1):255–261.
Djordjevic, M. V., and K. A. Doran. 2009. Nicotine content and delivery across tobacco products. Handbook of Experimental Pharmacology (192):61–82.
D’Ruiz, C. D., D. W. Graff, and X. S. Yan. 2015. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health 15:991. https://doi.org/10.1186/s12889-015-2349-2 (accessed February 5, 2018).
D’Ruiz, C. D., D. W. Graff, and E. Robinson. 2016. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health 16:543. https://doi.org/10.1186/s12889-016-3236-1 (accessed February 5, 2018).
Ehmer, U., A. Vogel, J. K. Schutte, B. Krone, M. P. Manns, and C. P. Strassburg. 2004. Variation of hepatic glucuronidation: Novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology 39(4):970–977.
Eissenberg, T. 2010. Electronic nicotine delivery devices: Ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control 19(1):87–88.
El-Hellani, A., R. El-Hage, R. Baalbaki, R. Salman, S. Talih, A. Shihadeh, and N. A. Saliba. 2015. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chemical Research in Toxicology 28(8):1532–1537.
England, L. J., K. Aagaard, M. Bloch, K. Conway, K. Cosgrove, R. Grana, T. J. Gould, D. Hatsukami, F. Jensen, D. Kandel, B. Lanphear, F. Leslie, J. R. Pauly, J. Neiderhiser, M. Rubinstein, T. A. Slotkin, E. Spindel, L. Stroud, and L. Wakschlag. 2017. Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neuroscience & Biobehavioral Reviews 72(Supplement C):176–189.
Etter, J. F. 2016. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug and Alcohol Dependence 160:218–221.
Etter, J. F., and A. Bugey. 2017. E-cigarette liquids: Constancy of content across batches and accuracy of labeling. Addictive Behaviors 73:137–143.
Etter, J. F., and C. Bullen. 2011. Saliva cotinine levels in users of electronic cigarettes. European Respiratory Journal 38(5):1219–1220.
Etter, J. F., E. Zather, and S. Svensson. 2013. Analysis of refill liquids for electronic cigarettes. Addiction 108(9):1671–1679.
Farsalinos, K. E., A. Spyrou, C. Stefopoulos, K. Tsimopoulou, P. Kourkoveli, D. Tsiapras, S. Kyrzopoulos, K. Poulas, and V. Voudris. 2015. Nicotine absorption from electronic cigarette use: Comparison between experienced consumers (vapers) and naïve users (smokers). Scientific Reports. https://www.nature.com/articles/srep11269 (accessed February 5, 2018).
Fearon, I. M., A. Eldridge, N. Gale, C. J. Shepperd, M. McEwan, O. M. Camacho, M. Nides, K. McAdam, and C. J. Proctor. 2017. E-cigarette nicotine delivery: Data and learnings from pharmacokinetic studies. American Journal of Health Behavior 41(1):16–32.
Flouris, A. D., M. S. Chorti, K. P. Poulianiti, A. Z. Jamurtas, K. Kostikas, M. N. Tzatzarakis, A. W. Hayes, A. M. Tsatsakis, and Y. Koutedakis. 2013. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology 25(2):91–101.
Galitovskiy, V., A. I. Chernyavsky, R. A. Edwards, and S. A. Grando. 2012. Muscle sarcomas and alopecia in A/J mice chronically treated with nicotine. Life Sciences 91(21–22): 1109–1112.
Ghosheh, O., and E. M. Hawes. 2002. N-Glucuronidation of nicotine and cotinine in humans: Formation of cotinine glucuronide in liver microsomes and lack of catalysis by 10 examined UDP-glucuronosyltransferases. Drug Metabolism & Disposition 30(9):991–996.
Goney, G., I. Cok, U. Tamer, S. Burgaz, and T. Sengezer. 2016. Urinary cotinine levels of electronic cigarette (e-cigarette) users. Toxicology Mechanisms and Methods 26(6):414–418.
Goniewicz, M. L., T. Kuma, M. Gawron, J. Knysak, and L. Kosmider. 2013. Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research 15(1):158–166.
Goniewicz, M. L., P. Hajek, and H. McRobbie. 2014. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: Regulatory implications. Addiction 109(3):500–507.
Goniewicz, M. L., R. Gupta, Y. H. Lee, S. Reinhardt, S. Kim, B. Kim, L. Kosmider, and A. Sobczak. 2015. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the U.S., Korea, and Poland. International Journal of Drug Policy 26(6):583–588.
Goniewicz, M. L., M. Gawron, D. M. Smith, M. Peng, P. Jacob, 3rd, and N. L. Benowitz. 2017. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: A longitudinal within-subjects observational study. Nicotine & Tobacco Research 19(2):160–167.
Gori, G. B., N. L. Benowitz, and C. J. Lynch. 1986. Mouth versus deep airways absorption of nicotine in cigarette smokers. Pharmacology Biochemistry and Behavior 25(6):1181–1184.
Gotti, C., L. Riganti, S. Vailati, and F. Clementi. 2006. Brain neuronal nicotinic receptors as new targets for drug discovery. Current Pharmaceutical Design 12(4):407–428.
Grando, S. A. 2014. Connections of nicotine to cancer. Nature Reviews Cancer 14(6):419–429.
Gundisch, D. 2000. Nicotinic acetylcholine receptors and imaging. Current Pharmaceutical Design 6(11):1143–1157.
Hajek, P., M. L. Goniewicz, A. Phillips, K. Myers Smith, O. West, and H. McRobbie. 2015. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine & Tobacco Research 17(2):175–179.
Hajek, P., D. Przulj, A. Phillips, R. Anderson, and H. McRobbie. 2017. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology 234(5):773–779.
Haussmann, H. J., and M. W. Fariss. 2016. Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se. Critical Reviews in Toxicology 46(8):701–734.
Hecht, S. S. 1998. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology 11(6):559–603.
Hecht, S. S., S. G. Carmella, D. Kotandeniya, M. E. Pillsbury, M. Chen, B. W. Ransom, R. I. Vogel, E. Thompson, S. E. Murphy, and D. K. Hatsukami. 2015. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine & Tobacco Research 17(6):704–709.
Henningfield, J., J. Pankow, and B. Garrett. 2004. Ammonia and other chemical base tobacco additives and cigarette nicotine delivery: Issues and research needs. Nicotine & Tobacco Research 6(2):199–205.
HHS (U.S. Department of Health and Human Services). 1988. The health consequences of smoking: Nicotine addiction: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
HHS. 2010a. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
HHS. 2010b. How tobacco smoke causes disease: What it means to you: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
HHS. 2014. The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
Hukkanen, J., P. Jacob, 3rd, and N. L. Benowitz. 2005. Metabolism and disposition kinetics of nicotine. Pharmacological Reviews 57(1):79–115.
IARC (International Agency for Research on Cancer). 2012. Chromium(VI) compounds. https://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-9.pdf (accessed September 29, 2017).
IOM (Institute of Medicine). 2001. Clearing the smoke: Assessing the science base for tobacco harm reduction. Washington, DC: National Academy Press.
Kaivosaari, S., P. Toivonen, L. M. Hesse, M. Koskinen, M. H. Court, and M. Finel. 2007. Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Molecular Pharmacology 72(3):761–768.
Klaassen, C. D., and J. B. Watkins, eds. 2015. Casarett & Doull’s essentials of toxicology, third edition. New York: McGraw-Hill Medical.
Knezevich, A., J. Muzic, D. K. Hatsukami, S. S. Hecht, and I. Stepanov. 2013. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N’-nitrosonornicotine in humans. Nicotine & Tobacco Research 15(2):591–595.
Leon, M. E., A. Lugo, P. Boffetta, A. Gilmore, H. Ross, J. Schuz, C. La Vecchia, and S. Gallus. 2016. Smokeless tobacco use in Sweden and other 17 European countries. European Journal of Public Health 26(5):817–821.
Lerman, C., R. Tyndale, F. Patterson, E. P. Wileyto, P. G. Shields, A. Pinto, and N. Benowitz. 2006. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical Pharmacology & Therapeutics 79(6):600–608.
Lerman, C., C. Jepson, E. P. Wileyto, F. Patterson, R. Schnoll, M. Mroziewicz, N. Benowitz, and R. F. Tyndale. 2010. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clinical Pharmacology & Therapeutics 87(5):553–557.
Lisko, J. G., H. Tran, S. B. Stanfill, B. C. Blount, and C. H. Watson. 2015. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine & Tobacco Research 17(10):1270–1278.
Lopez, A. A., M. M. Hiler, E. K. Soule, C. P. Ramoa, N. V. Karaoghlanian, T. Lipato, A. B. Breland, A. L. Shihadeh, and T. Eissenberg. 2016. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: A preliminary report. Nicotine & Tobacco Research 18(5):720–723.
Luo, J., W. Ye, K. Zendehdel, J. Adami, H. O. Adami, P. Boffetta, and O. Nyren. 2007. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: A retrospective cohort study. Lancet 369(9578):2015–2020.
Margham, J., K. McAdam, M. Forster, C. Liu, C. Wright, D. Mariner, and C. Proctor. 2016. Chemical composition of aerosol from an e-cigarette: A quantitative comparison with cigarette smoke. Chemical Research in Toxicology 29(10):1662–1678.
Maskos, U., B. E. Molles, S. Pons, M. Besson, B. P. Guiard, J. P. Guilloux, A. Evrard, P. Cazala, A. Cormier, M. Mameli-Engvall, N. Dufour, I. Cloez-Tayarani, A. P. Bemelmans, J. Mallet, A. M. Gardier, V. David, P. Faure, S. Granon, and J. P. Changeux. 2005. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436(7047):103–107.
McRobbie, H., A. Phillips, M. L. Goniewicz, K. M. Smith, O. Knight-West, D. Przulj, and P. Hajek. 2015. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prevention Research 8(9):873–878.
Mobarrez, F., L. Antoniewicz, J. A. Bosson, J. Kuhl, D. S. Pisetsky, and M. Lundback. 2014. The effects of smoking on levels of endothelial progenitor cells and microparticles in the blood of healthy volunteers. PLoS ONE 9(2):e90314. https://doi.org/10.1371/journal.pone.0090314 (accessed February 5, 2018).
Murray, R. P., W. C. Bailey, K. Daniels, W. M. Bjornson, K. Kurnow, J. E. Connett, M. A. Nides, and J. P. Kiley. 1996. Safety of nicotine polacrilex gum used by 3,094 participants in the Lung Health Study. Chest 109(2):438–445.
Murray, R. P., J. E. Connett, and L. M. Zapawa. 2009. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine & Tobacco Research 11(9):1076–1082.
Neunteufl, T., S. Heher, K. Kostner, G. Mitulovic, S. Lehr, G. Khoschsorur, R. W. Schmid, G. Maurer, and T. Stefenelli. 2002. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. Journal of the American College of Cardiology 39(2):251–256.
Nides, M. A., S. J. Leischow, M. Bhatter, and M. Simmons. 2014. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. American Journal of Health Behavior 38(2):265–274.
O’Connell, G., D. W. Graff, and C. D. D’Ruiz. 2016. Reductions in biomarkers of exposure (BOE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicology Mechanisms and Methods 26(6):453–464.
Oncken, C. A., M. D. Litt, L. D. McLaughlin, and N. A. Burki. 2015. Nicotine concentrations with electronic cigarette use: Effects of sex and flavor. Nicotine & Tobacco Research 17(4):473–478.
Pankow, J. F. 2001. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chemical Research in Toxicology 14(11):1465–1481.
Pankow, J. F., B. T. Mader, L. M. Isabelle, W. Luo, A. Pavlick, and C. Liang. 1997. Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environmental Science & Technology 31(8):2428–2433.
Pankow, J. F., K. Kim, K. J. McWhirter, W. Luo, J. O. Escobedo, R. M. Strongin, A. K. Duell, and D. H. Peyton. 2017. Benzene formation in electronic cigarettes. PLoS ONE 12(3):e0173055. https://doi.org/10.1371/journal.pone.0173055 (accessed February 5, 2018).
Papaseit, E., M. Farre, S. Graziano, R. Pacifici, C. Perez-Mana, O. Garcia-Algar, and S. Pichini. 2016. Monitoring nicotine intake from e-cigarettes: Measurement of parent drug and metabolites in oral fluid and plasma. Clinical Chemistry and Laboratory Medicine 55(3): 415–423.
Pellegrino, R. M., B. Tinghino, G. Mangiaracina, A. Marani, M. Vitali, C. Protano, J. F. Osborn, and M. S. Cattaruzza. 2012. Electronic cigarettes: An evaluation of exposure to chemicals and fine particulate matter (PM). Annali di Igiene 24(4):279–288.
Perez-Stable, E. J., B. Herrera, P. Jacob, 3rd, and N. L. Benowitz. 1998. Nicotine metabolism and intake in black and white smokers. Journal of the American Medical Association 280(2):152–156.
Picciotto, M. R. 1998. Common aspects of the action of nicotine and other drugs of abuse. Drug and Alcohol Dependence 51(1–2):165–172.
Pitarque, M., O. von Richter, B. Oke, H. Berkkan, M. Oscarson, and M. Ingelman-Sundberg. 2001. Identification of a single nucleotide polymorphism in the TATA box of the CYP2A6 gene: Impairment of its promoter activity. Biochemical and Biophysical Research Commununications 284(2):455–460.
Pulvers, K., A. S. Emami, N. L. Nollen, D. R. Romero, D. R. Strong, N. L. Benowitz, and J. S. Ahluwalia. 2016. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine & Tobacco Research 20(2):206–214.
Ramoa, C. P., M. M. Hiler, T. R. Spindle, A. A. Lopez, N. Karaoghlanian, T. Lipato, A. B. Breland, A. Shihadeh, and T. Eissenberg. 2016. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: A preliminary report. Tobacco Control 25(e1):e6–e9. http://dx.doi.org/10.1136/tobaccocontrol-2015-052447 (accessed February 5, 2018).
Rivenson, A., D. Hoffmann, B. Prokopczyk, S. Amin, and S. S. Hecht. 1988. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and areca-derived N-nitrosamines. Cancer Research 48(23):6912–6917.
Rose, J. E., S. D. Rose, J. E. Turner, and T. Murugesan. 2008. Device and method for delivery of a medicament. U.S. Patent US20080241255 A1, filed March 25, 2008, and issued October 2, 2008.
Schnoll, R. A., F. Patterson, E. P. Wileyto, R. F. Tyndale, N. Benowitz, and C. Lerman. 2009. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: A validation study. Pharmacology Biochemistry and Behavior 92(1):6–11.
Schoedel, K. A., E. B. Hoffmann, Y. Rao, E. M. Sellers, and R. F. Tyndale. 2004. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics 14(9):615–626.
Shahab, L., M. L. Goniewicz, B. C. Blount, J. Brown, A. McNeill, K. U. Alwis, J. Feng, L. Wang, and R. West. 2017. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: A cross-sectional study. Annals of Internal Medicine 166(6):390–400.
Spindle, T. R., A. B. Breland, N. V. Karaoghlanian, A. L. Shihadeh, and T. Eissenberg. 2015. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine & Tobacco Research 17(2):142–149.
Spindle, T. R., M. M. Hiler, A. B. Breland, N. V. Karaoghlanian, A. L. Shihadeh, and T. Eissenberg. 2017. The influence of a mouthpiece-based topography measurement device on electronic cigarette user’s plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine & Tobacco Research 19(4):469–476.
St.Helen, G., C. Havel, D. A. Dempsey, P. Jacob, 3rd, and N. L. Benowitz. 2016a. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111(3):535–544.
St.Helen, G., K. C. Ross, D. A. Dempsey, C. M. Havel, P. Jacob, 3rd, and N. L. Benowitz. 2016b. Nicotine delivery and vaping behavior during ad libitum e-cigarette access. Tobacco Regulatory Science 2(4):363–376.
St.Helen, G., D. A. Dempsey, C. M. Havel, P. Jacob, 3rd, and N. L. Benowitz. 2017. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug and Alcohol Dependence 178:391–398.
Stepanov, I., J. Jensen, D. Hatsukami, and S. S. Hecht. 2006. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research 8(2):309–313.
Stepanov, I., S. G. Carmella, A. Briggs, L. Hertsgaard, B. Lindgren, D. Hatsukami, and S. S. Hecht. 2009a. Presence of the carcinogen N’-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Research 69(21):8236–8240.
Stepanov, I., S. G. Carmella, S. Han, A. Pinto, A. A. Strasser, C. Lerman, and S. S. Hecht. 2009b. Evidence for endogenous formation of N’-nitrosonornicotine in some long-term nicotine patch users. Nicotine & Tobacco Research 11(1):99–105.
Stiles, M. F., L. R. Campbell, D. W. Graff, B. A. Jones, R. V. Fant, and J. E. Henningfield. 2017. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: Implications for abuse liability. Psychopharmacology (Berl) 234(17):2643–2655.
Strasser, A. A., V. Souprountchouk, A. Kaufmann, S. Blazekovic, F. Leone, N. L. Benowitz, and R. A. Schnoll. 2016. Nicotine replacement, topography, and smoking phenotypes of e-cigarettes. Tobacco Regulatory Science 2(4):352–362.
Talih, S., Z. Balhas, T. Eissenberg, R. Salman, N. Karaoghlanian, A. El Hellani, R. Baalbaki, N. Saliba, and A. Shihadeh. 2015. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine & Tobacco Research 17(2):150–157.
Teichert, A., P. Brossard, L. Felber Medlin, L. Sandalic, M. Franzon, C. Wynne, M. Laugesen, and F. Ludicke. 2017. Evaluation of nicotine pharmacokinetics and subjective effects following use of a novel nicotine delivery system. Nicotine & Tobacco Research. https://doi.org/10.1093/ntr/ntx093 (accessed February 5, 2018).
Tierney, P. A., C. D. Karpinski, J. E. Brown, W. Luo, and J. F. Pankow. 2016. Flavour chemicals in electronic cigarette fluids. Tobacco Control 25(e1):e10–e15. http://dx.doi.org/10.1136/tobaccocontrol-2014-052175 (accessed February 5, 2018).
Timberlake, D. S., D. Nikitin, N. J. Johnson, and S. F. Altekruse. 2017. A longitudinal study of smokeless tobacco use and mortality in the United States. International Journal of Cancer 141(2):264–270.
Trehy, M. L., W. Ye, M. E. Hadwiger, T. W. Moore, J. F. Allgire, J. T. Woodruff, S. S. Ahadi, J. C. Black, and B. J. Westenberger. 2011. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography and Related Technologies 34(14):1442–1458.
Vansickel, A. R., and T. Eissenberg. 2013. Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine & Tobacco Research 15(1):267–270.
Vansickel, A. R., C. O. Cobb, M. F. Weaver, and T. E. Eissenberg. 2010. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention 19(8):1945–1953.
Vansickel, A. R., M. F. Weaver, and T. Eissenberg. 2012. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 107(8):1493–1500.
Wagener, T. L., E. L. Floyd, I. Stepanov, L. M. Driskill, S. G. Frank, E. Meier, E. L. Leavens, A. P. Tackett, N. Molina, and L. Queimado. 2017. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control 26(e1):e23–e28. http://tobaccocontrol.bmj.com/content/26/e1/e23 (accessed February 5, 2018).
Walele, T., G. Sharma, R. Savioz, C. Martin, and J. Williams. 2016. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B: Safety and subjective effects. Regulatory Toxicology and Pharmacology 74:193–199.
Wang, H., W. Tan, B. Hao, X. Miao, G. Zhou, F. He, and D. Lin. 2003. Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco-specific carcinogen NNK. Cancer Research 63(22):8057–8061.
Westenberger, B. 2009. Evaluation of e-cigarettes. http://web.archive.org/web/20170210071043/fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf (accessed February 13, 2018).
Wickholm, S., A. Lahtinen, A. Ainamo, and M. Rautalahti. 2012. [Adverse effects of Swedish smokeless tobacco “snus”]. Duodecim 128(10):1089–1096.
Yan, X. S., and C. D’Ruiz. 2015. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology 71(1):24–34.
Yuan, J. M., A. D. Knezevich, R. Wang, Y. T. Gao, S. S. Hecht, and I. Stepanov. 2011. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 32(9):1366–1371.


































































