1986b). When the level of exposure was not constant throughout the study period, a time-weighted average daily dose was used to determine the TD50. Although more precise methods of estimating carcinogenic potency with time-dependent exposure patterns are available (Murdoch & Krewski, 1988), this is not critical for our present purposes (cf. Murdoch et al., 1992).
The CPDB includes data on over 3700 experiments on 975 different chemicals conducted under the National Cancer Institute/National Toxicology Program and by other investigators who have reported their results in the scientific literature (Gold et al., 1989). For each chemical, the CPDB may include studies done on different sexes, species and strains; by various routes of exposure; or under other experimental conditions. For each experiment, the doses and crude tumor response rates for each lesion demonstrating evidence of a dose-related effect are provided, thereby affording the opportunity for secondary analyses of the experimental results.
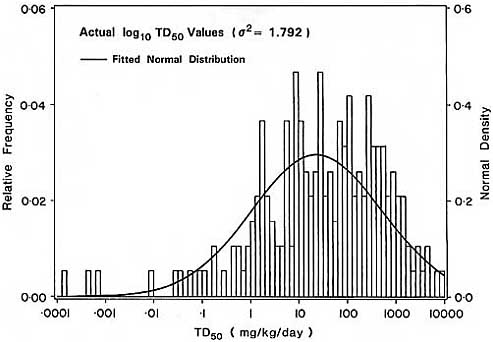
2.3 Variation in Carcinogen Potency
The CPDB includes data on potent chemical carcinogens such as 2,3,7,8-tetraclorodibenzo-p-dioxin (TCDD), as well as less potent compounds such as DDT. Gold et al. (1984) noted that the TD50 value in the CPDB for chemicals inducing tumors in rats varied by seven orders of magnitude or 10 million-fold.
In studying the distribution of carcinogenic potency, Rulis (1986) noted that the TD50 values for 343 rodent carcinogens selected from the CPDB were roughly lognormally distributed. (In cases where more than one experiment was done on the same chemical or where more than one lesion was dose-related in a single study, the minimum TD50 value was used in this analysis.) Similar distributions have been observed using other subsets of the CPDB (Krewski et al., 1990b). For example, consider the distribution of TD50 values shown in Figure 1a for 191 of the 217 compounds considered previously by Krewski et al. (1990b). These compounds were selected to satisfy a number of criteria, including the requirements that the experiment have at least two doses in addition to unexposed controls and demonstrate clear evidence of carcinogenicity. The 26 experiments omitted from the current analysis included only one