16
Union of Phylogeography and Landscape Genetics
LESLIE J. RISSLER
Phylogeography and landscape genetics have arisen within the past 30 years. Phylogeography is said to be the bridge between population genetics and systematics, and landscape genetics the bridge between landscape ecology and population genetics. Both fields can be considered as simply the amalgamation of classic biogeography with genetics and genomics; however, they differ in the temporal, spatial, and organismal scales addressed and the methodology used. I begin by briefly summarizing the history and purview of each field and suggest that, even though landscape genetics is a younger field (coined in 2003) than phylogeography (coined in 1987), early studies by Dobzhansky on the “microgeographic races” of Linanthus parryae in the Mojave Desert of California and Drosophila pseudoobscura across the western United States presaged the fields by over 40 years. Recent advances in theory, models, and methods have allowed researchers to better synthesize ecological and evolutionary processes in their quest to answer some of the most basic questions in biology. I highlight a few of these novel studies and emphasize three major areas ripe for investigation using spatially explicit genomic-scale data: the biogeography of speciation, lineage divergence and species delimitation, and understanding adaptation through time and space. Examples of areas in need of study are highlighted, and I end by advocating a union of phylogeography and landscape genetics under the more general field: biogeography.
__________________
Division of Environmental Biology, Directorate of Biological Sciences, National Science Foundation, Arlington, VA 22230. Email: lrissler@nsf.gov.
Two similar fields have arisen within the past 30 years: phylogeography (Avise et al., 1987) and landscape genetics (Manel et al., 2003). Both fields, to varying degrees, integrate theory and methods from population genetics, biogeography, and ecology. It is my contention that these fields, though somewhat independent in terms of history, methods, scale, and application, do not address fundamentally distinct questions. As such, ecologists and evolutionary biologists who combine genealogical and spatially explicit approaches to the study of biodiversity patterns and processes would benefit from a conceptual unification of phylogeography and landscape genetics. Both fields can be considered as an amalgamation of classic biogeography and genetics. I begin by briefly summarizing the history of each field, especially landscape genetics because phylogeography has been discussed at length elsewhere (e.g., Hickerson et al., 2010), and highlighting a few examples typical of each field. I then summarize the supposed differences between them and move on to a discussion of what I consider the fundamental questions that fall under a general biogeographic framework encompassing both phylogeography and landscape genetics. I end by advocating for a unification of the fields and point out basic areas of inquiry that are ripe for future investigation by biogeographers at all spatial and temporal scales.
A HISTORY OF THE FIELDS OF PHYLOGEOGRAPHY AND LANDSCAPE GENETICS
Phylogeography (including comparative phylogeography) and landscape genetics are considered separate fields: But are they? One of the earliest papers examining phenotypic variation (albeit not genetic variation, although this was assumed) in an explicitly spatial way was by Epling and Dobzhansky (1942), who focused on the “microgeographic races” of Linanthus parryae in the Mojave Desert of California. The distribution of the relative frequencies of blue and white flowers were mapped every half mile along a 200-mile roadway as well as every 25 feet along a 750-foot transect. These data were used to test Wright’s shifting balance theory of evolution (Wright, 1931, 1937), in particular, whether random drift could act in large populations distributed over uniform environments. Lewontin et al. (2003) in their synthesis of Dobzhansky’s works note that, in personal correspondence between Sewall Wright and Theodosius Dobzhansky, Wright told Dobzhansky how to analyze the data to test for isolation by distance (IBD) before Wright had even published a formal statistical model for IBD. Wright later used the Linanthus data in his development of F-statistics (Wright, 1943); these metrics are central to describing the structure of genetic variation within and among natural populations and can be used to infer demographic history. More recently, they have been
used to pinpoint genomic regions under strong selection (reviewed in Holsinger and Weir, 2009). Thus, this paper (Epling and Dobzhansky, 1942) can be considered the birth of landscape genetics although, at the time of Wright, “landscape” rarely meant a literal landscape but rather a figurative one describing the relation between the genotypes of individuals and their fitness or the relation between the allele frequencies in a population and its mean fitness (reviewed in Coyne et al., 1997). That said, those blue and white flowers of the Linanthus system studied by Epling, Dobzhansky, and Wright helped the leaders of the Modern Synthesis to merge theory with empirical data and spawned many studies investigating evolutionary processes (reviewed in Schemske and Bierzychudek, 2001, 2007; Lewontin et al., 2003). Putting those processes on a map and understanding how selection, drift, and gene flow operate on a changing landscape, varying across space and time, remain a focus of evolutionary biology today.
Another paper that presages the fields of phylogeography and landscape genetics is Dobzhansky et al. (1966) on the geographic distribution of chromosomal inversions in Drosophila pseudoobscura in the western United States. That 1966 paper summarizes Dobzhansky’s earlier work in the 1940s and 1950s on the system and compares temporal and spatial patterns among populations of gene arrangements on the third chromosome. Surprisingly, the frequencies of different inversions showed a consistent geographic break that separated populations in the Pacific Coast states (e.g., California, Oregon, and Washington) from those in the Great Basin, Rocky Mountains, and Texas (Fig. 16.1). This biogeographic pattern was not the focus of the paper, but, today, due to comparative phylogeographic and biogeographic studies across the western United States, we recognize this region as a major suture zone (i.e., an area where multiple hybridizing species and/or phylogeographic lineages come into contact) (Anderson, 1948, 1953; Remington, 1968; Swenson and Howard, 2004, 2005; Rissler and Smith, 2010). Remington, who first mapped suture zones across North America, was strongly influenced by Dobzhansky’s ideas on reinforcement during speciation (Dobzhansky, 1940), and later researchers like Hewitt studying species in Europe (e.g., Hewitt, 1999, 2001), Avise in the southeastern United States (Avise, 2000), and Moritz et al. (2009) in the Australian Wet Tropics rainforest also suggested that shared suture zones were ideal natural laboratories to study the processes driving divergence, reproductive isolation, and speciation. Unknown for most suture zones is whether their location is due to strong selection or ecological processes arising from abiotic conditions that vary between physiographic areas, or to historical processes as midpoints between glacial refugia where secondary contact between lineages resulted in either introgression or reinforcement (e.g., Swenson, 2010). In fact, the last sentence in Dobzhansky et al.’s 1966 paper was, “The causation of the genetic changes observed remains
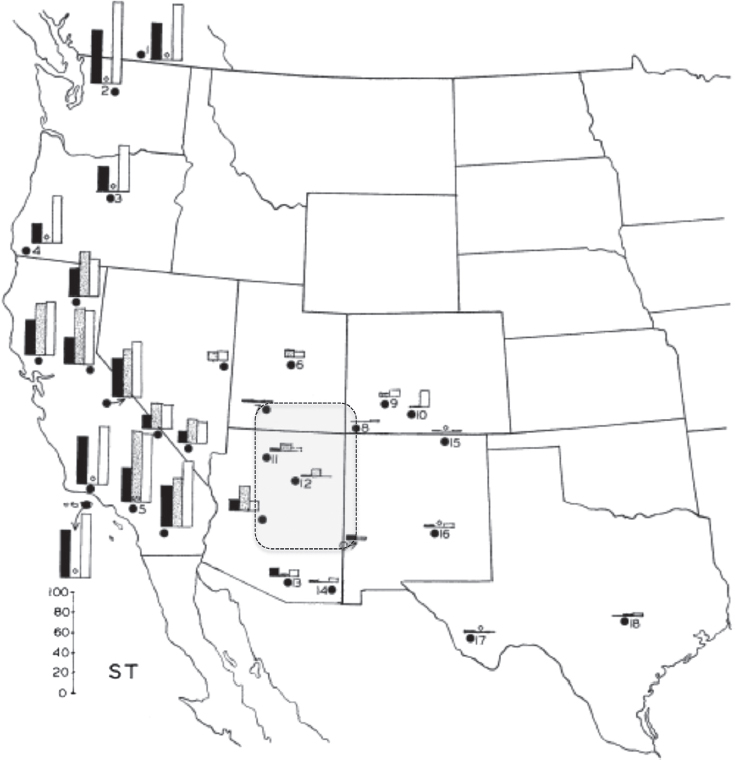
problematic.” It is in answering this question and similar questions about the relative influence of ecological and evolutionary processes on biogeographic patterns that a union of phylogeography and landscape genetics has much to offer.
Phylogeography derives from a synergy between systematics (specifically, molecular phylogenetics and population genetics) because phylogeographers study the geographic distributions of genealogical lineages, rather than species, per se. This integration was possible because of the technical developments of the past half-century that allow the measurement of genetic variation at fine spatial scales, along with the growing realization that the diversification of species can be explained by population-level evolutionary processes (reviewed in Coyne and Orr, 2004). Landscape genetics, on the other hand, grew up mostly as a sub-discipline of ecology and macroecology, with the goal of tracking the movement of organisms across space and over generations, but not deep time (Manel et al., 2003). The proliferation and ubiquity of molecular genetics created an opportunity for ecologists (and conservation biologists) to incorporate population genetics into their studies of the distribution and abundance of organisms. This union spawned landscape genetics (Manel et al., 2003; Storfer et al., 2007; Holderegger and Wagner, 2008; Belkenhol et al., 2009; Sork and Waits, 2010; Dyer, 2015).
Although the use of molecular genetics within ecology goes back decades, the moniker “landscape genetics” was not coined until 2003 (Manel et al., 2003). The history of the field is one that looks primarily to ecology and biogeography, rather than evolution and the Modern Synthesis, as sources of inspiration (but see Dyer, 2015). As Manel et al. (2003) pointed out, landscape genetic studies focus on individuals (in preference to populations, lineages, or species) to find genetic discontinuities in space, which are then correlated with landscape or environmental features. This phylogeny-free view, at least in the early days, was distinct from phylogeography and comparative phylogeography (Bermingham and Avise, 1986; Bermingham and Moritz, 1998; Avise, 2008; Gutierrez-Garcia and Vazquez-Dominguez, 2011). Most landscape genetic studies focus on explaining contemporary rather than historic causes of intraspecific genetic variation (Storfer et al., 2010; Wang, 2010; but see Bohonak and Vandergast, 2011) although this focus is a recurring problem for the field (known as the “time lag” problem) because genetic variation may not be reflective of very recent changes in population size or connectivity (reviewed in Epps and Keyghobadi, 2015).
It is only recently and rarely that researchers have attempted to combine phylogeography and landscape genetics, specifically to account for historical processes when explaining responses of organisms to contemporary ecological perturbations. For example, Swaegers et al. (2014, 2015),
studying genetic diversity and structure of the damselfly Coenagrion scitulum, used a combination of mitochondrial gene sequencing, microsatellite analyses, and single-nucleotide polymorphisms across the entire species’ range to disentangle the role of historical range expansions from the effect of recent range shifts due to global warming. Their multilocus polygenic analysis identified candidate loci potentially under thermal selection, and their studies highlight the usefulness of integrating genomic, phenotypic, and environmental data to disentangle historical and contemporary evolutionary and ecological processes.
THE WAYS IN WHICH PHYLOGEOGRAPHY AND LANDSCAPE GENETICS ARE THOUGHT TO DIFFER
Both phylogeography and landscape genetics fall squarely within the realm of biogeography—“the science that attempts to document and understand spatial patterns of biological diversity” (Lomolino et al., 2010). Species numbers can increase due to both dispersal into an area and in situ speciation. Therefore, phylogeography, with its emphasis on geographic patterns of genealogical lineages (often incipient species), and landscape genetics, with its emphasis on dispersal and gene flow across space, are two sides of the biogeography coin. Today, we know that explaining patterns of genetic variation and organismal traits is both a historical (evolutionary) and an ecological question and an ecological question that lies at the junction of major microevolutionary and macroevolutionary disciplines (summarized in Avise, 2000). Unifying population genetics with explicitly spatial dimensions of time and space is what landscape genetics and phylogeography are all about. It is the recognition that the same ecological and evolutionary processes that cause lineage divergence can also drive speciation that links population genetics and ecology with systematics and biogeography. Thus, the fields of phylogeography and landscape genetics differ not because the questions they address are fundamentally different (Dyer, 2015), but because they arose from different subdisciplines in biology, with one emphasizing history and one ecology. Their union is inevitable as molecular genetics further permeates all of the biological sciences, enabling us to distinguish ancient and contemporary influences on the genome and the resulting consequences for the phenotype, populations of organisms, and their divergence (i.e., speciation). Because of these differences in emphases and disciplinary roots, phylogeography and landscape genetics are considered to differ in three major ways: temporally, methodologically, and spatially.
Temporal Scale
The temporal scale is one of the more common ways of differentiating the fields, with phylogeography examining timescales on the order of millions of years (more typical of lineage divergence and speciation events), but landscape genetics examining more shallow timescales closer to thousands of years BP. However, the advent and proliferation of next-generation sequencing and nonmodel organism genomics now allow evolutionary biologists to investigate both neutral and adaptive genetic diversity at a variety of temporal scales (Bohonak and Vandergast, 2011; Epps and Keyghobadi, 2015). Both historic and contemporary environmental, geographic, and ecological factors influence patterns of genetic variation (e.g., Garrick et al., 2008; Duncan et al., 2015) so the artificial separation of two fields by these criteria creates a situation in which ecologists (who more often focus on current patterns of diversity) and evolutionary biologists (who more often focus on historic mechanisms of diversity) fail to integrate their research (e.g., Knowles and Alvarado-Serrano, 2010; He et al., 2013).
Molecular Markers and Methodology
At the time of phylogeography’s inception (the mid-1980s), the state of the art for genetic analysis was mtDNA. This material was useful for intraspecific studies, but mtDNA has many peculiarities (e.g., incomplete lineage sorting between hybridizing taxa, predominately maternal inheritance, ease of introgression compared with nuclear genes, etc.) that limit its relevance to speciation research. In contrast, landscape genetics studies tended to rely on microsatellite data. The growing use of multilocus and single-nucleotide polymorphisms (SNPs) will help bridge these fields. Modern phylogeography uses all manner of genetic diversity data to ascertain relationships between lineages and geography, and landscape genetics uses highly variable genetic data to test the relative influence of landscape composition and configuration, most often in contemporary settings (Storfer et al., 2007). Therefore, the choice of marker is no longer a useful way to distinguish the fields.
Although a detailed discussion of analytical methods used in the two fields is beyond the scope of this chapter (Storfer et al., 2007), I note that phylogeographic studies tend to emphasize genealogical lineages with a more explicit appreciation of history whereas landscape genetics studies focus on spatially explicit population genetic analyses, with little regard for genealogical relationships of closely related lineages or species (but see Vandergast et al., 2007; Koscinski et al., 2009; Swaegers et al., 2014, 2015). As our ability improves to (i) model past environmental and landscape changes, (ii) describe the tree of life, and (iii) estimate demo-
graphic responses of populations and lineages to spatial and temporal perturbations, the union of phylogeography and landscape genetics will strengthen—one more reason to consider these fields under the single rubric of biogeography.
Space: Which Landscape?
The “adaptive landscape” from the Modern Synthesis is essentially geography-free. Phylogeography, on the other hand, is as much about understanding the physiography of an area as the process of lineage diversification, so the “landscape” is a particular region of interest, like California (e.g., Lapointe and Rissler, 2005), or watersheds in the eastern United States (e.g., Avise et al., 1987), or mountains in Australia (Moritz et al., 2009), or the North Atlantic intertidal (e.g., Wares and Cunningham, 2001). The landscape for landscape genetics studies is also any geographic space, albeit usually at a much smaller scale, encompassing only a portion of any species’ range. Typical landscape genetics studies are less interested in physiography, history, climate, and geology and more focused on a particular species’ or population’s dispersal across space and how those factors influence movement and gene flow. These spaces are often distilled into distance matrices that can be ecological (e.g., environmental climate data—past, present, or future), habitat types (e.g., wetlands, agricultural fields, roads), or any other data layer that represents how an organism may disperse across areas that are more or less permeable to movement. Landscape genetics studies often assess the level of isolation by distance (IBD) (Wright, 1943), isolation by environment (IBE) (Wang and Summers, 2010; Wang and Bradburd, 2014), or isolation by adaptation (IBA) (reviewed in Wang, 2013). Fine-scale ecological data are more likely to provide clear association among ecology, geography, and local adaptation, if it exists, because the spatial scale of genetic variation matches that of the landscape of interest, thus linking ecological and evolutionary pattern and process (e.g., Bermingham and Avise, 1986; Manel et al., 2010; Eckert and Dyer, 2012).
EXAMPLES OF RECENT ADVANCES IN UNDERSTANDING THE BIOGEOGRAPHY OF LIFE
Biogeography, broadly construed, is an integrative science that spans vast temporal and spatial scales encompassing the purview of both phylogeography and landscape genetics. Recent advances in theory, models, and methods have allowed researchers to better synthesize ecological and evolutionary processes in their quest to answer some of the most basic questions in biology, such as why species have range limits in the absence
of geographic barriers. For example, niche theory (Chase, 2011) and ecological niche modeling (e.g., Elith et al., 2006; Kozak et al., 2008; Warren, 2012; Alvarado-Serrano and Knowles, 2014) have been responsible for an exponential increase in studies that use Geographic Information Systems (GIS) and various algorithms (e.g., Phillips et al., 2004, 2006; Elith et al., 2011) to quantify the relationship between species’ distributions and associated environmental parameters (past, current, or future) and project it onto geographic space. Ecological niche models (ENMs) use as input georeferenced specimen data, typically from natural history collections (e.g., www.gbif.org/), and spatially explicit environmental data, often from publically accessible sites like WorldClim (www.worldclim.org/; Hijmans et al., 2005), to examine where organisms should be distributed based solely on the input data, that is, without biogeographic barriers, ecological interactions, or adaptation. Additional investigations using reciprocal transplants (e.g., between allopatric sister species) can illuminate whether ecogeographic isolation mirrors geographic separation, providing insight into the relative roles of ecological adaptation and divergence vs. niche conservatism (discussed in Divergence, Speciation, and Species Delimitation) (Sobel et al., 2010). These kinds of studies that mesh niche theory and modeling are relevant to a vast array of questions in biogeography, ecology, evolutionary biology, and even systematics (e.g., Rissler and Apodaca, 2007).
Another basic question in biogeography that has recently benefited from an interdisciplinary approach is the following: Why are there more species in the tropics? Wiens and coworkers in several publications (e.g., Kozak and Wiens, 2007; Wiens et al., 2009; Hua and Wiens, 2010; Pyron and Wiens, 2013; Chejanovski and Wiens, 2014) have highlighted the importance of investigating the three mechanisms responsible for increased species richness in a region—increased speciation rates, decreased extinction rates, and/or increased dispersal rates (Ricklefs, 1987, 2004; Wiens and Donoghue, 2004). Pyron and Wiens (2013) used phylogenetic comparative methods on a tree of 2,871 species of amphibians and found that tropical regions had higher speciation rates and lower extinction rates, which were strongly linked to ecological factors such as climate. Even within just the temperate zone, Chejanovski and Wiens (2014) found that areas with higher species richness had species with narrower climatic niches, which follows the hypothesis of Janzen (1967), where regions with reduced seasonality (specifically temperature) promote the evolution of species with narrow climatic tolerances. The explicit link among niche breadth, dispersal, and speciation rates (reviewed in Ghalambor et al., 2006) is centered on the idea that species with narrower niche breadths are less able to disperse across environmental gradients, resulting in increased opportunities for allopatric speciation. In summary, the increased availability of large-scale
phylogenetic and phylogenomic data, global environmental data, georeferenced specimen data, and spatial statistics has allowed researchers to more robustly address fundamental and long-standing questions in ecology, evolution, and biogeography.
Additional advances have come in the realm of better and faster computer algorithms. For example, understanding current species distributions and genetic diversity requires accounting for variation in population sizes and migration rates across a species’ temporal and spatial range, such as in the studies of Knowles and Alvarado-Serrano (2010) and Brown and Knowles (2012). Through the use of approximate Bayesian computation (ABC) (Beaumont et al., 2002; Wegmann et al., 2010), they tested the likelihood of different dispersal and demographic scenarios [see He et al. (2013) for an example using the Australian lizard Lerista lineopunctulata], resulting in explanations for current population genetic patterns. He et al. (2013) argue that this approach can result in completely different conclusions than those from more common correlative approaches in landscape genetics, like those of IBD or associated matrices that do not consider the temporal variation in habitat suitability. Such variation can affect population sizes and dispersal patterns resulting from organisms tracking environmental shifts due to niche requirements or moving out of glacial refugia during climate change.
Approaches that consider demographic history have recently been expanded to community-level assemblages (Ghalambor et al., 2006). Again, the advance is mainly a technological one using computationally intensive model testing [e.g., hierarchical approximate Bayesian computation (hABC)] and high-resolution genomic data to estimate demographic scenarios across multiple codistributed species. For example, Xue and Hickerson (2015) developed a new way to leverage single-nucleotide polymorphism datasets to estimate the extent of temporal synchronicity in range expansions across multiple taxa. But simply acknowledging that species interactions within a community can influence genetic diversity of co-occurring species (e.g., Criscione et al., 2006; James et al., 2011) is an important advance in biogeography. Hand et al. (2015) even advocate for a new field—“landscape community genomics”—which would explicitly consider both the biotic and abiotic factors that affect genomic variation. But the same basic questions in biogeography remain. For example, how stable are species associations through space and time? Have multiple species in a community responded similarly to past and current geographic and environmental conditions? Can we predict how species individually, and communities collectively, will respond to climate change? To better answer these questions, we need to integrate ideas, theory, models, and methods across ecology and evolutionary biology rather than balkanize
and separate subdisciplines, as if understanding biogeography is possible without a holistic, interdisciplinary approach (e.g., Riddle et al., 2008).
ECOLOGICAL AND EVOLUTIONARY QUESTIONS THAT WOULD BENEFIT FROM A MORE HOLISTIC VIEW OF BIOGEOGRAPHY
Phylogeography is loosely analogous to historical biogeography (Arbogast and Kenagy, 2001), relying more heavily on history than current ecological conditions, and landscape genetics is loosely analogous to ecological biogeography, relying more heavily on the latter than the former. Modern biogeography, on the other hand, must necessarily consider both historic and current ecological and evolutionary processes. As such, all of the processes that drive the distribution and abundance of genes, organisms, traits, populations, species, and communities are the purview of biogeography. Below are three major, but related, topics that would benefit from synergy among ecologists, evolutionary biologists, and biogeographers.
The Biogeography of Speciation
The modern evolutionary synthesis of the late 1930s made it clear that understanding genetic variation within species is essential for understanding the evolution of higher taxa. Speciation is a predominately geographic phenomenon (Coyne and Orr, 2004). Understanding the relative roles of isolation, gene flow, divergence, and selection on the process of speciation remains a promising area of research. Geographically isolated populations are often viewed as incipient species (Mayr, 1963), and the extent of gene flow between populations is a central focus of landscape genetics. Although Avise in his classic text Phylogeography (Avise, 2000) has a section titled “Genealogical Concordance: Toward Speciation and Beyond,” surprisingly few empirical studies in biogeography explicitly investigate speciation.
The biogeography of speciation is of relatively recent interest (e.g., Coyne and Price, 2000; Losos and Schluter, 2000). For example, Kisel and Barraclough (2010) quantified the speciation-area relationship by examining the probability of in situ speciation on islands of angiosperms, bats, birds, carnivorous mammals, ferns, lizards, butterflies and moths, and land snails. The minimum island size necessary to support speciation was found to scale linearly with the strength of gene flow across almost all of these groups. Snails have speciated on islands smaller than 1 km2, but bats show no in situ speciation on any island except for Madagascar (>500,000 km2) (Kisel and Barraclough, 2010). More recent studies show even stronger associations between island area and the probability of speciation by
using phylogenetic information rather than taxonomy-based approaches (Igea et al., 2015). Despite extensive effort, there are still only a handful of cases of substantial genetic divergence in the face of strong gene flow (e.g., Rhagoletis, Crater Lake cichlids) and even fewer of divergence proceeding to full speciation (reviewed in Coyne, 2011). Accurate assessments of gene flow and phylogenies, increasingly made possible by molecular genetics, will play a crucial role in this endeavor. Spatial statistical methods common to landscape genetics (e.g., isolation by environment while controlling for isolation by distance) have been used to assess the relative influence of divergent selection vs. geographic isolation on speciation. Thus, comparing the relative influence of geography, history (isolation), and ecology (divergent selection) in speciation and biogeography remains an exciting and current question in evolutionary biology at the confluence of phylogeography and landscape genetics (e.g., Papadopulos et al., 2011, 2014; Nosil, 2012; Shafer and Wolf, 2013; Wang, 2013).
Divergence, Speciation, and Species Delimitation
The important role of ecology in evolution was recognized by both Mayr and Dobzhansky (reviewed in Sobel et al., 2010), but, as pointed out by Coyne and Orr (2004), it was not until the 1980s that new tools (e.g., molecular genetics, phylogenetic methods, comparative analyses) allowed molecular systematists to observe fine-scaled genetic differences within species and allowed evolutionary ecologists to apply knowledge of natural selection and biogeographic barriers in nature to questions about speciation. Today, there are many studies of ecological speciation (e.g., Hendry, 2009) defined as reproductive isolation driven by ecological selection, in the presence or absence of gene flow (Schluter, 2001, 2009; Sobel et al., 2010; Nosil, 2012). Importantly, landscape genetics has added many methods that allow one to examine isolation by distance using different metrics (e.g., resistance, geographic barriers, environmental factors, community interactions, and local adaptation) (Nosil et al., 2008; Shafer and Wolf, 2013; Wang, 2013; Wang et al., 2013; Papadopulos et al., 2014) although the link between these measures and broader ideas about how adaptation and natural selection in different environments drive speciation is less appreciated in the field (but see Papadopulos et al., 2014). That said, isolation due to local adaptation is something that links landscape genetics with work on speciation and is often suggested to be a signature of sympatric speciation (e.g., Nosil et al., 2008; Funk et al., 2011; Shafer and Wolf, 2013). But how different abiotic conditions must be to ensure divergent selection even in the face of gene flow, and at what spatial scale, is an open and interesting question (Nosil, 2008).
Ecology and geography may also play a role in speciation, not by causing divergent selection in separated populations, but by the inability of separated populations to adapt to intervening, unsuitable habitat due to strong “niche conservatism” (Wiens, 2004). One study that shows the power of combining phylogeographic analyses with spatially explicit climate data to address questions regarding speciation is that of Cadena et al. (2012). They examined 93 pairs of sister species (mammals, birds, amphibians, and reptiles) that were restricted either to the New World tropics or North Temperate Zone to understand whether niche conservatism or divergence was more likely to explain higher speciation rates in the tropics. They predicted that, if niche conservatism was more important, then they should see greater overlap of the thermal niches of sister species in the tropics, relative to sister species in the temperate zone. Alternatively, if ecological divergence was more important, then the greater climatic stratification along elevational gradients in the tropics would create sister species with less overlap in their thermal niche. Using the WorldClim database (Hijmans et al., 2005), the authors extracted climate data from over 33,000 georeferenced collection localities. They found that tropical sister species had narrower thermal regimes and were more evolutionarily conserved than sister species in the temperate zone, suggesting that populations in the tropics should experience more opportunities for isolation and subsequent speciation via niche conservatism. Recent modeling (Hua and Wiens, 2013) has also advanced our understanding of the link between climate and speciation by making specific predictions about when and where you might expect niche conservatism vs. divergence to be more important in speciation. These studies and future studies that investigate the relative importance of mechanisms (e.g., geographic isolation, strong natural selection, sexual selection) (reviewed in Richardson et al., 2014) capable of initiating and enhancing lineage divergence, at various spatial scales under different environmental conditions, will be key to a better appreciation of the role of ecology and geography in the origination of organismal and trait diversity.
Even systematics has been infused with more biogeography in recent years because of the ubiquity of spatially explicit environmental data and publicly accessible georeferenced specimen data from natural history collections. An early comparative phylogeographic analysis of California by Lapointe and Rissler (2005) took multiple species’ phylogenies (bird, plant, insect, mammal, amphibian, and reptile) and developed a new statistical method to assess concordancy of multiple phylogeographic trees and synthesize the data into a regional supertree. They found that the geographic location of breaks between lineages was correlated with sharp changes in climatic conditions. Later ecological studies in the same geographic region found that the contact zones between parapatrically separated lineages of
an endemic Californian salamander and potential incipient species were inhospitable for migrants and thus barriers to gene flow (Rissler and Apodaca, 2007). These kinds of phylogeographic and landscape genetics studies, which integrate geographic and ecological analyses, have spawned new methods in species delimitation that explicitly consider the extent of ecological divergence when diagnosing species (Raxworthy et al., 2007; Rissler and Apodaca, 2007; Leaché et al., 2009; Pelletier et al., 2015). Thus, understanding the role of ecological and evolutionary processes across a landscape can be illuminating to the study of both the processes and the resulting biogeographic patterns of speciation.
Understanding Adaptation Through Time and Space
Much current research is focused on understanding patterns of adaptive genetic diversity and the ecological and evolutionary processes that cause those patterns (Hancock et al., 2011; Schoville et al., 2012; Jones et al., 2013; Savolainen et al., 2013). Landscape genetics and genomics (Joost et al., 2007) have been touted as providing a unique perspective on local adaptation because space is considered explicitly (Holderegger and Wagner, 2008). A recent example by Vincent et al. (2013) studied gene–environment interactions driving adaptation in 54 North American populations of Atlantic salmon. They found that regional genetic structure and ecological structure (described by 49 environmental variables) were correlated, and, in particular, they noted a signature of thermal local adaptation linked to counter gradient selection imposed by growing season length. However, because landscape genomic studies are generally only correlative, additional studies are needed to confirm adaptation.
New spatial modeling methods have improved our ability to assess the geography of adaptation. For example, Fitzpatrick and Keller (2015), using balsam poplar as a case study, took methods more common to ecology—generalized dissimilarity modeling (Ferrier et al., 2007) and gradient forests (Ellis et al., 2012) [which are regression-based models that map turnover in biological composition (e.g., species) using nonlinear functions of environmental gradients]—and instead mapped the turnover of thousands of single-nucleotide polymorphisms. They found several threshold gene–environment relationships along temperature gradients (e.g., circadian clock gene GIGANTEA-5), suggesting strong local adaptation to temperature. These methods also can be used to forecast temporal changes in genetic composition due to climate change.
SPECIFIC AREAS IN NEED OF GROWTH TO ADVANCE INTEGRATION
Theory and Analytic Methods
Theory and analytic methods are underdeveloped aspects of the growing unification of ecology and evolutionary biology, given the diffusion of genetics into individual, population, community, and even ecosystem questions. The analysis of evolutionary processes should continue to better integrate spatial and ecological data (e.g., Fitzpatrick and Keller, 2015). True integration may require the development of new theory, as well as the development of new analytical methods (Petren, 2013). Populations comprising a single species can vary in multiple traits (e.g., demography, morphology, physiology, connectivity to other populations, genetic diversity, etc.) across a species’ range, a condition that should be more formally integrated into theories and methods. Integrating theory of species range dynamics (Scheiner and Willig, 2011) with microevolutionary processes (genetic drift, mutation, gene flow, and natural selection) is challenging but would advance fields interested in understanding the distribution of organisms (e.g., biogeography, ecology, evolution, and conservation) (Vellend, 2010).
Data: What We Need, Where to Get It, and How to Add to It
A lack of sufficient data plagues many fields, but it is particularly insidious for biogeography because species are currently experiencing an extinction rate at least 1,000 times the background rate (De Vos et al., 2015). We are losing species before they are even formally described (the Linnean shortfall), and, for those that are described (<10% of species on Earth), we have little to no knowledge of their geographic distributions, and even less knowledge of life history characteristics and traits of populations, lineages, and species (the Wallacean shortfall) (Lomolino, 2004; Whittaker et al., 2005). Another impediment is a lack of fine-scale environmental and ecological data, which severely limits our understanding of the biogeography of life on this planet and how it may change given human-caused perturbations.
Large-scale mapping initiatives (e.g., The Global Map project, www.iscgm.org/index.html) have been ongoing for the past 50 years, but those focused on biodiversity data are only about a decade old (e.g., Global Biodiversity Information Facility, www.gbif.org). Linking this information with natural history information is an ongoing challenge but is being tackled by databases like iDigBio (www.idigbio.org), Morphobank (www.morphobank.org), and Phenoscape (www.phenoscape.org). Phylogenetic information is increasing through programs like the National Science
Foundation’s Assembling, Visualizing, and Analyzing the Tree of Life (Avatol, www.avatol.org) and the newer Genealogy of Life, whose aim is an open-access tree of life that encompasses other information, such as traits, geographic distributions, and associated environmental parameters for comparative biological questions. Contributing to the acquisition and synthesis of genomic, phenotypic, and ecological data across populations and lineages within a species, and across species through time and space, is an important endeavor for biodiversity informatics and is critical for advancing our knowledge of life on an ever-changing planet (Losos et al., 2013).
WHAT EXCITING QUESTIONS STILL REMAIN?
Following are a few fundamental questions that span the biogeography–phylogeography–landscape genetics spectrum (Fig. 16.2); they are not new but are examples of important areas of inquiry bridging ecology and evolutionary biology that are ripe for investigation using spatially explicit analyses of genomic-scale data: (i) How much reduction of gene flow, if any, is required to generate new species, and is that gene flow most often reduced by geographic distance, natural selection, or other factors? (ii) Why do species have range limits in the absence of
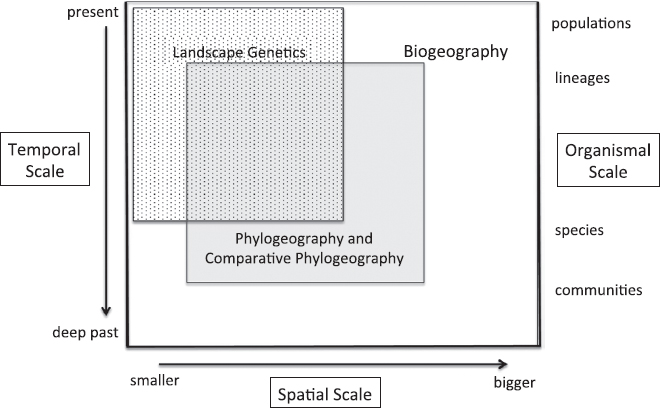
geographic barriers? (iii) What is the relative role of history vs. contemporary processes on genetic diversity patterns of species and thus communities? (iv) Can adaptive evolution and genetic diversity keep pace with the scale of current environmental variability and shifts? (v) Can we predict species’ responses to climate change (including degree of adaptation or phenotypic plasticity), and how might species in a community differ and thus influence coevolutionary or eco-evolutionary dynamics? (vi) How constrained by phylogenetic history and ecology are the evolution of species’ traits, and what explains convergent evolution among suites of traits (functional or otherwise) along environmental gradients across codistributed species?
SUMMARY
Biogeography is the union of ecology and evolutionary biology. It can provide insight into speciation, species delimitations, adaptation, and the future of species in a rapidly changing world. For studies linking geography, ecology, and history in their understanding of genetic variation in nature, we should be using all methods that allow us to gain access to, and understanding of, the processes influencing biodiversity. Why continue to separate fields by the scale of study (temporal or spatial) or the method of analysis? This false separation leads to a real separation in thinking, in training, in practice, and in effort. We owe it to the next generation of scientists to show them the links between the fields and how an integrative approach can yield stronger inferences about some of the most fundamental questions in biology.
ACKNOWLEDGMENTS
I thank J. Avise and F. Ayala for the invitation to contribute to this colloquium; S. Scheiner and two anonymous reviewers for careful reviews of the manuscript; and J. Coyne for discussions on speciation and comments on an earlier draft of this manuscript. The views expressed in this paper do not necessarily reflect those of the U.S. National Science Foundation or U.S. government.


















