2
Precursor Chemicals Used to Make Homemade Explosives
The number of precursor chemicals that can be used to make homemade explosives (HMEs) is large. To prioritize those chemicals, the committee compiled a long list of precursor chemicals; then it established a short list of chemicals of particular concern; and lastly, it applied a set of criteria to the chemicals on the short list and, according to those criteria, it ranked the chemicals in three separate groups: A, B, and C. The Group A precursor chemicals appear to pose the most immediate threat in terms of their potential for use in improvised explosive devices (IEDs), though shifts in bomb makers’ tactics could elevate the status of Group B and C chemicals without warning.
PAST AND RECENT ATTACKS INVOLVING EXPLOSIVES
The committee produced a list of selected explosives incidents, both realized and thwarted, starting with the 1970 Sterling Hall Bombing at the University of Wisconsin (Table 2-1).34-36 This incident was chosen as a logical starting point as it was the first major attack in the United States that employed precursor chemicals to produce the IED’s main charge, specifically, ammonium nitrate (AN) mixed with fuel oil (AN/FO). The main charge of an IED contains the largest amount of explosive; a description of the main charge used in each attack is shown in Table 2-1, along with the estimated mass.
The majority of domestic incidents have used and continue to use commercial explosives, smokeless powder, black powder, flash powder, and pyrotechnic fillers as a main charge likely due to their ease of acquisition (e.g., purchasing 50 pounds of black powder requires no federal license or permit).37,38 These materials have been used in high-profile incidents like the Boston Marathon bombing.4 However,
TABLE 2-1 Selected Attacks Involving Explosives from 1970 to 2016
| Event (Location) | Main Charge | Mass† (lb) |
|---|---|---|
| 1970-Sterling Hall Bombing (Madison, WI) | AN/FO | 2,000 |
| 1983-Beirut Barracks Bombing *Beirut, London) | PETN | 20,000 |
| 1983-US Embassy Bombings (Beirut, Lebanon) | AN/FO | 2,000 |
| 1992-St. Mary Axe Bombing (London, United Kingdom) | CAN/IS | 2,000 |
| 1993-World Trade Center Bombing (New York, NY) | Urea Nitrate | 1,200 |
| 1993-Bishopsgate Bombing (London, United Kingdom) | CAN/IS | 4,000 |
| 1995-Oklahoma City Bombing (Oklahoma City, OK) | AN/NM | 5,000 |
| 1996-Manchester Shopping Mall (Manchester, United Kingdom) | CAN/IS | 3,000 |
| 1996-South Quay Bombing (London, United Kingdom) | CAN/IS | 3,000 |
| 1996-Khobar Towers Bombing (Khobar, Saudi Arabia) | C4 | 20,000 |
| 1998-US Embassy Bombings (Tanzania, Kenya) | TNT | 2,000 |
| 1999-Millennial Bomber Interdiction (Port Angeles, WA) | Urea Nitrate | 500 |
| 2000-USS Cole Bombing (Aden, Yemen) | Mil. Exp. | 1,000 |
| 2001-Shoe Bomber (AA Flight 63) | PETN | 1 |
| 2002-Bali Nightclub Bombing (Bali, Indonesia) | KClO3/S/AI | 2,000 |
| 2003-Marriott Hotel Jakarta Bombing (Jakarta, Indonesia) | KClO3/S/AI | 100 |
| 2003-Britsh Consulate Bombing (Istanbul, Turkey) | AN/AI | 2,000 |
| 2003-Casablanca Bombings (Casablanca, Morocco) | TATP/AN | 20 |
| 2004-Australian Embassy Attack (Jakarta, Indonesia) | KClO3/S/AI | 2,000 |
| 2004-US Consulate Failed Attack (Karachi, Pakistan) | CHP/Flour | 2,000 |
| 2004-Distrupted Jordanian Attack (Amman, Jordan) | CHP/Cumin | 10,000 |
| 2004-US Embassy Attack (Tashkent, Uzbekistan) | AN/AI | 20 |
| 2004-Madrid Train Bombings (Madrid, Spain) | Dynamite | 20 |
| 2005-7/7 Underground Bombing (London, United Kingdom) | CHP/Black Pepper | 20 |
| 2005-7/21 Bombing (London, United Kingdom) | CHP/Flour | 20 |
| 2006-Operation Overt (London, United Kingdom) | CHP/Tang | 1 |
| 2006-Disrupted Plot (Ontario, Canada) | AN/FO | 7,000 |
| 2007-Disrupted Bomb (Ramstein, Germany) | CHP/Flour | 1,000 |
| 2008-US Embassy Attack (Sana’a, Yemen) | TNT | 100 |
| 2009-Underwear Bomber (NWA Flight 253) | PETN | 1 |
| 2009-Operation Highrise Interdiction (Denver, CO/New York, NY) | CHP/Flour | 10 |
| 2010-Printer Bombs (United Kingdom, United Arab Emirates) | PETN | 1 |
| 2010-Failed Times Square Plot (New York, NY) | AN/IS/Sawdust | 100 |
| 2011-Khalid Ali-M Aldawsari Plot (Lubbock, TX) | Picric Acid | 20 |
| 2011-Osla Bombing (Oslo, Norway) | AN/FO/CAN/AI/MB | 2,000 |
| 2002-Aurora Theater Shooting (Aurora, CO) | BP | 20 |
| 2013-Boston Marathon Bombings (Boston, MA) | Pyrotechnic Filler | 20 |
| 2015-Paris Attacks (Paris, France) | TATP | 20 |
| 2016-Brussels Attacks (Brussels, Belguim) | TATP | 40 |
| 2016-Ahmad Khan Rahami (New York/New Jersey) | AN ET/BP/HMTD | 10 |
NOTE: AN: ammonium nitrate, AN/FO: ammonium nitrate/fuel oil, BP: black powder, CAN: calcium ammonium nitrate, CHP: concentrated hydrogen peroxide, HMTD: hexamethylene triperoxide diamine, IS: icing sugar, NM: nitromethane, PETN: pentaerythritol tetranitrate, TATP: triacetone triperoxide, TNT: trinitrotoluene.
†Upper limit of charge mass.
Gray: event involving precursor chemicals. White: event using commercial or military explosives. Black: event with ambiguous sources.
See Appendix C for an expanded table with boosters and initiators.
as shown in Table 2-1, precursor chemicals have played an important role in many bombing incidents over the past several decades.
Events that occurred prior to the Sterling Hall attack primarily relied on commercial explosives (mainly dynamite), with bombers only adopting precursor-based HMEs once commercial explosives became less accessible.
It would be highly impractical to attempt to compile a list of all explosive attacks over the nearly 50-year span covered by Table 2-1. The committee chose to highlight the events in the table for one or more of three reasons:
- events were either high-profile terrorist attacks that garnered appreciable political or public attention, or struck high-profile U.S. targets outside active war zones;
- events used HMEs; and
- events had reliable forensic data with which to identify the charge.
In the 1970s, a large number of small dynamite bombs (less than 20 lb) were used in the United States. While incidents, such as the Harvey’s Casino bombing, that involved dynamite in larger-scale devices garnered significant attention at the time, such incidents are not listed in Table 2-1. Moreover, this list also does not reflect the use of IEDs in active military theaters.
Between the 1970s and 2000, a series of larger vehicle bombs emerged in terrorist attacks with main charges in the thousands of pounds range, but in the
following decade, bombs with smaller charges like those seen in the 1970s started to appear again. By the 2010s, the use of HMEs in smaller charges was growing. Similarly, there was a related expansion from fertilizer-based materials to a more diverse range of possible precursor chemicals.
HMEs are produced either by blending or cooking. Blending is the most common form of manufacture, and the simplest, as it requires only physically mixing the precursor chemicals together. To make a blended explosive, at least one precursor chemical must be an oxidizer (a chemical source of oxygen) and one must be a fuel (a chemical or compound that can react with oxygen in a combustion-like process). The blasting agent AN/FO and flash powder are both examples of blended mixtures.
Cooking, a term borrowed from the narcotics enforcement community, is a more complicated manufacturing process to make HMEs wherein multiple precursor chemicals are mixed together and chemically react to form an explosive material. Triacetone triperoxide (TATP), urea nitrate, and ethylene glycol dinitrate (EGDN) are all made through cooking reactions. For many HMEs, more than one synthetic route is possible, involving different precursor chemicals.
Groups involved in explosive attacks and the types of explosives employed by each are shown in Figure 2-1. Both the Unabomber39 and the Provisional Irish Republican Army (PIRA)40 represent bombing campaigns with roots traced back to the 1970s, and the Fuerzas Armadas Revolucionarias de Colombia (FARC) has a similarly storied history. The remainder of the groups shown in Figure 2-1 include bomb builders in the Iraq and Afghanistan conflicts as well as the newer factions encountered with the rise of ISIS and other extremists. All of these groups use precursor chemicals to produce their HME charges. History has shown that the tactics developed by groups like Al-Qaeda in the Arabian Peninsula have migrated across the world. For example, the trend of using concentrated hydrogen
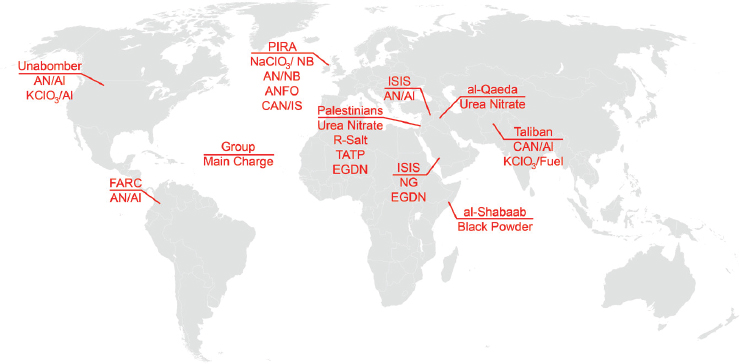
peroxide (CHP) to produce IEDs emerged in Pakistan and rapidly transitioned to Jordan, the United Kingdom, Germany, and, eventually, the United States.41
Case Study: The Evolving Tactics of a Terrorist Group
The attempt to solve a problem by making policy in the midst of or in response to a crisis can create even greater difficulties. Perhaps one of the best historical examples of the pitfalls of narrowly focusing on immediate events, at least in the context of precursor chemicals, is that of the response of the United Kingdom to the explosives produced by PIRA during its bombing campaign.42
The PIRA bombing campaign began around 1971 and employed devices filled with readily available dynamite stolen from quarries and mines. In parallel, during this time frame in the United States, groups such as Weather Underground, Fuerzas Armadas de Liberación Nacional (FALN), and United Freedom Front (UFF) also conducted many attacks using dynamite. Responding narrowly to these events, both the United Kingdom and the United States increased controls on dynamite. In the United States, bombers migrated to readily accessible low-explosive fillers like black powder and smokeless powder (which remain popular choices to this day). Such materials were not accessible in the United Kingdom, but PIRA was able to obtain farm chemicals to replace the dynamite.
The first chemical PIRA used to produce HME mixtures and replace dynamite was sodium chlorate, a strong oxidizer used as a weed killer. Sodium chlorate was mixed with the energetic fuel nitrobenzene to make small explosive charges. To counter the threat of chlorate explosives, the United Kingdom government mandated the addition of a diluent to weed killer to reduce its explosive potential. After chlorate was no longer an option, PIRA turned to AN. Many farmers in Northern Ireland possessed large quantities of AN as it was a chief fertilizer found in agriculture. In addition, with the heavy equipment required for farming, many of the same farmsteads were equipped with diesel tanks and pumps. This combination made for the logical progression of PIRA developing AN/FO-based IEDs.
The transition to AN/FO-based devices by PIRA from its earlier dynamite and chlorate charges had some logistical and tactical consequences. Unlike dynamite, AN/FO is not cap sensitive (the sensitivity of an explosive to initiation by a #8 detonator), does not function properly in small charges, and requires some confinement to reliably function. As a result, the devices produced from AN/FO tended to be larger than the previous dynamite and chlorate devices, and often incorporated metal containers to produce greater confinement. The net result was larger, fragment-producing bombs. These larger, heavier IEDs had to be delivered by vehicles due to their mass. Thus, efforts to keep terrorists from accessing dynamite and chlorate resulted in PIRA’s development of the vehicle bomb.
The United Kingdom, under pressure to address the trend of vehicle-borne IEDs (VBIEDs), passed legislation in 1972 that outlawed the possession of AN fertilizers that contained more than 27.5% nitrogen by mass.25 To replace
the outlawed AN, farmers selected the fertilizer calcium ammonium nitrate (CAN). CAN consisted of AN combined with dolomitic limestone (a blend of calcium and magnesium carbonate). This mixture contained 21% diluent (by weight) to the 79% AN, and was tested and found incapable of being used to produce AN/FO.
It did not take long for PIRA explosives chemists to exploit a simple physical weakness in the new CAN formulation. AN was soluble in water, and the dolomite diluent was not. By mixing the CAN in hot water the AN could be dissolved and separated from the insoluble carbonate component. Once the solid was filtered out, the remaining liquid could be driven off to isolate nearly pure AN. During this time period, United Kingdom authorities came across caches of AN in three purity ranges (100%, 80–90%, and 60%). It is notable that the 60% AN product was actually more dilute than the CAN the terrorists were trying to pull AN out of. The use of CAN in farming did not stop PIRA, but it did make the production of AN-based devices more time consuming and removed the least-adept bomb makers from the picture. Thus, the countermeasure had some limited effect.
Initially, the AN recovered from the recrystallization process was not ideal for AN/FO production. It was coarse and crystalline and would not absorb an optimum amount of diesel. To compensate for this change PIRA began using alternative fuels. One very popular formulation developed was a mixture of AN and nitrobenzene (referred to as ANNIE).
In 1991, approximately 19 years after its introduction, PIRA discovered that crushing the CAN prills into a powdered form using either industrial strength coffee grinders or barley crushers eliminated the need to isolate purified AN. The pulverized CAN could be mixed with a variety of fuels to make an effective explosive filler. Two fuels surfaced as constants: aluminum powder and powdered (icing) sugar. Aluminum was applied consistently for smaller, mortar-borne charges, and sugar was used in the larger-scale VBIEDs. During the 1990s, PIRA perfected the CAN and icing sugar mixture and used it in four major bombings in England. Three of these bombs were deployed against the city of London, and one the city of Manchester. The largest was approximately 4,000 pounds (roughly equivalent to the bomb used in Oklahoma City).
The Taliban’s development of explosives in Afghanistan in recent years and PIRA’s development of countermeasures to overcome attempts to regulate precursor chemicals in the United Kingdom bear striking similarity. The Taliban conducted the same processing operations to weaponize AN as PIRA. However, the Taliban developed its methods in the span of years instead of the decades it took PIRA.
PIRA’s transition from commercial dynamite to a variety of AN-based HME mixtures resembles the paths of other determined terrorist groups. Initially, groups attempt to procure commercial or military explosives if such are accessible. In the absence of available explosives, they look for materials that can be blended
together, such as to make AN/FO. Denied the precursors for simple blends, they resort to processing materials to produce the feedstock of their explosives, such as by isolating AN from CAN. With each level of difficulty introduced into the process, fewer bombers will be successful in their endeavors. However, any government creating controls for precursor chemicals must consider the tactics that will be developed in response.
IDENTIFYING AND PRIORITIZING PRECURSOR CHEMICALS USED IN IED ATTACKS
Precursor chemicals used to produce HMEs for IEDs can be categorized by type and role as oxidizers, fuels (organic materials, energetic organic compounds, food products, or inorganic materials), and synthesis chemicals (including strong and weak acids; Figure 2-2). The figure, which constitutes the committee’s “long list” of precursor chemicals, is not exhaustive, as it would be impossible to list every precursor chemical that has been or can be used in an IED.
Charge Size Analysis
Not all precursor chemicals can be used to make the main charges for every bombing scenario. Figure 2-3 summarizes the various precursor chemicals seen as the main charges for different use-cases: VBIEDs, person-borne IEDs (PBIEDs), aircraft bombings, and detonators. These are not the only possible charges for each use-case.
VBIEDs use charges ranging in mass from approximately 40 pounds to tens of thousands of pounds, depending on the carrying capacity of the vehicle. Precursor chemicals used to produce these explosives tend to be fertilizers (e.g., AN and urea), potassium chlorate, and CHP, given the ability to amass these precursor chemicals in large quantities.
PBIEDs are typically encountered in backpacks, brief cases, small bags, and suicide bombing vests, belts, etc. The charge mass of these devices is predicated on what the individual delivering the charge is capable of carrying. Historically, the charge mass for PBIEDs ranges from approximately 1 to 40 pounds. PBIEDs typically also employ a mass of fragmentation material, such as nails or screws, that can weigh as much as the explosive charge itself.
Explosives used against aviation targets historically have been military formulations due to their reliability and power, although recent terrorist plots against aircraft have used HMEs, albeit below the mass seen in PBIEDs. Terrorists use precursor chemicals frequently in detonator construction, but they also opt for pre-made systems acquired from commercial sources when possible. Detonators use precursor chemicals in very small amounts, but the primary explosives they produce are often very sensitive and unstable. Thus, there is an inherent danger in making, handling, transporting, and storing improvised detonators.

NOTE: Ca2+: calcium; Na+: sodium; K+: potassium; Ba2+: barium; NH4+: ammonium; AN: ammonium nitrate; CAN: calcium ammonium nitrate.
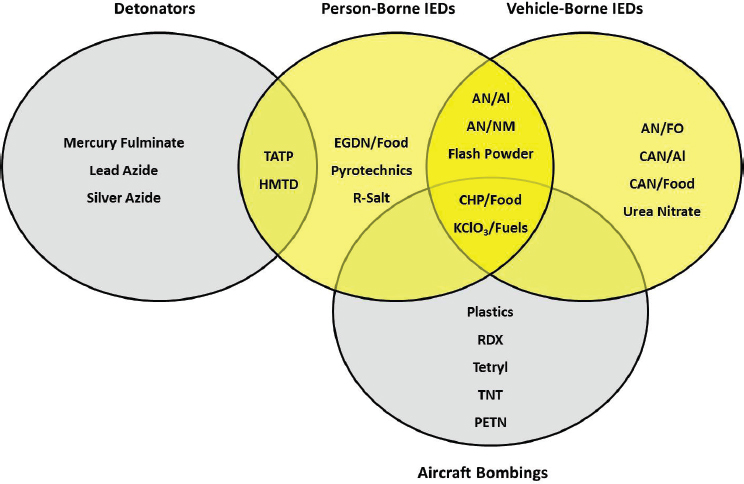
NOTE: TATP: triacetone triperoxide; HMTD: hexamethylene triperoxide diamine; EGDN: ethylene glycol dinitrate; AN: ammonium nitrate; NM: nitromethane; CHP: concentrated hydrogen peroxide; KClO3: potassium chlorate; R-salt: cyclotrimethylenetrinitrosamine. Food products include flour and icing sugar. For a fuller list of food products, refer to Figure 2-2. Fuels include diesel and saw dust.
Due to the lesser orders of magnitude in aviation IED and detonator charge masses—lesser as compared to the VBIEDs and PBIEDs, described above—the committee limited subsequent analysis to those VBIEDs and PBIEDs, both of which entail sufficient risk to merit consideration.
A scenario involving a larger-scale VBIED, such as a truck bomb, could entail substantially more damage than a scenario involving a smaller-scale PBIED, such as a backpack bomb, but be less likely to occur (Appendix B). Thus, the risk of either scenario might rate concern when both severity and probability are included in the assessment. Starting with these scenarios, one can (1) identify the chemicals that terrorists can use to produce each type of device and the conditions under which they can obtain them; (2) develop strategies to reduce the odds of malicious actors getting access to the precursor chemicals; and (3) ultimately, lessen the risk of either scenario by making both scenarios less likely to happen (i.e., lower probability). While beyond the scope of this study, it may also be possible to drive toward scenarios with less lethal or damaging consequence (i.e., lower severity) by changing access to different precursor chemicals.
Generating a Short List of Precursor Chemicals
Every exercise in prioritization, including this winnowing process, has an inherent degree of subjectivity. Any one of the precursor chemicals listed in Figure 2-2 could be used to produce another devastating attack. To generate a more-focused short list of precursor chemicals, the committee considered two variables: quantity required and ubiquity.
First, it was judged impractical to control very small amounts of any particular precursor chemical and, for this reason, precursor chemicals used only to construct charges for detonators (e.g., mercury and lead azide) and aviation IEDs were eliminated from further consideration.
Second, the committee eliminated certain chemicals on the basis of ubiquity. Ubiquity, for the purposes of this study, described chemicals that are present in high volumes and used in myriad common applications in research, industry, and personal use, such that their analysis by the committee was deemed intractable. All the food products (see Figure 2-2) were removed from consideration because of their ubiquity, as were common hydrocarbons such as diesel fuel. Acetone, however, posed a unique challenge. Acetone is slightly less common than household fuels such as kerosene, but its use in academia and chemical processes makes it one of the most ubiquitous general solvents in the world. While acetone can be reacted with hydrogen peroxide to produce the explosive TATP, the committee did not include acetone on the short list because it is not considered a threat if appropriate steps are taken to control the peroxide component.14
By removing precursor chemicals used only in very small amounts and ubiquitous materials, such as food products, the committee narrowed the list of chemicals under consideration to just 28 chemicals, the short list.
Criteria for Generating Groups A, B, and C
To group the short list by priority, the committee adopted three criteria:
- the size of the main charge resulting from the precursor chemical, and whether it can be employed in a VBIED, a PBIED, or both;
- the history of the precursor chemical’s use in IED construction; and
- whether the precursor chemical can be used independently, or is dependent on other precursors listed, for the chemical synthesis of an explosive.
Under the first criterion, the committee focused on precursor chemicals that can result in VBIEDs and PBIEDs. These IEDs have charge sizes ranging from several tons to about a pound, as described previously. Some explosives require a large mass to propagate a detonation, and the precursors needed to produce these explosives may not be suited for the production of smaller charges. Other types of explosives are highly susceptible to detonation, making them impractical or difficult to produce at the hundreds of pounds scale; the precursors for these
sensitive explosives may be limited to use in smaller quantities. Limitations of precursor availability also dictate usage, independent of the properties of the explosives they can make; some chemicals are simply not available in large quantities. Based on all of these factors, a precursor may have utility in either VBIEDs or PBIEDs, or in both.
Under the second criterion, past usage of a precursor was taken as an indicator of its continued potential to be applied in IEDs in the present and future. Some precursor chemicals have been consistently used in IEDs across the world for many decades, while others have seen only brief use by one isolated terrorist group or individual, only to quickly disappear from malicious use.
Under the third criterion, a precursor chemical merits greater priority if it is independent, that is, if the precursor chemical plays an essential part in the synthesis of an explosive material. For example, as seen in Table 2-1 and Figure 2-1, urea nitrate has been used in HMEs in VBIEDs. To synthesize urea nitrate, the precursor chemicals urea and nitric acid are both required; thus, urea nitrate production could be blocked in the absence of either. Of the two, urea is much more commonly available than nitric acid, and the only explosive it can be used to produce is urea nitrate. In contrast, nitric acid can be used to synthesize a variety of other explosive materials. Thus, in this situation, urea would be categorized as dependent (D) on nitric acid, while nitric acid would be judged independent (I).
Application of the Criteria to Precursor Chemicals
The committee assigned each chemical either a higher or lower priority for each criterion. For the first criterion, chemicals limited to use in either vehicle- or person-borne devices (V or P) were assigned lower priority, while those that could be reasonably anticipated to produce both VBIEDs and PBIEDs (V/P) were assigned higher priority. Aspects discussed earlier, such as the safety and commonality of the chemicals, were considered for this analysis (i.e., whether enough of the final main charge explosive material could be assembled from available materials and without killing the bomb maker).
For the criterion of historical usage, chemicals previously used to produce explosives (Y) were assigned higher priority, and those whose usage was either extremely rare or largely theoretical (N) were assigned lower priority. Ratings for this criterion introduced an element of professional judgment. Every chemical on the list had been used in a bombing or in IED production in some capacity at least once. Ratings were made in a conservative fashion when possible, with some chemicals that had been used by single groups, under very limiting circumstances, receiving a lower priority rating. In some cases, chemicals that had limited past usage were given a higher priority rating due to their versatility and potential for explosives production.
For the third criterion, chemicals judged independent in syntheses (I) were assigned a higher priority, and those judged dependent (D) were assigned a lower
priority. In some cases, the committee had to compare a chemical’s global utility to ensure that it rated as dependent for any explosive preparations in which it could be put to use.
The committee sorted the chemicals into three groups based on whether they met the conditions of the higher priority for one, two, or three criteria. The committee placed chemicals that met the conditions of a higher priority for three criteria in Group A; for two criteria in Group B; and for one criterion in Group C. The final evaluation is provided in Table 2-2. Coincidently, the precursor chemicals sorted into three groups of almost equal size. In this study, the committee chose to conduct an in-depth examination of the Group A precursor chemicals.
The decision to include urea ammonium nitrate (UAN) solution in Group A represents the only departure from a strict application of the committee’s ranking principles. UAN is considered a relatively new product with limited geographical distribution, but commercially available. There is a well-documented history of explosives production from analogous urea-nitrate salt solutions used in Iraq. While UAN has not been used historically to produce explosives, the ease of producing various explosives from nitrating urea solutions, as seen in Iraq, supports the notion of UAN as a future threat and justifies its inclusion in Group A.
There is an additional caveat for certain precursor chemicals insofar as they come in a diverse range of concentrations when contained in commercial products or bulk mixtures. For example, hydrogen peroxide as low as 35% can be quickly blended to make an explosive charge if mixed with the proper fuel. While some control strategies specify concentration thresholds (see Chapters 3 and 4), the lack of a scientific consensus on what those thresholds are precluded the committee from including concentration thresholds in the prioritized table (Table 2-2).
CONCLUSION
The National Academies’ 1998 short list, which was later applied by the Department of Homeland Security (DHS) to construct the list of chemicals in the Chemical Facilities Anti-Terrorism Standards (CFATS) Appendix A, only focused on precursor chemicals that made charges with larger mass sizes suitable for VBIEDs. Looking at the trend in Table 2-1, more bombing incidents are reporting smaller charge mass sizes, consistent with PBIEDs. Based on this trend, the committee chose to cast a wider net, by looking at precursor chemicals that can be used to manufacture VBIEDs or PBIEDs, and further prioritized the precursors using three criteria: suitability for large and small charge sizes, hence VBIEDs and PBIEDs; prior use; and dependency.
Every chemical in Table 2-2 is viewed as a viable precursor chemical and a viable threat, whether it has been sorted into Group A, B, or C. Group ranking is
TABLE 2-2 Ranking of Precursor Chemicals into Three Groups
| Charge Size | Prior Use | Dependency | ||
|---|---|---|---|---|
| Group A | Aluminum (powder, paste, flake) | V/P | Y | 1 |
| Ammonium nitrate | V/P | Y | 1 | |
| Calcium ammonium nitrate | V/P | Y | 1 | |
| Hydrogen peroxide | V/P | Y | 1 | |
| Nitric acid | V/P | Y | 1 | |
| Nitromethane | V/P | Y | 1 | |
| Potassium chlorate | V/P | Y | 1 | |
| Potassium perchlorate | V/P | Y | 1 | |
| Sodium chlorate | V/P | Y | 1 | |
| Urea ammonium nitrate solution | V/P | N* | 1 | |
| Group B | Calcium nitrate | V/P | N | 1 |
| Hydrochloric acid | V/P | N | 1 | |
| Potassium nitrate | V/P | N | 1 | |
| Potassium permanganate | P | Y | 1 | |
| Sodium nitrate | V/P | N | 1 | |
| Sodium nitrite | P | Y | 1 | |
| Sulfur | V/P | N | 1 | |
| Sulfuric acid | V/P | Y | D | |
| Urea | V/P | Y | D | |
| Zinc (powder) | P | Y | 1 | |
| Group C | Ammonium perchlorate | P | N | 1 |
| Antimony trisulfide | P | N | 1 | |
| Hexamine | P | Y | D | |
| Magnalium (powder) | P | N | 1 | |
| Magnesium (powder) | P | N | 1 | |
| Pentaerythritol | P | Y | D | |
| Phenol | P | Y | D | |
| Potassium nitrite | P | N | 1 |
NOTE: *See discussion for explanation of including UAN in Group A. V: VBIED, P: PBIED, Y: used historically, N: not used historically, I: independent, D: dependent.
somewhat subjective, and could change depending, for example, on the interpretation of existing data or a shift in terrorist tactics. Continuous reevaluation of the precursors is encouraged by the committee, as some of the rankings may change over time with an evolving threat environment. The committee concentrated its efforts on Group A chemicals when examining the supply chains and existing controls, both discussed in Chapter 3.














